Abstract
Olfactory impairment might be an important clinical marker and predictor of Alzheimer’s disease (AD). In the present study, we aimed to compare the degree of olfactory identification impairment in each mild cognitive impairment (MCI) subtype, subjective memory impairment, and early AD dementia and assessed the relationship between olfactory identification and cognitive performance. We consecutively included 50 patients with amnestic MCI, 28 patients with non-amnestic MCI, 20 patients with mild AD, and 17 patients with subjective memory impairment (SMI). All patients underwent clinical and neuropsychological assessments. A multiple choice olfactory identification cross-cultural smell identification test was also utilized. Controlling for age and gender, olfactory impairment was significantly more severe in patients with AD and amnestic MCI compared with the results from the non-amnestic MCI and SMI groups. Higher scores on MMSE, verbal and non-verbal memory, and frontal executive function tests were significantly related to olfactory identification ability. In conclusion, olfactory identification is impaired in amnestic MCI and AD. These findings are consistent with previous studies. In amnestic MCI patients, this dysfunction is considered to be caused by underlying AD pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of olfactory impairment in various neurodegenerative diseases has been documented. This is thought to be attributable to the deposition of pathological proteins and degeneration in the olfactory epithelium, olfactory bulb, entorhinal cortex, hippocampus, and secondary olfactory cortices [1].
Likewise, olfactory dysfunction in Alzheimer’s disease (AD), which is the most common cause of dementia [2], has been well established by numerous epidemiologic studies [3,4,5,6]. The impairment has also been supported by several imaging studies, which showed a correlation between hippocampal atrophy and olfactory dysfunction [7,8,9,10], and autopsy studies [11, 12], which revealed a relationship between olfactory impairment and accumulation of neurofibrillary tangles in central olfactory regions.
In AD, odor identification deficit is the earliest and most prevalent form of olfactory impairment [1, 13]. Consequently, olfactory identification has been repeatedly examined in amnestic mild cognitive impairment (aMCI), which is known to be highly associated with progression to AD [14, 15], and loss of odor identification has been proposed as a predictive marker of conversion from aMCI to AD [16,17,18,19,20].
Meanwhile, to the best of our knowledge, there have been only a few reports regarding associations between odor identification deficit and different subtypes of MCI including non-amnestic MCI (naMCI) [21, 22], which is reported to less frequently convert to AD [23]. In addition, odor identification in subjective memory impairment (SMI), a self-perception of progressive cognitive deterioration, has rarely been investigated, even though SMI is increasingly recognized as a possible preclinical stage of AD [24, 25].
In this study, we assessed odor identification performance in different subtypes of MCI and compared the results with those of participants with SMI and with early AD. We hypothesized that aMCI individuals would have lower odor identification scores than naMCI participants. We also expected that the olfactory function of SMI patients would be different from that of aMCI and AD patients. Furthermore, we analyzed the association between neuropsychological profile and olfactory identification score on the assumption that olfactory impairment is related to cognitive measures of the memory domain.
Methods
Subjects
A total of 115 participants with complaints of cognitive problems were recruited from the Department of Neurology, Seoul St. Mary’s Hospital from January 2016 to December 2016. Clinical information, including age, sex, education status, disease duration, history of hypertension, diabetes mellitus, and smoking status, was obtained. Laboratory tests for variables that can affect cognitive function, including complete blood counts, blood chemistry, homocysteine, vitamin B12, folate level, syphilis serology, and thyroid function, were performed, and patients with any abnormalities were excluded from the study. Apolipoprotein E (APO E) genotype was determined by real-time multiplex PCR blinded to participant status [26].
Subjects were excluded from the study if they presented with history of neurologic diseases (e.g., Parkinson’s disease, stroke, epilepsy, brain tumor), a current psychiatric diagnosis, or rhinological disorders that can have a negative effect on olfaction.
The present study was approved by the local ethics committee, and informed consent was obtained from all participants.
Neuropsychological measures
All patients underwent the following set of neuropsychological tests at the time of enrollment in the study: Mini Mental State Examination (MMSE), Clinical Dementia Rating (CDR), Global Deterioration Scale (GDS), Neuropsychiatric Inventory (NPI), Barthel Index of Activities of Daily Living (ADL), and Seoul Neuropsychological Screening Battery (SNSB) [27]. Quantifiable tests on the SNSB comprise the five domains of attention and working memory, language, visuo-constructive function, verbal and visual memory, and frontal/executive function.
A consensus diagnosis was established using clinical criteria for AD [28] and MCI [29]. Depending on the impaired cognitive domain, MCI was further stratified into MCI with memory impairment (amnestic MCI) and MCI without memory impairment (non-amnestic MCI). In addition, patients who had more than two abnormal domains in the neuropsychological tests were classified as multi-domain MCI, while all others were classified as single-domain MCI. A diagnosis of SMI was made on the basis of a complaint of memory decline, despite the absence of any objective neuropsychological explanation provided by neuropsychological tests [30].
Olfactory assessment
Olfaction was assessed using the Cross-Cultural Smell Identification Test (CCSIT) [31], a widely used test of odor identification involving a scratch-and-sniff test of 12 microencapsulated odorants. Before the CCSIT, nasal problems that evoked olfactory dysfunction were evaluated using an otorhinolaryngological evaluation. CCSIT scores were dichotomized as less than 8 (hyposmia) versus 8 or greater (normosmia).
Statistical analysis
Statistics were calculated using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Group differences were analyzed using chi-square test or independent sample t test for categorical variables and one-way analysis of variance (ANOVA) for continuous variables. Analysis of covariance (ANCOVA) was conducted to compare CCSIT scores after controlling for age, gender, smoking, education, diabetes mellitus, hypertension, and APO ε4 status as covariates. In addition, correlations between CCSIT score and cognitive measures, NPI, and Barthel Index of ADL were evaluated using Spearman correlation analysis. Statistical significance was noted when the p value was less than 0.05.
Results
The demographic and clinical characteristics of the study populations are summarized in Table 1. Of the 115 participants, 17 (14.8%) were classified into the SMI group, 50 (43.5%) were placed into the aMCI group, 28 (24.3%) were placed into the naMCI group, and 20 (17.4%) were classified as early AD. The mean MMSE and CDR scores of the subjects were 24.5 ± 4.3 and 0.5 ± 0.2, respectively. Fifty-three (46.1%) of the subjects were normosmic, whereas 62 (53.9%) had hyposmia. The mean score of CCSIT was 6.9 ± 2.6.
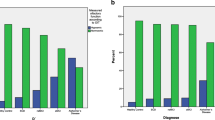
CCSIT scores varied across studied groups, including different subtypes of MCI (p < 0.001) (Fig. 1). The SMI, naMCIsd, and naMCImd groups performed better than the aMCIsd, aMCImd, and AD groups. This trend remained consistent after regrouping MCI patients into aMCI and naMCI. Furthermore, similar group differences were observed after dichotomizing odor identification scores into hyposmia and normosmia. However, ANCOVA showed that CCSIT score was not significantly influenced by APOE ε4 status [6.9 ± 2.6 for APOE ε4 non-carriers vs. 6.7 ± 2.6 for APOE ε4 carriers, p = 0.747].
CCSIT scores of subjects. Values represent the mean with standard error of the mean (SEM) or numbers of patients (percentage). SMI subjective memory impairment, aMCI amnestic mild cognitive impairment, naMCI non-amnestic mild cognitive impairment, sd single-domain, md multi-domain, AD Alzheimer’s dementia. Analyses were performed by the χ2 test and analysis of covariance (ANCOVA) controlling with age, sex, hypertension, diabetes mellitus, and smoking. *p < 0.05; **p < 0.01
Spearman correlation analyses were conducted for the groups of participants to analyze the associations between neuropsychological measures and olfactory performance (Table 2). Moderate correlations were found between CCSIT score and memory tests, immediate and delayed recall in verbal learning test, as well as immediate and delayed recall in RCFT (r = 0.395, 0.467, 0.334, and 0.385, respectively); all were statistically significant (p < 0.001). A moderate correlation was also observed with semantic COWAT (r = 0.507, p < 0.001), while only a weak association was detected with phonemic COWAT (r = 0.246, p = 0.011). Additionally, results of the Boston naming test and Stroop tests (word and color) were moderately correlated with odor identification score (r = 0.358, p = 0.002; r = 0.322, p = 0.001; and r = 0.309, p = 0.001). On the other hand, calculation was not significantly correlated with CCSIT score (p = 0.565). Also, there were no meaningful associations between olfactory performance and digit span tests (forward and backward), which were designed to evaluate attention (p = 0.809 and 0.052, respectively).
In comparison with hyposmic participants, subjects with normosmia had better profiles in global cognition as measured by CDR and GDS (Table 3). Among neuropsychological measures, normosmic participants were significantly outperformed as compared with hyposmic subjects in delayed recall in verbal (5.0 ± 3.1 vs. 2.2 ± 2.7, p = 0.006) and visuospatial (11.9 ± 6.4 vs. 7.9 ± 5.6, p = 0.039) memory tests and semantic COWAT (28.0 ± 10.4 vs. 19.9 ± 7.1, p = 0.014). Results from digit span tests, calculation, and Rey Copy test were not significantly different between the two groups, consistent with the correlation analysis. While hyposmic participants were significantly older than subjects with normosmia (74.7 ± 6.9 vs. 69.6 ± 8.0, p < 0.001), the two groups were not different in other clinical characteristics, including gender, disease duration, diabetes mellitus, hypertension, smoking, and APOE ε4 status. Clinical and neuropsychological characteristics across different cognitive groups are summarized in the supplementary table.
Discussion
The important finding of our study is that the odor identification deficit of the aMCI group was comparable with that of the early AD group, while the naMCI group showed better olfactory performance, similar to that of the SMI group. These results are in line with results published in the literature, which found olfactory identification impairment in AD [11, 32,33,34] and its association with conversion from MCI to AD [8, 18, 35,36,37,38]. The relative preservation of odor identification in the naMCI group can be explained by the heterogeneous etiologies of naMCI, which might represent prodromal stages of dementias not related to AD, such as frontotemporal dementia (FTD) or dementia with Lewy bodies (DLB). This is in contrast with aMCI, most cases of which progress to AD dementia [15]. This explanation is supported in part by a previous study that reported milder impairment of olfactory identification in FTD patients compared with AD patients [39]. The differences in olfactory performance across MCI subtypes can also be explained by the results of our correlation analysis, which revealed significant associations between performance on memory tests and CCSIT score.
The present findings might be based on AD-related neurodegeneration of the olfactory bulb and brain regions, such as the entorhinal cortex and hippocampus, which are responsible for memory and olfaction. In fact, many studies have reported accumulation of AD pathology (amyloid plaques, neurofibrillary tangles, and neuropil threads) and corresponding degenerative changes in the olfactory system and related brain regions [9, 11, 40,41,42]. Moreover, a few neuropathological studies have addressed the association of odor identification with severity of AD pathology [12, 37, 43].
However, considering the results of our correlation analysis, which showed a significant correlation between olfactory performance and memory, pathologic changes in the central olfactory areas, rather than involvement of the peripheral olfactory system, might be the neurobiological basis of the present findings. It has been demonstrated in a meta-analysis that, among several aspects of olfaction, AD patients are more strongly impaired on high-order olfactory tasks compared with low-level perceptual tasks [44]. In addition, an image study demonstrated that there was no significant correlation between olfactory bulb volume and olfactory function, suggesting that hyposmia in AD might be related to degeneration of the central structures of the olfactory system and subsequent cognitive impairment [45].
In particular, the entorhinal cortex, which mediates olfactory information from the primary olfactory system to the hippocampus, might play a central role in AD-related odor identification deficit. While the entorhinal cortex, which is one of the earliest involved sites in AD pathogenesis [46, 47], is well known for its role in generation and retrieval of long-term memories [48, 49], it also plays an important role in integration of sensory input [47]. Furthermore, olfactory structures are the only primary sensory system components that have direct projections to the entorhinal cortex [50]. Thus, presumably, based on the degeneration of the entorhinal cortex, olfactory dysfunction can appear in the very early stage of the disease process in AD, distinct from other sensory modalities. This is consistent with previous studies that have shown that the entorhinal cortex correlates best with initial appearance of cognitive symptoms in AD [33, 51], and the volume of the entorhinal cortex well differentiates converters to AD from non-converters before the onset of overt dementia [47].
In the present study, subjects with SMI displayed olfactory test outcomes similar to those obtained by the naMCI group. Recently, SMI has been proposed as a potential indicator of AD, as associations of SMI with AD biomarkers have been increasingly reported [52,53,54,55]. A cross-sectional study indicated that subjective memory complaints correlated with olfactory identification in elderly individuals [56]. Nonetheless, results from the present study were not consistent with the abovementioned reports, as the olfactory performance of the SMI group was significantly better than those of the aMCI and early AD groups. Several considerations should be addressed regarding these findings. First, as previously reported, SMI is related to various conditions affecting older adults, such as personality traits, medical disorders, and medications, but these were not considered in our study. In particular, psychiatric conditions like depression can be associated with SMI [24, 56, 57] and should have been controlled for in the analysis. Second, according to recent recommendations, SMI with concerns should be distinguished from SMI without concerns [57, 58], which was not presumed to be associated with increased risk of AD dementia. In other words, SMI with concerns is truly relevant to AD dementia, and different outcomes might be revealed if we excluded SMI without concern.
Few studies have discussed odor identification in different subtypes of MCI. The results of this study are consistent with previous longitudinal cohort and case-control studies, which showed an association among olfactory impairment, incident aMCI, and progression from aMCI to AD dementia [21, 38]. In contrast to previous findings, other cross-sectional studies [8, 22] have observed a conflicting result which showed similar degree of odor identification deficit in naMCI patients and aMCI patients. Some authors also reported the olfactory impairment of naMCI as a condition precedent of FTLD, or DLB, which appears to accompany profound olfactory impairment [59, 60]. However, a noteworthy finding of each study is that the degree of olfactory identification impairment was consistently correlated with the results of memory tests among various cognitive measures. Further, even in the study that directly compared aMCI and naMCI with odor identification [22], olfactory deficit in naMCI was not associated with any cognitive correlates. Namely, on the basis of the correlation analysis, it can be postulated that aMCI, which is characterized by deficit in memory measures, rather than naMCI has a legitimate relationship with impairment of odor identification.
There was no significant influence of the APOE ε4 allele in odor identification. Some studies have reported that APOE ε4 allele carriers have stronger associations between impaired olfaction and cognitive impairment than do non-carriers [61,62,63], while several longitudinal studies [8, 17, 38] did not find a significant association between olfactory performance and APOE genotype. Overall, the interaction between odor identification and APOE genotype is not consistent or strong, and the pathophysiological basis for such an association remains unclear [2].
Several limitations of the present study must be noted. First, there are questions regarding generalizability, because of the relatively small sample size, restricted ethnicity, and large proportion of female participants. Second, as the study design was cross-sectional, we could not make precise conclusions regarding the disease trajectory of our MCI subjects. Third, we did not conduct odor detection tests, and some degree of odor detection deficit could have influenced our results. The results of odor detection tests are known to be highly correlated with those of odor identification tests [5]. Lastly, it is well documented that many conditions, including drug intake, exposure to chemical agents, head trauma, systemic diseases like chronic kidney and liver diseases, and chronic alcohol abuse, can interfere with olfactory function [64,65,66,67], but we were unable to exclude people with those conditions.
In conclusion, our findings demonstrated that degree of olfactory impairment is different across MCI subtypes and is mainly driven by deficit in memory measures. Based on these results, it can be suggested that olfactory impairment is related to the pathological process of AD. Longitudinally designed studies with larger sample sizes are needed to validate this suggestion.
References
Attems J, Walker L, Jellinger KA (2014) Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol 127:459–475
Velayudhan L (2015) Smell identification function and Alzheimer's disease: a selective review. Curr Opin Psychiatry 28(2):173–179. https://doi.org/10.1097/YCO.0000000000000146
Doty RL, Reyes PF, Gregor T (1987) Presence of both odor identification and detection deficits in Alzheimer's disease. Brain Res Bull 18(5):597–600. https://doi.org/10.1016/0361-9230(87)90129-8
Morgan CD, Nordin S, Murphy C (1995) Odor identification as an early marker for Alzheimer's disease: impact of lexical functioning and detection sensitivity. J Clin Exp Neuropsychol 17:793–803
Mesholam RI, Moberg PJ, Mahr RN, Doty RL (1998) Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol 55(1):84–90. https://doi.org/10.1001/archneur.55.1.84
Chan A, Tam J, Murphy C, Chiu H, Lam L (2002) Utility of olfactory identification test for diagnosing Chinese patients with Alzheimer's disease. J Clin Exp Neuropsychol 24:251–259
Murphy C, Jernigan TL, Fennema-Notestine C (2003) Left hippocampal volume loss in Alzheimer's disease is reflected in performance on odor identification: a structural MRI study. J Int Neuropsychol Soc 9(3):459–471. https://doi.org/10.1017/S1355617703930116
Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R (2010) Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging 31(9):1593–1600. https://doi.org/10.1016/j.neurobiolaging.2008.09.008
Vasavada MM, Wang J, Eslinger PJ, Gill DJ, Sun X, Karunanayaka P, Yang QX (2015) Olfactory cortex degeneration in Alzheimer's disease and mild cognitive impairment. J Alzheimers Dis 45(3):947–958. https://doi.org/10.3233/JAD-141947
Vassilaki M, Christianson TJ, Mielke MM, Geda YE, Kremers WK, Machulda MM, Knopman DS, Petersen RC, Lowe VJ, Jack CR Jr, Roberts RO (2017) Neuroimaging biomarkers and impaired olfaction in cognitively normal individuals. Ann Neurol 81: 871–882, 6, DOI: https://doi.org/10.1002/ana.24960
Attems J, Lintner F, Jellinger K (2005) Olfactory involvement in aging and Alzheimer's disease: an autopsy study. J Alzheimers Dis 7:149–157
Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA (2007) The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 78(1):30–35. https://doi.org/10.1136/jnnp.2006.099721
Serby M, Larson P, Kalkstein D (1991) The nature and course of olfactory deficits in Alzheimer's disease. Am J Psychiatry 148:357–360
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7(3):270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Petersen RC (2011) Mild cognitive impairment. N Engl J Med 364(23):2227–2234. https://doi.org/10.1056/NEJMcp0910237
Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, Bell K, Stern Y, Mayeux R (2000) Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry 157(9):1399–1405. https://doi.org/10.1176/appi.ajp.157.9.1399
Swan GE, Carmelli D (2002) Impaired olfaction predicts cognitive decline in nondemented older adults. Neuroepidemiology 21(2):58–67. https://doi.org/10.1159/000048618
Conti MZ, Vicini-Chilovi B, Riva M, Zanetti M, Liberini P, Padovani A, Rozzini L (2013) Odor identification deficit predicts clinical conversion from mild cognitive impairment to dementia due to Alzheimer's disease. Arch Clin Neuropsychol 28:391–399
Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK (2014) Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc 20(02):209–217. https://doi.org/10.1017/S1355617713001409
Devanand D, Lee S, Manly J, Andrews H, Schupf N, Doty RL, Stern Y, Zahodne LB, Louis ED, Mayeux R (2015) Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 84(2):182–189. https://doi.org/10.1212/WNL.0000000000001132
Westervelt HJ, Bruce JM, Coon WG, Tremont G (2008) Odor identification in mild cognitive impairment subtypes. J Clin Exp Neuropsychol 30(2):151–156. https://doi.org/10.1080/13803390701287408
Vyhnalek M, Magerova H, Andel R, Nikolai T, Kadlecova A, Laczo J, Hort J (2015) Olfactory identification in amnestic and non-amnestic mild cognitive impairment and its neuropsychological correlates. J Neurol Sci 349:179–184
Busse A, Hensel A, Gühne U, Angermeyer M, Riedel-Heller S (2006) Mild cognitive impairment long-term course of four clinical subtypes. Neurology 67:2176–2185
Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, Buckley RF, Chételat G, Dubois B, Ellis KA, Gifford KA, Jefferson AL, Jessen F, Katz MJ, Lipton RB, Luck T, Maruff P, Mielke MM, Molinuevo JL, Naeem F, Perrotin A, Petersen RC, Rami L, Reisberg B, Rentz DM, Riedel-Heller SG, Risacher SL, Rodriguez O, Sachdev PS, Saykin AJ, Slavin MJ, Snitz BE, Sperling RA, Tandetnik C, van der Flier WM, Wagner M, Wolfsgruber S, Sikkes SA (2015) Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis 48(s1):S63–S86. https://doi.org/10.3233/JAD-150154
Fernandez-Blazquez MA, Avila-Villanueva M, Maestu F, Medina M (2016) Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J Alzheimers Dis 52(1):271–281. https://doi.org/10.3233/JAD-150956
Park HD, Park KU, Kim KW, Song J, Chang HE, Heo SR, Lee HJ, Kim JQ (2007) Real-time multiplex PCR assay for genotyping of three apolipoprotein E alleles and two choline acetyltransferase alleles with three hybridization probes. Clin Chem Lab Med 45(3):346–350. https://doi.org/10.1515/CCLM.2007.075
Kang Y, Na D, Hahn S (2003) Seoul neuropsychological screening battery. Human brain research & consulting co., Incheon
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer Dement 7: 263–269, 3, DOI: https://doi.org/10.1016/j.jalz.2011.03.005
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256(3):183–194. https://doi.org/10.1111/j.1365-2796.2004.01388.x
Stewart R (2012) Subjective cognitive impairment. Curr Opin Psychiatry 25(6):445–450. https://doi.org/10.1097/YCO.0b013e3283586fd8
Doty RL, Marcus A, William Lee W (1996) Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 106:353–356
Talamo BR, Rudel R, Kosik KS, Lee VM-Y, Neff S, Adelman L, Kauer JS (1989) Pathological changes in olfactory neurons in patients with Alzheimer's disease. Nature 337(6209):736–739. https://doi.org/10.1038/337736a0
Braak H, Braak E (1994) Morphological criteria for the recognition of Alzheimer's disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging 15(3):355–356. https://doi.org/10.1016/0197-4580(94)90032-9
Thompson MD, Knee K, Golden CJ (1998) Olfaction in persons with Alzheimer's disease. Neuropsychol Rev 8:11–23
Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H (2008) Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol Aging 29(5):693–706. https://doi.org/10.1016/j.neurobiolaging.2006.11.014
Lehrner J, Pusswald G, Gleiss A, Auff E, Dal-Bianco P (2009) Odor identification and self-reported olfactory functioning in patients with subtypes of mild cognitive impairment. Clin Neuropsychol 23:818–830
Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA (2009) Olfactory impairment in presymptomatic Alzheimer's disease. Ann N Y Acad Sci 1170(1):730–735. https://doi.org/10.1111/j.1749-6632.2009.04013.x
Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, Alhurani RE, Geda YE, Knopman DS, Petersen RC (2016) Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol 73(1):93–101. https://doi.org/10.1001/jamaneurol.2015.2952
Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA (2007) Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia 45(8):1823–1831. https://doi.org/10.1016/j.neuropsychologia.2006.12.008
Kovacs T, Cairns N, Lantos P (1999) Beta-amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer's disease. Neuropathol Appl Neurobiol 25:481–491
Kovács T, Cairns NJ, Lantos PL (2001) Olfactory centres in Alzheimer's disease: olfactory bulb is involved in early Braak's stages. Neuroreport 12(2):285–288. https://doi.org/10.1097/00001756-200102120-00021
Arnold SE, Lee EB, Moberg PJ, Stutzbach L, Kazi H, Han LY, Lee VM, Trojanowski JQ (2010) Olfactory epithelium amyloid-β and paired helical filament-tau pathology in Alzheimer disease. Ann Neurol 67(4):462–469. https://doi.org/10.1002/ana.21910
Growdon ME, Schultz AP, Dagley AS, Amariglio RE, Hedden T, Rentz DM, Johnson KA, Sperling RA, Albers MW, Marshall GA (2015) Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology 84(21):2153–2160. https://doi.org/10.1212/WNL.0000000000001614
Rahayel S, Frasnelli J, Joubert S (2012) The effect of Alzheimer's disease and Parkinson's disease on olfaction: a meta-analysis. Behav Brain Res 231:60–74
Servello A, Fioretti A, Gualdi G, Di Biasi C, Pittalis A, Sollaku S, Pavaci S, Tortorella F, Fusetti M, Valenti M, Masedu F, Cacciafesta M, Marigliano V, Ettorre E, Pagliarella M (2015) Olfactory dysfunction, olfactory bulb volume and Alzheimer's disease: is there a correlation? A pilot study1. J Alzheimers Dis 48:395–402
Gómez-Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT (1996) Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci 16(14):4491–4500
Daulatzai MA (2015) Olfactory dysfunction: its early temporal relationship and neural correlates in the pathogenesis of Alzheimer's disease. J Neural Transm 122(10):1475–1497. https://doi.org/10.1007/s00702-015-1404-6
Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA (1989) Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci 9(12):4355–4370
Zola-Morgan S, Squire LR, Ramus SJ (1994) Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus 4(4):483–495. https://doi.org/10.1002/hipo.450040410
Insausti R, Marcos P, Arroyo-Jimenez M, Blaizot X, Martınez-Marcos A (2002) Comparative aspects of the olfactory portion of the entorhinal cortex and its projection to the hippocampus in rodents, nonhuman primates, and the human brain. Brain Res Bull 57:557–560
Braak H, Braak E (1997) Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 18(4):351–357. https://doi.org/10.1016/S0197-4580(97)00056-0
Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, Schild HH, Scheef L (2006) Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging 27(12):1751–1756. https://doi.org/10.1016/j.neurobiolaging.2005.10.010
Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, Suliman H, Wagner M, Schild HH, Jessen F (2010) Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord 29(1):75–81. https://doi.org/10.1159/000264630
Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ (2012) Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol 69(2):223–229. https://doi.org/10.1001/archneurol.2011.666
van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, van der Flier WM (2013) Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement 9(5):481–487. https://doi.org/10.1016/j.jalz.2012.08.004
Sohrabi HR, Bates KA, Rodrigues M, Taddei K, Laws SM, Lautenschlager NT, Dhaliwal SS, Johnston AN, Mackay-Sim A, Gandy S, Foster JK, Martins RN (2009) Olfactory dysfunction is associated with subjective memory complaints in community-dwelling elderly individuals. J Alzheimers Dis 17(1):135–142. https://doi.org/10.3233/JAD-2009-1020
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chetelat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M; Subjective Cognitive Decline Initiative (SCD-I) Working Group (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 10: 844–852, 6, DOI: https://doi.org/10.1016/j.jalz.2014.01.001
Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mosch E, Kaduszkiewicz H, Pentzek M, Riedel-Heller SG, Luck T, Fuchs A, Weyerer S, Werle J, van den Bussche H, Scherer M, Maier W, Wagner M German Study on Aging, Cognition and Dementia in Primary Care Patients (2014) AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement 10: 76–83
Westervelt HJ, Stern RA, Tremont G (2003) Odor identification deficits in diffuse Lewy body disease. Cogn Behav Neurol 16(2):93–99. https://doi.org/10.1097/00146965-200306000-00002
Rami L, Loy CT, Hailstone J, Warren JD (2007) Odour identification in frontotemporal lobar degeneration. J Neurol 254(4):431–435. https://doi.org/10.1007/s00415-006-0379-5
Bacon AW, Bondi MW, Salmon DP, Murphy C (1998) Very early changes in olfactory functioning due to Alzheimer's disease and the role of apolipoprotein E in olfaction. Ann N Y Acad Sci 855(1 OLFACTION AND):723–731. https://doi.org/10.1111/j.1749-6632.1998.tb10651.x
Graves AB, Bowen J, Rajaram L, McCormick W, McCurry S, Schellenberg G, Larson EB (1999) Impaired olfaction as a marker for cognitive decline interaction with apolipoprotein E ε4 status. Neurology 53: 1480–1487
Wang QS, Tian L, Huang YL, Qin S, He LQ, Zhou JN (2002) Olfactory identification and apolipoprotein E ε4 allele in mild cognitive impairment. Brain Res 951(1):77–81. https://doi.org/10.1016/S0006-8993(02)03137-2
Hastings L, Miller M (2003) Influence of environmental toxicants on olfactory function. Neurol Dis Ther 57:575–592
Murphy C, Doty R, Duncan H (2003) Clinical disorders of olfaction. Neurol Dis Ther 57:461–478
Rupp CI, Kurz M, Kemmler G, Mair D, Hausmann A, Hinterhuber H, Fleischhacker WW (2003) Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcohol Clin Exp Res 27:432–439
Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM (2011) Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope 121(4):873–878. https://doi.org/10.1002/lary.21416
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Park, SJ., Lee, JE., Lee, KS. et al. Comparison of odor identification among amnestic and non-amnestic mild cognitive impairment, subjective cognitive decline, and early Alzheimer’s dementia. Neurol Sci 39, 557–564 (2018). https://doi.org/10.1007/s10072-018-3261-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3261-1