Abstract
Multiple sclerosis (MS) is an auto-immune disease whose etiology remains controversial. Both genetic and environmental factors are thought to be involved in the risk of developing the disease. The purpose of our study was to assess the association of Vitamin D receptor (VDR) polymorphisms with MS and to investigate the interaction of these polymorphisms with vitamin D levels. A total of 179 Sicilian subjects, including 104 MS patients and 75 healthy controls, were studied. The most common VDR polymorphisms (Fok-I, Bsm-I, Taq-I and Apa-I) were genotyped by polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) analyses in both groups and serum 25-hydroxyvitamin D [25(OH)D] levels were determined in MS patients by high-performance liquid chromatography (HPLC). The distribution of genotype and allele frequencies of the four VDR polymorphisms did not differ significantly between MS patients and healthy controls, and were unrelated to the forms and the course of MS. Low serum levels of 25(OH)D were observed in MS patients but no association was observed between VDR and 25(OH)D levels except for Fok-I. Moreover, MS patients with FF and Ff genotype had a significantly lower serum levels of 25(OH)D compared with ff carriers (P < 0.05 FF vs Ff and Ff vs ff). Our findings showed no association between VDR polymorphisms and risk of MS. Interestingly, F allele could confer a genetic predisposition to lower 25(OH)D levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic neurodegenerative disease, characterized by demyelination in different areas of the central nervous system. It is the leading cause of non-traumatic disability in young adults aged 20–40 years in North America and Europe. MS is a complex disease whose pathological mechanisms are determined by autoimmune and degenerative processes where genetic and environmental risk factors are supposed to interact differently during individual development and growth [1]. Epidemiological data support an association between poor vitamin D status and higher risk for chronic illness including cancers, cardiovascular morbidity and autoimmune diseases such as systemic lupus erythematosus, type 1 diabetes and multiple sclerosis [2, 3]. Vitamin D is a fat-soluble pro-hormone supplied in the diet and/or formed in the skin upon exposure to ultraviolet radiation (UVR). In the liver, Vitamin D is metabolized to 25-hydroxyvitamin D [25(OH)D], which represents the major circulating vitamin D metabolite and a reliable indicator of vitamin D status. Following the hepatic hydroxylation, 25(OH)D is converted, in the kidney, to its bioactive form, 1,25-dihydroxyvitamin D [1,25(OH)2D], which controls a wide variety of biological responses, including calcium homeostasis, bone formation, cellular growth, proliferation, apoptosis and immune homeostasis [4]. 1,25(OH)2D initiates biological responses via binding to the nuclear vitamin D receptor (VDR), a phosphoprotein which binds the hormone with high affinity and regulates the gene expression via zinc finger-mediated DNA binding and protein–protein interactions. The mechanism underlying the correlation between Vitamin D and autoimmunity is its immunomodulatory function as well as the presence of the VDR on the most immune cells. VDR polymorphisms could provide a genetic basis to MS susceptibility and shed light on the relation between vitamin D and MS. Impaired vitamin D signaling, inadequate vitamin D intake and/or environmental factors (e.g. insufficient sunlight exposure) may contribute to the onset and progression of autoimmunity in MS patients.
In humans, the VDR gene is located at chromosome 12q13.1 and contains nine exons and eight introns. Although over 30 polymorphisms within the VDR gene have been described, only 4 are the most widely studied: Apa-I (rs7975232), Bsm-I (rs1544410), Fok-I (rs10735810) and Taq-I (rs731236). Apa-I (G/T substitution), Bsm-I (A/G substitution) and Taq-I (T/C substitution) are located at the 3′ end of the gene, do not result in structural changes of the VDR protein and they are in strong linkage disequilibrium (LD). The LD extends into the 3′ untranslated region (UTR) of the VDR gene, a region involved in regulating mRNA stability and therefore gene expression. The Fok-I polymorphism is located in the translation initiation site in exon 2 of the VDR gene and results in two different translation initiation sites on the VDR. A thymine (T) to cytosine (C) substitution in the first translation initiation codon ATG generate long (ff) and short variants (FF) of the VDR protein [5].
Studies investigating VDR polymorphisms did not show conclusive results about their possible role in MS susceptibility [6, 7]. Various factors have been claimed to explain these discrepancies such as methodological concerns or possible genetic differences related to the different population investigated. We designed a controlled cross-sectional study to investigate the role of four known common VDR genetic polymorphisms in Sicilian individuals affected by MS. In addition, we have determined 25(OH)D levels in MS patients to evaluate a potential association with VDR polymorphisms and clinical characteristics of the disease.
Materials and methods
Subjects
From June 2013 to December 2014, a total of 104 multiple sclerosis patients who were resident in Sicily, were recruited at the Department of Neurology, University Hospital in Palermo.
VDR polymorphisms were analyzed and compared with those of 75 healthy controls, blood donors, from the same geographical area. The study population characteristics are shown in Table 1.
The protocol was approved by the local medical ethics committee and all subjects agreed to participate after informed consent.
Genetic analysis
Genomic DNA was extracted from 200 μl of whole peripheral blood using a commercial Kit (Qiagen, Valencia, CA, USA) according to manufacturer’s instructions. The DNA quality was evaluated by electrophoresis in a 0.8 % agarose gel, quantified by using absorbance spectrophotometric analysis and stored at −20 °C for subsequent analysis.
The VDR polymorphisms, Fok-I, Bsm-I, Taq-I and Apa-I, were genotyped [8]. Briefly, the polymorphisms were detected by polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) analysis. Primers for VDR gene polymorphisms are listed in Table 2. PCR was performed in a reaction volume of 50 µl containing 300 ng genomic DNA, 50 pmol primers (forward/reverse), 1.5 mM MgCl2, 1.5U Taq Polymerase (EuroClone) and 25 µl sterile double-distilled water.
The following conditions were used for both Fok-I and Bsm-I amplification: 94 °C for 5′, 35 cycles of 94 °C for 1′, 60 °C for 1′ and 72 °C for 1′, followed by 72 °C for 10′. The PCR products were separately restricted in a 25 µl reaction volume for 2 h with Fok-I at 55 °C and Bsm-I at 37 °C, respectively.
Fok-I and Bsm-I genotyping was made as follows FF = 272 bp, Ff = 272, 191 and 81 bp, ff = 191 and 81 bp; BB = 191 bp, Bb = 191,115 and 76 bp, bb = 115 and 76 bp, respectively.
The amplification for Apa-I and Taq-I was performed according to the following program. The mixture was first heated at 93 °C for 10 min and then amplified for 35 cycles by 93 °C for 45 s, 66 °C for 30 s, 72 °C for 45 s, and a final extension at 72 °C for 10 min. PCR product were digested for 2 h with APA I at 25 °C and TAQ I at 65 °C, respectively. APA-I digestion revealed genotypes denoted as AA (740 bp), aa (520 and 220 bp) and Aa (740, 520 and 220 bp); TAQ-I digestion revealed genotypes denoted as TT (245 and 495 bp), tt (290, 245 and 205 bp) and Tt (495, 290, 245 and 205 bp).
All PCR products were separated by electrophoresis on a 2 % agarose gel stained with eurosafe (Euroclone).
Approximately 20 % of the samples were randomly selected and genotyped in duplicate and 10 % of the samples were confirmed by DNA sequencing. All results were concordant.
Measurement of serum 25(OH)D
Serum 25(OH)D levels were determined by high-performance liquid chromatography (HPLC) using a Chromosystem reagent kit (Chromsystems Instruments & Chemicals GmbH; Grafelfing, Munich, Germany) and a chromatographic system equipped with a Waters 1525 Binary HPLC pump connected to a photo diode array detector; detection was carried out at 265 nm. Chromatographic separation was performed as follows: C18 analytical column, column temperature 25 °C, flow rate 0.7 ml min−1, wavelength 265 nm and sample injection volume 50 µl. Chromatographic separation was performed with isocratic elution with retention time of 4.2 min.
In accordance to the kit’s instructions, a serum 25(OH)D concentration of 30 μg/l was considered the threshold value for identifying low levels of vitamin D.
Statistics
Hardy–Weinberg equilibrium (HWE) test of all VDR polymorphism was performed using Michael H. Courts 2005–2008. All statistical analyses were performed using NCSS 10. Differences among groups were evaluated using Kruskal–Wallis test and Mann–Whitney U test for continuous variables.
Any association between a specific genotype or allele and multiple sclerosis was investigated using the Chi-square test. A two-tailed p < 0.05 was considered statistically significant. Odds ratio (OR) was derived with 95 % confidence interval (CI).
Results
Clinical characteristics of MS patients and healthy controls are summarized in Table 1. Both groups were in Hardy–Weinberg equilibrium.
The analysis of VDR polymorphisms did not reveal significant differences in genotypic and allelic frequencies among subjects examined (Table 3). Furthermore, no significant differences between MS patients and healthy controls were detected using dominant and recessive genetic models (Table 4).
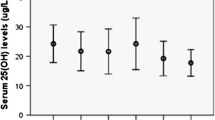
In 104 MS patients we observed low serum levels of 25(OH)D (median 20, interquartile range 15–26 µg/l). We found a trend towards low levels of 25(OH)D in MS patients with progressive forms (SP and PP) compared to the remitting relapsing form, but it was not statistically significant (Table 5). We analyzed VDR polymorphisms for their potential influence on ex vivo levels of 25(OH)D in MS patients but no significant difference was observed among the VDR gene variants of Bsm-I, Taq-I and Apa-I polymorphisms; a significant association between Fok-I and 25(OH)D levels was found. Interestingly, MS patients with FF and Ff genotype had a significantly lower serum levels of 25(OH)D (median 19, interquartile range 14.75–26 µg/l and median 19, interquartile range 15–24 µg/l, respectively) compared with ff carriers (median 26.5, interquartile range 21–35.5 µg/l) (P < 0.05 FF vs. Ff and Ff vs. Ff) Fig. 1.
We did not find any association between serum levels of 25(OH)D, VDR polymorphisms and clinical characteristics of MS such as forms (relapsing-remitting, secondary progressive and primary progressive) and outcome in terms of Expanded Disability Status Scale (EDSS), Multiple Sclerosis Severity Score (MSSS) and Annualized Relapse Rates (ARR).
Discussion
A poor vitamin D status has been documented in MS patients. Although Vitamin D is a potent immune modulator of MS both in vitro and in animal models, the causality of its association with MS is still matter of debate. Since the VDR plays a main role in the vitamin D metabolism, not only as a mediator of biological effects, but also in the regulation of the Vitamin D metabolism itself, the VDR and its genetic polymorphisms may be interesting actors to consider in the MS susceptibility. In our study, the distribution of genotypic and allelic frequencies of Fok-I, Bsm-I, Taq-I and Apa-I polymorphisms was not significantly different between MS patients and healthy controls. Our results are in agreement with studies performed in Caucasian, Spanish [6], Grecian [9], Canadian [10] and American populations [11]. On the contrary, Tunisian, Japanese and Australian studies [12–14] showed a correlation between VDR polymorphisms and MS susceptibility. The heterogeneity of these findings could be related to different ethnic origins of study groups or possibly, to the degree of genetic admixture of the population investigated. As reported by Uitterlinden et al. [15], the f allele of Fok-I and A allele of Apa-I had a lower frequency in the Africans than in Caucasians and Asians, while the B allele of Bsm-I and T allele of Taq-I occurred with lower frequency in the Asians than in Caucasians and Africans.
We found low levels of 25(OH)D in MS patients, in accordance to previous reports [16]. Although 25(OH)D is not the bioactive metabolite of Vitamin D, it best reflects the vitamin D status. A combination of insufficient vitamin D intake and limited solar UV radiation (UVR) exposition due to increasing disability could explain decreased vitamin D levels detected in MS patients. However, it is unknown if the high prevalence of vitamin D deficiency is the cause or the effect of MS. By investigating the potential influence of VDR polymorphisms on serum levels of 25(OH)D in MS patients, we found a significant association only with Fok-I. In particular, lower serum levels of 25(OH)D were observed in MS patients with FF and Ff genotype compared with ff genotypes (Fig. 1). Thus, we can assume that the presence of at least an F allele is associated to lower levels of 25(OH)D. This association has already been described by Orton in a study on twins with multiple sclerosis [17] and by Smolders in a case–control study [18]. Although VDR is downstream of 25(OH)D synthesis in the Vitamin D pathway, it could influence its expression through feed-back regulation mechanisms. After binding to 1,25(OH)2D, the VDR is active and heterodimerization with the retinoid X receptor (RXR) occurs. This heterodimer translocates to the nucleus where binds to vitamin D response elements (VDREs) through transcription factor IIB (TFIIB), resulting in a up or down regulation of vitamin D responsive genes transcription [19]. Several studies showed that short VDR isoform (F allele) is associated with a higher transcriptional activity than long isoform (f allele) [20, 21]. Some authors suggested a role of the VDR in the regulation of enzymes involved in the vitamin D metabolism. Experimental studies, both in vitro and in animal models, revealed that the VDR-1,25(OH)2D complex is a positive regulator of 1,25(OH)2D-24-hydroxylase (also known as CYP24A1), the vitamin D inactivating enzyme, and a negative regulator of both vitamin D-25-hydroxylase (also known as CYP27A1), which catalyzes the 25-hydroxylation of vitamin D, and 25(OH)D-1α-hydroxylase (also known as CYP27B1), which catalyzes the conversion of 25(OH)D to 1,25(OH)2D [22-25]. According to these evidences, we have hypothesized that the F isoform limited serum levels of 25(OH)D, enhancing the inhibitory effect of the VDR on CYP27A1. This could also provide an explanation of contradictory results of randomized trials on beneficial effects of Vitamin D supplementation in MS patients [26–28]. To date there is no evidence of the efficacy and safety of MS treatment with high doses of vitamin D. However, although we have found low serum levels of 25(OH)D in MS patients and an association between the Fok-I VDR polymorphism and 25(OH) levels, we did not observe any expected association of the polymorphism with MS. Thus, probably, genetic VDR variants are a determinant of the Vitamin D status that acts in concert with other genetic and environmental factors in MS susceptibility. Moreover, not only the genetic background, but also personal, social, and cultural factors have been shown to be important determinants of the vitamin D status via their influence on sun exposure and diet [29, 30]. It is reasonable that genetic variants have a main role in maintaining a Vitamin D adequacy in regions where UVR exposure is limited or is seasonally limited [17]. Indeed, it is well known that the MS prevalence is higher in areas with lower solar UVR exposure that represents the main source of vitamin D; an inverse correlation between outdoor activity (thus sunlight exposure) and MS susceptibility has been documented [31, 32].
Vitamin D has been associated, not only with MS risk, but also with the disease course. Smolders and colleagues reported that both the 25(OH)D and the 1,25(OH)2D were significantly lower in the progressive forms compared to the relapsing remitting (RR)MS phenotype. In RRMS patients, high 25(OH)D levels were associated with a high chance of remaining relapse-free and low 25(OH)D levels were associated with high EDSS-scores [33]. We observed a trend towards low 25(OH)D levels in RR MS patients compared to SP and PP MS patients but it was not statistically significant, probably because our MS population included few patients with progressive MS forms. Moreover, we failed to replicate the association of low 25(OH)D levels with a greater degree of disability; MS patients included in our study had low EDSS, MSSS and ARR scores.
A genome-wide association study (GWAS) found a relationship with genetic region containing enzymes involved in the vitamin D metabolism [34] but few data are available on the potential influence of VDR genetic variants on the disease course. We found that the distribution of genotypic frequencies of Fok-I, Bsm-I, Apa-I and Taq-I did not differ among the MS forms, progression and severity of the disease evaluated by EDSS, MSSS and ARR.
This study has some limitations, such as, in particular, the cross-sectional nature of the study and the lack of assessment of average sun exposure in the study sample. In addition, it was carried out on a relatively small sample size of populations; thus further studies involving large cohorts are required. It was not an exhaustive examination of variants in the VDR gene and the selected polymorphisms did not provide a full tagging coverage. Therefore, we cannot exclude that other genetic variants of the VDR could be associated to MS susceptibility. Moreover, the analyses of other genes involved in the Vitamin D metabolism, such as the Vitamin D-binding protein, Vitamin D 25-hydroxylase and CYP27A1 and CYP27B1 could be interesting. The MS study group had mild disability and as a consequence, in order to assess the course of the disease, a cohort including patients with a more severe disease should be analyzed. Finally, we measured serum levels of 25(OH)D only in MS patients.
In summary, our findings showed no direct association of Fok-I, Bsm-I, Taq-I and Apa-I polymorphisms of the VDR with MS risk in Sicilian population. With regard to the Fok-I polymorphism, we observed an association of the F allele with low serum levels of 25(OH)D in MS patients. We did not identify a role of the Vitamin D metabolism, both in terms of serum 25(OH)D levels and VDR variant genetics, in the disease course.
Abbreviations
- EDSS:
-
Expanded disability status scale
- MSSS:
-
Multiple sclerosis severity score
- ARR:
-
Annualized relapse rates
References
Ascherio A, Munger KL, Lünemann JD (2012) The initiation and prevention of multiple sclerosis. Nat Rev Neurol 8:602–612
Agmon-Levin N, Theodor E, Segal RM, Shoenfeld Y (2013) Vitamin D in systemic and organ-specific autoimmune diseases. Clin Rev Allergy Immunol 45(2):256–266
Burton JM, Costello FEJ (2015) Vitamin D in multiple sclerosis and central nervous system demyelinating disease—a review. Neuroophthalmol 35(2):194–200
Huang J, Xie ZF (2012) Polymorphisms in the vitamin D receptor gene and multiple sclerosis risk: a meta-analysis of case-control studies. J Neurol Sci 313(1–2):79–85
Ingles SA, Haile RW, Henderson BE, Kolonel LN, Nakaichi G, Shi CY, Yu MC, Ross RK, Coetzee GA (1997) Strength of linkage disequilibrium between two vitamin D receptor markers in five ethnic groups: implications for association studies. Cancer Epidemiol Biomark Prev 6(2):93–98
García-Martín E, Agúndez JA, Martínez C, Benito-León J, Millán-Pascual J, Calleja P, Díaz-Sánchez M, Pisa D, Turpín-Fenoll L, Alonso-Navarro H, Ayuso-Peralta L, Torrecillas D, Plaza-Nieto JF, Jiménez-Jiménez FJ (2013) Vitamin D3 receptor (VDR) gene rs2228570 (Fok1) and rs731236 (Taq1) variants are not associated with the risk for multiple sclerosis: results of a new study and a meta-analysis. PLoS One 8(6):e65487
Tizaoui K, Kaabachi W, Hamzaoui A, Hamzaoui K (2015) Association between vitamin D receptor polymorphisms and multiple sclerosis: systematic review and meta-analysis of case-control studies. Cell Mol Immunol 12(2):243–252
Györffy B, Vásárhelyi B, Krikovszky D, Madácsy L, Tordai A, Tulassay T, Szabó A (2002) Gender-specific association of vitamin D receptor polymorphism combinations with type 1 diabetes mellitus. Eur J Endocrinol 147(6):803–808
Sioka C, Papakonstantinou S, Markoula S, Gkartziou F, Georgiou A, Georgiou I, Pelidou SH, Kyritsis AP, Fotopoulos A (2011) Vitamin D receptor gene polymorphisms in multiple sclerosis patients in northwest Greece. J Negat Results Biomed 5(10):3
Steckley JL, Dyment DA, Sadovnick AD, Risch N, Hayes C, Ebers GC (2000) Genetic analysis of vitamin D related genes in Canadian multiple sclerosis patients. Canadian Collaborative Study Group. Neurology 54(3):729–732
Simon KC, Munger KL, Yang Xing, Ascherio A (2010) Polymorphisms in vitamin D metabolism related genes and risk of multiple sclerosis. Mult Scler 16(2):133–138
Ben-Selma W, Ben-Fredj N, Chebel S, Frih-Ayed M, Aouni M, Boukadida J (2015) Age- and gender-specific effects on VDR gene polymorphisms and risk of the development of multiple sclerosis in Tunisians: a preliminary study. Int J Immunogenet 42(3):174–181
Fukazawa T, Yabe I, Kikuchi S, Sasaki H, Hamada T, Miyasaka K, Tashiro K (1999) Association of vitamin D receptor gene polymorphism with multiple sclerosis in Japanese. J Neurol Sci 166(1):47–52
Tajouri L, Ovcaric M, Curtain R, Johnson MP, Griffiths LR, Csurhes P, Pender MP, Lea RA (2005) Variation in the vitamin D receptor gene is associated with multiple sclerosis in an Australian population. J Neurogenet 19(1):25–38
Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338(2):143–156
Duan S, Lv Z, Fan X, Wang L, Han F, Wang H, Bi S (2014) Vitamin D status and the risk of multiple sclerosis: a systematic review and meta-analysis. Neurosci Lett 570:108–113
Orton SM, Morris AP, Herrera BM, Ramagopalan SV, Lincoln MR, Chao MJ, Vieth R, Sadovnick AD, Ebers GC (2008) Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr 88(2):441–447
Smolders J, Damoiseaux J, Menheere P, Tervaert JW, Hupperts R (2009) Fok-I vitamin D receptor gene polymorphism (rs10735810) and vitamin D metabolism in multiple sclerosis. J Neuroimmunol 207(1–2):117–121
Smolders J, Peelen E, Thewissen M, Menheere P, Tervaert JW, Hupperts R, Damoiseaux J (2009) The relevance of vitamin D receptor gene polymorphisms for vitamin D research in multiple sclerosis. Autoimmun Rev 8(7):621–626
Jurutka PW, Remus LS, Whitfield GK, Thompson PD, Hsieh JC, Zitzer H, Tavakkoli P, Galligan MA, Dang HT, Haussler CA, Haussler MR (2000) The polymorphic N terminus in human vitamin D receptor isoforms influences transcriptional activity by modulating interaction with transcription factor IIB. Mol Endocrinol 14(3):401–420
Gross C, Krishnan AV, Malloy PJ, Eccleshall TR, Zhao XY, Feldman D (1998) The vitamin D receptor gene start codon polymorphism: a functional analysis of FokI variants. J Bone Miner Res 13(11):1691–1699
Dusso AS, Brown AJ, Slatopolsky E (2005) Vitamin D. Am J Physiol Renal Physiol 289(1):F8–F28
Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S (1997) 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Scienc. 277(5333):1827–1830
Theodoropoulos C, Demers C, Delvin E, Ménard D, Gascon-Barré M (2003) Calcitriol regulates the expression of the genes encoding the three key vitamin D3 hydroxylases and the drug-metabolizing enzyme CYP3A4 in the human fetal intestine. Clin Endocrinol (Oxf) 58(4):489–499
Theodoropoulos C, Demers C, Petit JL, Gascon-Barre M (2003) High sensitivity of rat hepatic vitamin D3-25 hydroxylase CYP27A to 1,25-dihydroxyvitamin D3 administration. Am J Physiol Endocrinol Metab 284(1):E138–E147
Leitner HH (2012) A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 78(11):840–841
Soilu-Hänninen M, Aivo J, Lindström BM, Elovaara I, Sumelahti ML, Färkkilä M, Tienari P, Atula S, Sarasoja T, Herrala L, Keskinarkaus I, Kruger J, Kallio T, Rocca MA, Filippi M (2012) A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 83(5):565–571
Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L (2012) Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler 18(8):1144–1151
Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O’Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD (2010) Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 17 376(9736):180–188
Lucas RM, Ponsonby AL, Dear K, Valery PC, Taylor B, van der Mei I, McMichael AJ, Pender MP, Chapman C, Coulthard A, Kilpatrick TJ, Stankovich J, Williams D, Dwyer T (2013) Vitamin D status: multifactorial contribution of environment, genes and other factors in healthy Australian adults across a latitude gradient. J Steroid Biochem Mol Biol 136:300–308
Kampman MT, Wilsgaard T, Mellgren SI (2007) Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 254(4):471–477
Van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, Butzkueven H, Kilpatrick T (2003) Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 9 327(7410):316
Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R (2008) Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler 14(9):1220–1224
Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 10 476(7359):214–219
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No financial support has been used.
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Agnello, L., Scazzone, C., Ragonese, P. et al. Vitamin D receptor polymorphisms and 25-hydroxyvitamin D in a group of Sicilian multiple sclerosis patients. Neurol Sci 37, 261–267 (2016). https://doi.org/10.1007/s10072-015-2401-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2401-0