Abstract
Technology-supported training is emerging as a solution to support therapists in their efforts providing high-intensity, repetitive, and task-specific treatment, in order to enhance the recovery process. The aim of this review is to assess the effectiveness of different robotic devices (end-effector and exoskeleton robots) in comparison with any other type of intervention. Furthermore, we aim to assess whether or not better improvements are obtained in the sub-acute phase after stroke onset than in the chronic phase. A research was conducted in the electronic bibliographic databases Cochrane, MEDLINE, and EMBASE. A total of 17 studies were included: 14 randomized controlled trials, 2 systematic reviews, and one meta-analysis. Fugl-Meyer and modified Ashworth scale were selected to measure primary outcomes, i.e., motor function and muscle tone. Functional independence measure and motor activity log were selected to measure secondary outcomes, i.e., activities of daily living. In comparison with conventional therapy, the robot-assisted rehabilitation is more effective in improving upper limb motor function recovery, especially in chronic stroke patients. No significant improvements are observed in the reduction of muscle tone or daily living activities. The present systematic review shows that the use of robotic devices can positively affect the recovery of arm function in patients with stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) defines stroke as “rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 hours or longer or leading to death, with no apparent cause other than of vascular origin” [1]. Currently, stroke is the leading cause of adult disability in Western countries [2], and one of the most common causes of death in the world [3]: 80% out of them are first event and 20% relapses. The annual stroke incidence in Italy is approximately 200,000 patients, and the disease is the third cause of death, after cardiovascular diseases and neoplasia.
Incidence increases progressively with age: 75% of strokes affect people over 65 years of age [4]. The majority of people with stroke live with long-term disabilities, leading to serious social and economic impacts. The most frequent impairment caused by stroke is the restriction of motor activity, which reduces muscle movement and mobility [5], although stroke may also lead to a variety of sensory and cognitive disabilities as well. Moreover, the ability to carry out the activities of daily living in an autonomous way and to be engaged in social and community participation is strongly reduced.
More than two-third of all patients affected by stroke have impaired upper limb motor function and have difficulty in independently performing activities of daily living [6]. Six months after stroke, approximately 50% of patients remain with a chronic reduction of arm function [7]. This lack of functional recovery restricts patients’ activities in daily living, decreases productivity, affects social re-integration, and leads to economic burden. Therefore, one of the challenging aspects of stroke rehabilitation is upper limb intervention. While the initial degree of stroke and paresis severity is a good predictor of upper limb function recovery, task-specific, high-intensity exercises in an active, functional, and highly repetitive manner over a large number of trials have been shown to enhance motor recovery [8], even in chronic stages of stroke [9].
Technology-supported training is emerging as a solution to support therapists in their efforts by providing high-intensity, repetitive, and task-specific treatment, to enhance the recovery process and facilitate the restoration of arm function and to relieve pressure on the health system. Electromechanical devices for arm training can be differentiated into exoskeletons and end-effector robots.
End-effector robots hold the patient’s hand or forearm at one point and generate forces at the interface. The system designs for the end-effector trajectories match the hand’s natural trajectory in space for the required task [10]. The joints of end-effector robots do not match with that of the human limb [11]; thus, these devices do not allow the upper limb intersegmentary control. For this reason, end-effector systems are suitable for patients with residual motor skills sufficient to control their movement and should be employed after exoskeleton robots in rehabilitation. Examples of upper limb end-effector robots include the InMotion robot (Massachusetts Institute of Technology, MIT-Manus), the Mirror Image Motion Enabler (MIME), the Bi-Manu-Track, and the Neuro-Rehabilitation-Robot (NeReBot).
Exoskeletons have a structure, which resembles the human upper limb, as robot joint axes match the upper limb joint axes. These devices are designed to operate side by side with the human upper limb, and therefore can be attached to the upper limb at multiple locations [11]. Exoskeletons offer a larger range of motion (up to 7 degrees of freedom) compared to end-effector robots, with guaranteed optimal control of the arm and wrist movement [10]. These systems are suitable for the early stage of rehabilitation, as they do not require significant motor abilities. Examples of exoskeleton robots are ARMin and T-WREX.
The aim of this review is to assess the effectiveness of robot-assisted arm training for improving arm function and activities of daily living in patients after stroke in comparison with any other intervention. Attention is also paid to the different efficacy of exoskeleton and end-effector devices. Finally, we aim to assess if better improvements are obtained in the sub-acute phase after stroke onset, or in the chronic phase.
Methods
Study selection
We included randomized controlled trials (RCTs), randomized controlled cross-over trials (we only analyzed the first period as a parallel group trial), systematic reviews, and meta-analysis. All included studies investigated the effects of robot-assisted training on upper limb recovery after stroke.
Pre-post design studies, as well as case-report and case-series, were excluded for lack of sustainability of the results. We included the randomized controlled trial which met the following criteria: (i) use of robotic device in the experimental therapeutic treatment; (ii) robot therapy was aimed to the recovery of motor control and functional abilities of the upper limb; (iii) control group received any other type of non-robotic intervention (conventional therapy, usual care, etc.). Therefore, studies that compared the effects of two different types of robotic devices or robot-assisted protocols of therapy were excluded.
Systematic reviews that investigated the effects of robot-assisted therapy on motor and functional recovery of the upper limb in patients with stroke were included. We excluded the systematic reviews that selected a variety of study design rather than only randomized controlled trials.
Data extraction
In order to identify studies that potentially fulfill the inclusion criteria, a research was conducted in the electronic bibliographic databases Cochrane, MEDLINE, and EMBASE, without language restriction, using the following MeSH keywords: stroke, robotics, and upper extremity. Studies were collected up to December 21, 2015.
For an initial selection, it was decided to read the titles and the abstracts (if available) of the identified publications. When the abstract was not available, the entire article was read.
Based on the types of studies, participants, interventions, and outcome measures, articles were ranked as relevant, irrelevant, or possibly relevant.
At the first stage, we excluded all articles ranked as irrelevant. Then, we examined the full text for the remaining studies, identifying the ones that fulfilled the inclusion criteria.
The selected publications were reviewed and the following information were extracted:
-
1.
Descriptive information about subjects: number of patients included in the experimental and the control groups, time from stroke onset.
-
2.
Intervention information in both groups: type of robot, methodology, and duration of the treatment interventions.
-
3.
The average gain evaluations at the end of the intervention phase of outcomes, given as the difference between the mean value at T0 and at T1 (mean T1-mean T0), and their standard deviation.
Outcomes
The primary outcome was the impairment in motor function and muscle tone, measured through the Fugl-Meyer score (FM) and the Modified Ashworth Scale (MAS). FM test involves 33 items that assess voluntary movement, reflex activity, grasp, and coordination on a scale, with total scores ranging from 0 (no function) to 66 points (normal function) [12]. MAS (score 0–5) assess the tone of nine muscle groups: shoulder abductors, flexors and extensors of elbow, wrist, fingers, and thumb [13].
The secondary outcome was the activities of daily living (ADL), measured by the functional independence measure (FIM) or the motor activity log (MAL). FIM contains 18 items, and it is divided into six subscales that measure self-care, sphincter control, transfer, locomotion communication, and social cognition ability. Each item is rated from 1 to 7, which is based on the required level of assistance to perform the basic ADL [14]. In the randomized controlled trials included, the motor sections of self-care and transfers specifically involving the upper limb activity (maximum score = 63) were used. MAL is a semi-structured interview in which patients are asked how much (amount of use) and how well (quality of movement) they use their impaired arm to accomplish 30 daily tasks listed on the questionnaire. It uses a 6-point scale, rated from 0 to 5, with higher score indicating better performance [14]. This instrument had good interrater reliability and construct validity [15].
Data analysis
The methodological quality of the RCTs was rated with the PEDro scale [16] and with the AMSTAR tool [17] for the systematic reviews.
Two different comparative analyses were conducted: (i) robot-assisted therapy versus any other intervention and (ii) exoskeleton robot versus end-effector robot. Moreover, we performed a subgroup analysis by subdividing the studies according to the elapsed time from stroke: patients in the sub-acute phase (within 6 months) and patients in the chronic phase (more than 6 months).
Since in many settings different studies used different outcome scales, the treatment effect of an intervention was estimated by pooling the standardized mean difference (SMD) with 95% confidence interval (CI). Heterogeneity was quantified by the estimated between-study variance τ2 and I 2. When the level of heterogeneity was higher than 75%, we considered the results obtained by the application of the random effects model. The meta-analysis was performed using the meta package of R 3.2.3, setting at alpha = 0.05 the statistical significance.
Results
Search results
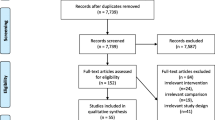
A total of 492 records were identified from the systematic literature search using keywords.
After reading title and abstracts, and removing duplicates, 75 articles were identified.
At the end of selection, two RCTs were excluded as in both the studies only non-parametric measures were employed. Thus, 14 randomized controlled trials, 2 systematic reviews, and 1 meta-analysis met the inclusion criteria and have been included in the present study.
The study selection process is represented in Fig. 1.
The trials selected for the meta-analysis are reported in Table 1, where a detailed description of their duration, frequency of treatment, device employed, and types of participants based on phase after stroke onset is provided for each single study.
Design of included trials
One trial [20] used a crossover design: subjects were randomized to start with conventional or robotic therapy and then crossed over to the other therapy following a washout period. In this case, we used the data of the first period before crossover. All other trails used a parallel group design with randomization to group allocation.
Trial characteristics
The analysis included 14 trials, with a total of 576 participants. One study [28] had four arms and used three treatment groups (robot) and one control group. Two other studies [21, 23] used three arms: two experimental groups (robot) and one control group.
Given that we were interested in the effects of robot therapy versus any other control intervention, we combined the results of the experimental groups in one collapsed group (robot) and compared these with the results of the control group.
One study [24] used three arms: one experimental group (robot) and two control groups (conventional therapy and usual care). Patients receiving usual care were enrolled in the study for 16 months, and those receiving robot-assisted therapy were enrolled for 24 months. The analysis of robot-assisted therapy versus usual care in the study included only patients receiving therapy during the same time period, while the analysis of robot-assisted therapy versus intensive conventional therapy included all patients. Therefore, we decided to keep the analysis data separated.
For the primary outcomes of arm function and muscle tone, Fugl Meyer (FM) was a common scale of all included studies, whereas the Modified Ashworth Scale was represented in eight studies. Concerning the secondary outcome, the trials used different assessment scales in order to measure the activities of daily living. The FIM was used in five studies whereas the MAL in three trials.
Comparisons of robot-assisted therapy versus any other intervention
Figures 2 and 3 show meta-analysis of robot-assisted therapy (treatment) versus any other intervention (control) for both primary outcomes.
All the 14 studies employed the FM scale to measure the arm function at the end of the intervention phase. Although the estimates of the measures of heterogeneity (τ2 = 0.14 and I 2 = 54.9% [19.2%, 74.8%]) indicated that a moderate statistical heterogeneity is present, the fixed model showed a statistically significant improvement of the arm function robot-assisted arm training (0.21 [0.04; 0.38], p value = 0.01).
Eight trials, including 385 participants, employed the MAS to measure the muscle tone at the end of the intervention phase. τ2 = 0 and I 2 = 0% [0%; 42.9%] indicated an absence of statistical heterogeneity. Both fixed effect and random effect models showed that robot-assisted training did not significantly reduce muscle tone in patients (−015 [−0.35; 0.05], p value = 0.15).
Eight trials, including 242 participants, employed two different scales to measure ADL at the end of the intervention phase. The estimates of the measures of heterogeneity τ2 = 0.74 and I 2 = 81.9% [65.5%; 90.5%] indicated a very large statistical heterogeneity across studies, as confirmed by the forest plot in Fig. 4, where controversial effects of the robotic treatment are visible. The overall estimate of the random effects model showed that the robot-assisted therapy did not significantly improve activities of daily living (0.51 [−0.15; 1.17], p value = 0.13). Furthermore, only 2 out of 8 trials showed a worthwhile effect of the robotic treatment on ADL.
Subgroup analysis between sub-acute and chronic phase
We subdivided the trials according to the elapsed time from stroke. Thus, we found six trials including 237 patients in the sub-acute phase (G1) and 8 trials including 339 patients in the chronic phase (G2).
Results of the meta-analysis for FIM (Fig. 5) showed a high heterogeneity across studies in G1 (τ2 = 0.52, I 2 = 81.3% [60%; 91.3%]) with controversial effects of the robotic treatment on FIM. The random effects model indicated a non-significant improvement of the arm function in patients performing the robot-assisted therapy (0.12 [−0.52; 0.76], p value = 0.70). On the contrary, on patients in G2 who were treated with the robot-assisted training, a significant arm function improvement was observed (0.26 [0.05; 0.47], p value = 0.01) (Fig. 6).
All the six trials in G1 and 2 out of 8 trials in G2 used MAS as measure of muscle tone for the 237 and 148 patients included, respectively. We found no evidence that the robot-assisted therapy reduces the muscle tone neither in sub-acute patients (−0.12 [−0.39; 0.14], p value = 0.35) nor in chronic patients (−0.17 [−0.48; 0.13], p value = 0.25).
Four trials in G1 and four trials in G2 measured ADL in their study, including 140 and 102 patients, respectively. We found no evidence that the robot-assisted significantly improves activities of daily living both in sub-acute patients (0.72 [−0.61; 2.05], p value = 0.28) and in chronic patients (0.27 [−0.12; 0.66], p value = 0.17).
Comparisons of end-effector robot versus exoskeleton robot
Since all included studies measured the arm function at the end of the intervention phase by means of the FM scale, we used such a measure for comparing end-effector robot with exoskeleton robot.
Eleven trials, involving 453 participants, employed end-effector devices in the experimental robot-assisted treatment (Fig. 7). The estimates of the measures of heterogeneity (τ2 = 0.19 and I 2 = 62% [28.8%; 79.7%]) indicated a moderate statistical heterogeneity. The fixed effect model showed that end-effector robot training did not significantly improve arm function (0.15 [−0.04; 0.34], p value = 0.11).
The remaining three trials, involving 123 participants, employed exoskeleton devises in their robot-assisted training (Fig. 8). τ2 = 0 and I 2 = 0% [0%; 36%] indicated an absence of statistical heterogeneity. The fixed effect model showed that exoskeleton robot did significantly improve arm function (0.42 [0.06; 0.78], p value = 0.01).
Discussion
The main finding of this systematic review derives from the FM scores of 14 experimental-control trials, with 576 participants. Indeed, the analysis demonstrates a significant effect of the robot-assisted therapy versus all the other different interventions compared. Hence, the use of robotic devices in rehabilitation may improve arm function, in particular in chronic stroke patients. We instead found no significant benefit of robot-assisted therapy over conventional therapy or any other intervention in the sub-acute phase after stroke. Recovery from a stroke event is a complex process that occurs through a combination of spontaneous and mediated processes. Partial structural and functional impairment likely recovers through a potentiation and extension of residual brain areas, whereas complete lesions of specific brain areas require a substitution by functionally related systems [31, 32].
We need to be aware of such processes and related outcomes to better understand when to expect recovery, plan the most appropriate treatment, and determine the timing of rehabilitation [33, 34]. Although it is widely recognized that most spontaneous behavioral recovery tends to occur within the first 3 months after stroke onset, different patterns of recovery may then emerge depending on many complex factors. Indeed, chronic stroke patients may experience cerebral plasticity, as evaluated by transcranial magnetic stimulation and advanced neuroimaging techniques [35]. We are not completely able to state the reason why, in our work, patients in the chronic phase had a better response to robotic treatment than those in the subacute phase, when neuralplasticity is expected to be more evident and efficient. However, we observed heterogeneity in the FM outcome that was mainly caused by the study of Burgar and colleagues [21]: this trial could have biased or hidden the effect size.
We did not found evidence that a robotic rehabilitation may improve activities of daily living, as well as reduce the muscle tone. Further studies with larger sample and focusing on these issues should be fostered to better evaluate whether or not the motor gain is translated in functional gain in the patient’s real life.
Exoskeleton robot-assisted training did significantly improve arm function. However, only 3 out of 14 studies employed exoskeleton devices and, for this reason, there could be a risk of bias due to the small sample size.
Noteworthy, a recent work has demonstrated that the exoskeleton ARMEO induced clinical and kinematic amelioration (i.e., flexor synergies, coordination and speed, passive joint motion, joint pain, sensation, and proprioception of the shoulder, arm, and forearm, as well as self-care functions, mood, and anxiety) through a potentiation of cortical plasticity within the affected hemisphere, besides a reduction of the interhemispheric inhibition. Further studies are thus needed to confirm the findings and to better understand the pathophysiology of post-stroke motor and functional recovery [36].
Scientific evidence shows that a multi-factorial approach, active repetitive practice of movements, and high intensity therapy are able to improve motor recovery of upper limbs in stroke rehabilitation. [37, 38].
The natural response to disability is to learn new ways of accomplishing daily activities, i.e., to develop compensatory behaviors. Stroke survivors with upper extremity impairments typically learn to rely on the non-paretic hand and arm for daily activities, leading to “learned-nonuse,” and thus exacerbating impairments. Moreover, when the paretic limb is forced to move, weakness, sensory impairments, and pain can prevent “normal” movement, and, often, compensatory strategies are used to complete the task [39].
The significant improvements in arm function found in this review confirm that robot-assisted therapy, involving intensive, repetitive, task-oriented exercises can have an effect on recovery from brain injury, enhancing a positive reorganization in the motor cortex and better outcomes.
The present review has a number of limitations. First, in the present study, we pooled functional independence measure scores with the scores from the motor activity log scale to calculate one overall summary effect size for activities of daily living outcome. Second, in two studies, we also pooled together two arms of control or experimental groups to obtain one overall effect size. Third, trials are subject to potential methodological limitations including inability to blind the therapist and participants. These potential limitations introduce the possibility of performance bias.
In summary, our systematic review confirms the potential for robotic-assisted devices to elicit improvements in upper limb function, while improvements in terms of ADLs could not be, to date, sustained. The amount of therapy a patient receives involving direct contact with rehabilitation therapists is often limited by cost considerations; however, an intensity-effect relationship exists between the amount of therapy individuals receive and movement gains achieved. Thus, integration of robot-assisted treatment with the conventional therapy may improve the quality of physical rehabilitation, providing the opportunity for more intensive and independent practice.
References
World Health Organization (2002) The world health report: 2002: reducing risks, promoting healthy life. WHO, Geneva http://www.who.int/whr/2002/en/
Carolei A, Sacco S, De Santis F, Marini C (2002) Epidemiology of stroke. Clin Exp Hypertens 24(7–8):479–483
World Health Organization (2008) The global burden disease: 2004 update. WHO, Geneva http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/
Società Italiana Ipertensione Arteriosa (2014). Ictus: i numeri in Italia. Istituto Superiore Sanità: Progetto cuore. http://siia.it/i-numeri-in-italia/
Mayo NE, Wood-Dauphinee S, Ahmed S, Gordon C, Higgins J, McEwen S, Salbach N (1999) Disablement following stroke. Disabil Rehabil 21(5–6):258–268
Nakayama H, Johrgensen HS, Raaschou HO, Olsen TS (1994) Recovery of upper extremity function in stroke patients: the Copenhagen stroke study. Arch Phys Med Rehabil 75(4):394–398
Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ (2003) Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 34(9):2181–2186
Van Peppen RP, Kwakkel G, Wood-Dauphinee S, Hendriks HJ, Van der Wees PJ, Dekker J (2004) The impact of physical therapy on functional outcomes after stroke: what’s the evidence? Clin Rehabil 18:833–862
Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N (2003) Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil 84(4):477–482
Pignolo L (2009) Robotics in neuro-rehabilitation. J Rehabil Med 41:955–960
Lo HS, Xie SQ (2012) Exoskeleton robots for upper-limb rehabilitation: state of the art and future prospects. Med Eng Phys 34:261–268
Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S (1975) The post-stroke hemiplegic patient 1: a method for evaluation of physical performance. Scand J Rehabil Med 7:13–31
Bohannon RW, Smith MB (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67:206–207
Liao WW, Wu CY, Hsieh YW, Lin KC, Chang WY (2012) Effects of robot-assisted upper limb rehabilitation on daily function and real-world arm activity in patients with chronic stroke: a randomized controlled trial. Clin Rehabil 26(2):111–120
Van der Lee JH, Beckerman H, Knol DL, de Vet HC, Bouter LM (2004) Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke 35:1410–1414
Blobaum P (2006) Physiotherapy evidence database (PEDro). Journal of the Medical Library Association 94(4):477–478
Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, Ramsay T, Bai A, Shukla VK, Grimshaw JM (2007) External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One 2(12):e1350
Sale P, Franceschini M, Mazzoleni S, Palma E, Agosti M, Posteraro F (2014) Effects of upper limb robot-assisted therapy on motor recovery in subacute stroke patients. J Neuroeng Rehabil 11:104
Klamroth-Marganska V, Blanco J, Campen K, Curt A, Dietz V, Ettlin T, Felder M, Fellinghauer B, Guidali M, Kollmar A, Luft A, Nef T, Schuster-Amft C, Stahel W, Riener R (2014) Three-dimensional, task-specific robot therapy of the arm after stroke: a multicentre, parallel-group randomized trial. Lancet Neurol 13(2):159–166
Brokaw EB, Nichols D, Holley RJ, Lum PS (2014) Robotic therapy provides a stimulus for upper limb motor recovery after stroke that is complementary to and distinct from conventional therapy. Neurorehabil Neural Repair 28(4):367–376
Burgar CG, Lum PS, Scremin AM, Garber SL, Van der Loos HF, Kenney D, Shor P (2011) Robot-assisted upper-limb therapy in acute rehabilitation setting following stroke: Department of Veterans Affairs multisite clinical trial. J Rehabil Res Dev 48(4):445–458
Masiero S, Armani M, Rosati G (2011) Upper-limb robot-assisted therapy in rehabilitation of acute stroke patients: focused review and results of new randomized controlled trial. J Rehabil Res Dev 48(4):355–366
Hsieh YW, Wu CY, Liao WW, Lin KC, Wu KY, Lee CY (2011) Effects of treatment intensity in upper limb robot-assisted therapy for chronic stroke: a pilot randomized controlled trial. Neurorehabil Neural Repair 25(6):503–511
Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, Ringer RJ, Wagner TH, Krebs HI, Volpe BT, Bever CT Jr, Bravata DM, Duncan PW, Corn BH, Maffucci AD, Nadeau SE, Conroy SS, Powell JM, Huang GD, Peduzzi P (2010) Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 362(19):1772–1783
Housman SJ, Scott KM, Reinkensmeyer DJ (2009) A randomized controlled trial of gravity-supported, computer-enhanced arm exercise for individuals with severe hemiparesis. Neurorehabil Neural Repair 23(5):505–514
Volpe BT, Lynch D, Rykman-Berland A, Ferraro M, Galgano M, Hogan N, Krebs HI (2008) Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair 22(3):305–310
Masiero S, Celia A, Rosati G, Armani M (2007) Robotic-assisted rehabilitation of the upper limb after acute stroke. Arch Phys Med Rehabil 88(2):142–149
Lum PS, Burgar CG, Van der Loos M, Shor PC, Majmundar M, Yap R (2006) MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: a follow-up study. J Rehabil Res Dev 43(5):631–642
Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML (2005) Computerized arm training improves the motor control of the severely affected arm after stroke: a single-blinded randomized trial in two centers. Stroke 36(9):1960–1966
Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M (2002) Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil 83(7):952–959
Jones TA, Adkins DL (2015) Motor system reorganization after stroke: stimulating and training toward perfection. Physiology (Bethesda) 30(5):358–370
Langhorne P, Coupar F, Pollock A (2009) Motor recovery after stroke: a systematic review. Lancet Neurol 8(8):741–754
Calabrò RS, De Cola MC, Leo A, Reitano S, Balletta T, Trombetta G, Naro A, Russo M, Bertè F, De Luca R, Bramanti P (2015) Robotic neurorehabilitation in patients with chronic stroke: psychological well-being beyond motor improvement. Int J Rehabil Res 38(3):219–225
Verheyden G, Nieuwboer A, de Wit L, Thijs V, Dobbelaere J, Devos H, Severijns D, Vanbeveren S, De Weerdt W (2008) Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil Neural Rep 22:173–179
Volz LJ, Sarfeld AS, Diekhoff S, Rehme AK, Pool EM, Eickhoff SB, Fink GR, Grefkes C (2014) Motor cortex excitability and connectivity in chronic stroke: a multimodal model of functional reorganization. Brain Struct Funct 220:1093–1097
Calabrò RS, Russo M, Naro A, Milardi D, Balletta T, Leo A, Filoni S, Bramanti P (2016) Who may benefit from Armeo power treatment? A neurophysiological approach to predict neurorehabilitation outcomes. PM R 8(10):971–978
Murphy TH, Corbett D (2009) Plasticity during stroke recovery: from synapse to behavior. Nat Rev Neurosci 10:861–872
Kwakkel G, van Peppen R, Wagenaar RC (2004) Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke 35:2529–2539
Raghavan P (2015) Upper limb motor impairment after stroke. Phys Med Rehabil Clin N Am 26(4):599–610
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Rights and permissions
About this article
Cite this article
Bertani, R., Melegari, C., De Cola, M.C. et al. Effects of robot-assisted upper limb rehabilitation in stroke patients: a systematic review with meta-analysis. Neurol Sci 38, 1561–1569 (2017). https://doi.org/10.1007/s10072-017-2995-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-2995-5