Abstract
Approximately 80% of individuals infected with West Nile virus (WNV) are asymptomatic, and less than 1% suffer from neuroinvasion that can result in permanent neurological deficits or mortality. Our institution’s location in southern California predisposes it to a sizable case volume of neuroinvasive WNV. A 2-year retrospective study was performed at the Olive View-UCLA Medical Center to identify patients with confirmed WNV infection with neuroinvasion. Patient demographics, neurological exam findings, and laboratory diagnostics were reviewed. Data were tabulated and are presented as percentage, mean ± standard deviation, or median [range]. Twenty-two patients (36.4% female, age 50.2 ± 10.6 years) were identified between 20 August 2012 and 24 September 2013. The most common positive findings on review of symptoms included fever (81.8%), nausea/vomiting (81.8%), and headache (68.2%). Thirteen patients (59.1%) presented with fever defined as ≥ 37.8 °C. Motor strength was reduced in nine patients (40.9%) and eight patients (36.4%) were hyporeflexive. Lumbar puncture was performed in all but three patients (cerebrospinal fluid [CSF] protein 76.8 ± 29.6 mg/dL and glucose 71.0 ± 18.8 mg/dL). Elevated CSF anti-WNV IgM and IgG antibody was detected in 93.8% and 62.5% of the 16 tested cases, respectively. Elevated serum anti-WNV IgM and IgG antibody was detected in 100% and 72.2% of the 18 tested cases, respectively. Encephalitic presentations, with or without focal neurological deficits (e.g., motor weakness, hypotonia), dominated this series. In endemic areas, seasonal presentation of such symptoms should raise suspicion for WNV with neuroinvasion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
West Nile virus (WNV), a Flavivirus genus member of the Japanese encephalitis virus antigenic complex, was first isolated in a blood sample in a patient from the West Nile province of Uganda in 1937 [1]. Since its introduction in North America in 1999, WNV has produced the three largest arboviral neuroinvasive disease outbreaks ever recorded in the United States (U.S.) that caused 62 cases of encephalitis and seven deaths [2]. WNV is now endemic throughout the contiguous U.S., with 16,196 neuroinvasive cases and 1549 deaths reported since 1999; more than 780,000 illnesses have likely occurred [3]. For the purposes of this study, neuroinvasion was defined as presenting symptoms of meningitis, encephalitis, or acute flaccid paralysis [3]. Since 2000, the ArboNET national surveillance system has tracked WNV in the U.S., and it is now the leading cause of domestically acquired arboviral disease [4].
Approximately 80% of individuals infected with WNV are asymptomatic, and less than 1% suffer from neuroinvasion that can result in permanent neurological deficits or mortality [5]. Our institution’s location predisposes it to a sizable case volume of neuroinvasive WNV, and the aim of this study was to characterize the presenting features, hospital course, and clinical sequelae of a southern California cohort of patients diagnosed with this disease.
Methods
A 2-year retrospective study (2012–2013) was performed at the Olive View-UCLA Medical Center in Sylmar, California (a WNV endemic area), to identify patients with confirmed WNV infection with neuroinvasion. This retrospective chart review study complied with the principles of the Declaration of Helsinki (1964). This retrospective case series study received approval from the UCLA IRB (IRB No. 16-000791). Laboratory diagnostic criteria for WNV infection included the detection of elevated WNV-specific IgM antibodies in the blood and/or CSF specimens. Detection of anti-WNV antibodies was performed using DxSelect™ enzyme-linked immunosorbent assay performed at Focus Diagnostics, Inc. (Cypress, CA). Patient demographics, neurological exam findings, and laboratory diagnostics were reviewed. Statistical analysis was performed in Intercooled Stata version 9.2 (Stata Corp., College Station, TX). Data were tabulated and are presented as percentage, mean ± standard deviation, or median [range].
Results
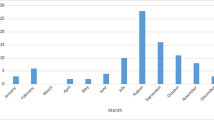
Twenty-two patients (36.4% female, age 50.2 ± 10.6 years) were identified between August 2012 and September 2013. The most common positive findings on review of symptoms included fever (81.8%), nausea/vomiting (81.8%), and headache (68.2%). Thirteen patients (59.1%) presented with fever defined as ≥ 37.8 °C. In regard to the three clinical presenting symptoms described by “neuroinvasion,” 13 patients had an encephalitic presentation (59.1%), five patients had a flaccid paralytic presentation (22.7%), and four patients had a meningitic presentation (18.1%). Table 1 summarizes patient demographics, review of symptoms, and vital signs upon presentation to the emergency room. Figure 1 displays the monthly incidence of hospital admissions for neuroinvasive WNV and monthly mean temperatures [6] during the study period.
Lumbar puncture was performed in all but three patients. The cerebrospinal fluid (CSF) analysis revealed protein 76.8 ± 29.6 mg/dL [reference 15–45 mg/dL] and glucose 71.0 ± 18.8 mg/dL [reference 40–70 mg/dL]. Elevated CSF anti-WNV IgM and IgG antibody was detected in 93.8% and 62.5% of the 16 tested cases, respectively. Elevated serum anti-WNV IgM and IgG antibody was detected in 100% and 72.2% of the 18 tested cases, respectively. Table 2 summarizes the cerebrospinal fluid and blood test results.
On neurological exam, motor strength was reduced in nine patients (40.9%) and eight patients (36.4%) were hyporeflexive. Table 3 summarizes the neurological exam findings upon hospital admission and discharge, as well as at post-hospitalization follow-up in a cohort of five patients (median follow-up 25 days, range 9–38 days). One patient presented with a cranial nerve III palsy that persisted at the time of discharge (hospital day 9). Two patients presented with a flaccid paraplegia that resolved by post-hospital follow-up. Two patients underwent electroencephalography that demonstrated diffuse slowing. None of the patients experienced a clinical seizure. There was a significant reduction in the modified Rankin Scale (mRS) score from hospital admission (median 1.5) to discharge (median 1), p = 0.02 (Wilcoxon rank-sum test). The mRS score improved or stayed the same after the time of discharge in the five patients who were assessed in follow-up clinic (4 → 4, 3 → 0, 3 → 0, 3 → 2, and 1 → 1).
Twelve patients (54.5%) were empirically treated with acyclovir for 1.4 ± 0.7 days prior to determination of the final diagnosis. Eighteen patients (81.8%) were empirically treated with antibiotics for 1.8 ± 0.6 days. In two cases (9.1%), intravenous immunoglobulin was administered. The majority of patients (77.3%) were discharged home after a mean length of stay of 14.7 ± 17.9 days [range 2–75 days], while 18.2% were discharged to an acute rehabilitation unit and one was discharged to a skilled nursing facility. Pearson’s correlation analysis of age and length of stay revealed no significant association (r = 0.18, p = 0.43). Table 4 summarizes the interventions, discharge disposition, and hospital length of stay.
Four patients (18.2%) underwent electromyelogram (EMG)/nerve conduction study (NCS). The baseline and 8-month follow-up EMG/NCS results for the patient who was intubated in the emergency room are presented in Table 5. The initial EMG/NCS in this patient demonstrated decreased amplitude but normal conduction velocity (CV) in the peroneal nerve, whereas both amplitude and CV were normal in the tibial nerve. EMG demonstrated no insertional activity in either nerve, but voluntary motor activity was absent in both. The second EMG/NCS at 8-month follow-up demonstrated unrecordable sensory and motor action potentials due to motor weakness and neuropathy, mostly of an axonal type. The EMG demonstrated positive insertional activities, fibrillations, and positive sharp waves. Voluntary motor units were absent because the patient was very weak and could not generate voluntary action potentials. In summary, the patient’s electrodiagnostic studies provided objective evidence of her poor neurological status since hospital discharge.
Discussion
Transmission of WNV to humans occurs predominantly after a bite from an infected mosquito, although when asked, the patients/family members in this study could not identify if/when they had been bitten by a mosquito. Approximately one in 150 patients infected with WNV develops central nervous system manifestations, including meningitis, encephalitis, and acute flaccid paralysis/poliomyelitis appearance. During the study period, we found that the incidence was highest from mid-August to October in the first year and from July to September in the second year. The earlier incidence of cases in the second year might be explained by the slightly elevated mean monthly temperatures in March, April, May, and June of that year compared to those in the first year. A 2012 mathematical model study by Hartley et al. of the spatiotemporal dynamics of WNV transmission from 2003 to 2009 in California demonstrated that the “temperature-dependent ratio of virus extrinsic incubation period to the mosquito gonotrophic period” and the “fundamental reproductive ratio” correlated with the intensity of WNV transmission [7]. Temperature-based factors that favor the increase of the Culex sp. mosquito populations, the most common species of mosquitoes in the greater Los Angeles, California area, should be considered to increase the risk of WNV infections. In the current study, men had a slightly higher incidence compared to women. The most common signs and symptoms were fever, nausea/vomiting, and headache, and to a lesser extent, dizziness, myalgias, and confusion.
The diagnosis of neuroinvasive WNV in this study was based on the detection of WNV-specific IgM antibodies in patient serum or cerebrospinal fluid. It should be noted that antigenic similarities among members of the Flavivirus genus render anti-WNV IgM-based detection methods susceptible to cross-reactivity, and thus potential false-positive results in the diagnosis of WNV infection. For example, a 2011 study by Papa et al. evaluated 22 patients (who were unvaccinated for Japanese encephalitis virus [JEV], yellow fever virus [YFV], and tick-borne encephalitis virus [TBEV]) who were suspected of having acute neuroinvasive WNV, and their 27 samples (25 sera and two CSF) all tested positive for WNV IgM (and 44.4% tested positive for WNV IgG); the cut-offs for a positive test in that study were ≥ 1.1 for IgM and ≥ 1.5 for IgG [8]. In those 22 patients, 0% tested positive for TBEV IgM, 9.1% tested positive for TBEV IgG, 77.2% tested positive for Dengue virus (DENV) IgM, and 18.2% tested positive for DENV IgG [8]. In this regard, eliminating cross-reactivity using antisera that are elicited by other flaviviruses may help to reduce the cross-reactivity and thus the false-positive rate of a WNV serological diagnosis [9, 10]. The WNV incidence rate in southern California is less than that of JEV, YFV, TBEV, and DENV [11], which reduces the potential for a false-positive diagnosis of neuroinvasive WNV in the current series although it cannot be totally ruled-out. The relationship between the emergence of Zika virus (another member of the Flavivirus genus) in the continental United States and possible cross-reactivity with anti-WNV antibodies used in routine diagnostic testing will need to be further explored over time.
A smaller study of seven patients in Montana reported a predominance of CSF neutrophilia instead of a lymphocytic pleocytosis, with 85.7% of patients having a CSF neutrophil differential greater than 50%; the percentage of patients in our series meeting this definition of neutrophilia was 36.8% [12]. That study also reported a mean serum WBC of 10.5 × 109/L (10.1 × 109/L in our series) and 85.7% of patients with serum sodium less than 135 mmol/L (45.5% in our series). They posited that the neutrophilic predominance may have been due to the early sampling of CSF within 3.5 days (6.9 days in our series), on average, after symptom onset [12]. In 2007, Arjona and colleagues reported that patients suffering from acute WNV infection presented with increased levels of the proinflammatory cytokine macrophage migration inhibitory factor (MIF) in their plasma and CSF [13]. They confirmed the role of MIF in WNV neuroinvasion by studying MIF knock-out mice, which compared to wild-type mice had a reduced viral load and inflammatory response in the brain that were found to be due to MIF-mediated compromise of the blood-brain barrier integrity [13]. We did not evaluate MIF levels in the plasma or CSF of the patients reported in this series.
The neurological exam improved in most patients from initial presentation to discharge, in regard to motor strength and deep tendon reflexes. Although disease severity is usually related to advanced age, there was no significant correlation between age and LOS in this study. The majority of patients were discharged to home. However, 80% of patients at follow-up still had residual weakness at a median of 25 days post-discharge, not inconsistent with the finding from an acute rehabilitation unit of 0% of patients being discharged with full recovery [14]. The main discrepancy between the residual weakness rate in our series and the 37% reported from the Houston West Nile Cohort is that the follow-up in that study was 1–3 years [15], suggesting that the patients from this cohort may go on to regain full motor strength over a longer follow-up time period. The rate and degree of recovery, however, is variable, as demonstrated by Cao and colleagues in 2005 [16].
With respect to morbidity and mortality, no fatalities were observed in this cohort, which is in contrast to the fatality rate reported in other case series [17]. Sixty percent of the patients who had post-hospital neurology follow-up fully recovered, and 20% had chronic complaints of fatigue or headaches. These rates are lower than those reported from the New York City outbreak of 1999 [18, 19]. The lower fatality rate may be explained by increasing recognition of the presenting symptoms of neuroinvasive WNV. Using a WNV strain collected during the 2000 outbreak in New York City, in 2016, Grubagh and colleagues demonstrated that recovered viral populations from avian cells infected with WNV had a higher mutational load relative to the input virus [20]. It is unclear if decreased relative fitness of WNV in the human host could be explained by genetic drift during host switching from the mosquito vector. The number of patients in this study presenting with confusion was low, but more than half of the patients had an abnormal neurological exam. The MRI findings of the patients described in this study have been reported separately [21].
The major limitation of this study is its retrospective nature. An attempt to reduce bias was made by consecutively including all patients in a given time period with diagnostic evidence of neuroinvasive WNV based on CDC criteria. However, controlling for different variables related to diagnosis, neurological exam, and follow-up was not possible. For example, clinically relevant risks for predisposition to neuroinvasive WNV (whether the patients were immunocompromised and/or had a history of domestic or international travel to high risk areas) could not be uniformly studied.
Encephalitic presentations, with or without focal neurological deficits (e.g., motor weakness, hypotonia), dominated this series. Patients had peripheral and central nervous system involvement. Despite the low fatality rate reported in this series, neuroinvasive WNV can cause significant morbidity and remains an important cause of infectious meningoencphalitis and infectious neurological disease in humans. In endemic areas, seasonal presentation of such symptoms should raise suspicion for WNV with neuroinvasion.
References
Smithburn KC, Hughes TP, Burke AW, Paul JH (1940) A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med 20(4):471–492
Nash D, Mostashari F, Fine A, Miller J, O’Leary D, Murray K, Huang A, Rosenberg A, Greenberg A, Sherman M, Wong S, Layton M (2001) The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344(24):1807–1814
Petersen LR, Brault AC, Nasci RS (2013) West Nile virus: review of the literature. JAMA 310(3):308–315
Lindsey NP, Lehman JA, Staples JE, Fischer M (2015) West Nile virus and other nationally notifiable arboviral diseases—United States, 2014. MMWR Morb Mortal Wkly Rep 64(34):929–934
Centers for Disease Control and Prevention (2015) Symptoms & Treatment: West Nile Virus. CDC. http://www.cdc.gov/westnile/symptoms/index.html. Accessed 20 Aug 2017
Past Weather in Sylmar, California, USA. Timeanddate. https://www.timeanddate.com/weather/usa/sylmar/historic. Accessed 27 Sept 2017
Hartley DM, Barker CM, Le Menach A, Niu T, Gaff HD, Reisen WK (2012) Effects of temperature on emergence and seasonality of West Nile virus in California. Am J Trop Med Hyg 86(5):884–894
Papa A, Karabaxoglou D, Kansouzidou A (2011) Acute West Nile virus neuroinvasive infections: cross-reactivity with dengue virus and tick-borne encephalitis virus. J Med Virol 83(10):1861–1865
Niedrig M, Donoso Mantke O, Altmann D, Zeller H (2007) First international diagnostic accuracy study for the serological detection of West Nile virus infection. BMC Infect Dis 7:72
Beck C, Jimenez-Clavero MA, Leblond A, Durand B, Nowotny N, Leparc-Goffart I, Zientara S, Jourdain E, Lecollinet S (2013) Flaviviruses in Europe: complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int J Environ Res Public Health 10(11):6049–6083
California Department of Public Health. CDPH. https://www.cdph.ca.gov. Accessed 27 Sept 2017
Crichlow R, Bailey J, Gardner C (2004) Cerebrospinal fluid neutrophilic pleocytosis in hospitalized West Nile virus patients. J Am Board Fam Pract 17(6):470–472
Arjona A, Foellmer HG, Town T, Leng L, McDonald C, Wang T, Wong SJ, Montgomery RR, Fikrig E, Bucala R (2007) Abrogation of macrophage migration inhibitory factor decreases West Nile virus lethality by limiting viral neuroinvasion. J Clin Invest 117(10):3059–3066
Rao N, Char D, Gnatz S (2005) Rehabilitation outcomes of 5 patients with severe West Nile virus infection: a case series. Arch Phys Med Rehabil 86(3):449–452
Weatherhead JE, Miller VE, Garcia MN, Hasbun R, Salazar L, Dimachkie MM, Murray KO (2015) Long-term neurological outcomes in West Nile virus-infected patients: an observational study. Am J Trop Med Hyg 92(5):1006–1012
Cao NJ, Ranganathan C, Kupsky WJ, Li J (2005) Recovery and prognosticators of paralysis in West Nile virus infection. J Neurol Sci 236(1–2):73–80
Brilla R, Block M, Geremia G, Wichter M (2004) Clinical and neuroradiologic features of 39 consecutive cases of West Nile Virus meningoencephalitis. J Neurol Sci 220(1–2):37–40
Petersen LR, Marfin AA (2002) West Nile virus: a primer for the clinician. Ann Intern Med 137(3):173–179
Asnis DS, Conetta R, Waldman G, Teixeira AA (2001) The West Nile virus encephalitis outbreak in the United States (1999-2000): from Flushing, New York, to beyond its borders. Ann N Y Acad Sci 951:161–171
Grubaugh ND, Weger-Lucarelli J, Murrieta RA, Fauver JR, Garcia-Luna SM, Prasad AN, Black WC 4th, Ebel GD (2016) Genetic drift during systemic arbovirus infection of mosquito vectors leads to decreased relative fitness during host switching. Cell Host Microbe 19(4):481–492
Patel CB, Trikamji B, Mathisen G, Yim C, Zipser B, Mishra S (2016) MRI ventral nerve root enhancement in five patients presenting with extremity weakness secondary to neuroinvasive West Nile virus. J Clin Neuromuscul Dis 18(1):41–43
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Retrospective study
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Patel, C.B., Trikamji, B.V., Mathisen, G.E. et al. Southern California neuroinvasive West Nile virus case series. Neurol Sci 39, 251–257 (2018). https://doi.org/10.1007/s10072-017-3164-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3164-6