Abstract
The value of C-reactive protein (CRP) as a prognostic tool in stroke patients is unclear. The aim of this study is to explore the prognostic impact of CRP levels assessed at different time points on functional outcome in a large cohort of thrombolysed acute stroke patients. All thrombolysed stroke patients admitted to our department were entered in an open, prospective database. Clinical and demographic data were recorded. CRP was measured upon admission, within 24 h, and in the following days. Functional outcome was assessed using the modified Rankin Scale (mRS) at 3 months. Among 1242 thrombolysed patients, we found a statistically significant difference in median CRP values upon admission, within 24 h, and follow-up with respect to outcome parameters (p < 0.001) including symptomatic intracerebral hemorrhage (sICH; p < 0.001). In regression models, follow-up CRP showed better predictive properties for outcome parameters compared to CRP assessed upon admission or within 24 h. The ROC analysis showed a good predictive value of follow-up CRP concerning dependent outcome [c-statistic 0.71 (95 % CI 0.67–0.75) p < 0.001] and mortality [c-statistic 0.70 (95 % CI 0.66–0.75) p < 0.001]. After adjustment for risk factors, follow-up CRP, but not admission CRP, was independently associated with dependent outcome (OR 2.67, 95 % CI 1.76–4.06; p < 0.001), mortality (OR 2.53, 95 % CI 1.50–4.25; p < 0.001), and sICH (OR 3.03, 95 % CI 1.51–6.06; p = 0.002). Follow-up CRP is strongly associated with functional outcome, sICH, and mortality after 90 days in thrombolysed stroke patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because the inflammatory response has been implicated in the pathogenesis of atherosclerosis and cerebral ischemia [1, 2], various markers of inflammation are under investigation as potential predictors of cerebrovascular events and as prognostic indicators for subsequent functional outcome [3–5]. C-reactive protein (CRP) is a frequently studied biomarker that is elevated in the acute phase of both myocardial infarction and ischemic stroke [6, 7]. However, CRP levels are also increased during the acute phase of infection [8], which often follows acute stroke.
Higher CRP levels in stroke patients are associated with mid- and long-term unfavorable functional outcome, larger infarct volume, and early neurological deterioration [4]. However, results of previously conducted research remain controversial, as some studies have been unable to establish a prognostic value and others convey high predictive value for CRP regarding stroke outcome [9–19].
Nonetheless, verification of the relevance of CRP as a robust prognostic tool for stroke outcome is of clinical relevance, as it is an easily and quickly measurable biomarker.
The aim of our study was to explore the prognostic impact that CRP levels assessed at different time points have on functional outcome in a large cohort of acute stroke patients. Because we have a well-organized and controlled prospective database of thrombolysis patients, we addressed this question using this cohort.
Materials and methods
Patients
All patients admitted to our tertiary care hospital who underwent intravenous thrombolysis for acute ischemic stroke were included into an open, prospective database. Initial stroke severity was assessed using the National Institute of Health Stroke Scale (NIHSS). Demographics, vascular risk factors, laboratory results, clinical characteristics, and previous medication were recorded. All patients provided venous blood samples for CRP evaluation upon emergency room admission, within 24 h of the acute event, and in the following days according to clinical routine. We defined follow-up CRP as the maximum CRP value obtained between days 2 and 7 (or discharge).
The presence of post-stroke infection was assessed using information collected from medical records. Based on these results, we defined infections according to the following criteria: (1) pneumonia, defined by clinical criteria, chest X-ray, and the presence of purulent sputum; (2) urinary tract infection, diagnosed based on leukocyte count >36/ml (indicated as threshold for pyuria by our local laboratory) and positive nitrites on urine screening; (3) presence of SIRS or sepsis as defined by the ACCP/SCCM Consensus Conference Committee; (4) thrombophlebitis or wound infection; and (5) when an empirical antibiotic treatment was started because of clinically suspected infection. Elevated leukocyte count, elevated CRP levels, or fever without any clinical signs of infection was not considered. Patients underwent a non-contrast brain CT or MRI scan before treatment and a routine brain CT scan 20–36 h after thrombolysis (or earlier in cases where clinical deterioration was present). Infarct size was defined as extension of the hypodensity involving the respective vessel territory and categorized as no infarct, small infarct (≤1/3 of the vessel territory), or large territorial infarct (>1/3 of the vessel territory). Stroke subtypes were classified according to the TOAST classification scheme. Symptomatic intracerebral hemorrhage (sICH) was assessed using ECASS II (European-Australasian Acute Stroke Study) criteria.
Outcome parameters
Functional outcome after 90 days was assessed using the modified Rankin Scale (mRS). Independent outcome was defined as mRS of 0–2 and dependent outcome as mRS 3–5. Neurological visits after 90 days were made in the outpatient setting or by standardized telephone interview.
Statistical analysis
We compared prevalence and extent of different characteristics in the groups of patients with independent 90-day outcome (mRS 0–2), dependent outcome (mRS 3–5) or death. Additionally, we compared patients with and without sICH. We used linear trend tests and ordinal logistic regression to assess differences in characteristics across mRS groups. Because age was strongly associated with 90-day functional outcome, we provided additional age-adjusted p values. To compare baseline characteristics across patients with and without sICH, we used the Fisher’s exact test (categorical variables) and Mann–Whitney U test (continuous variables). The maximum value of CRP after admission and during follow-up was chosen for further statistical analysis. In Table 1, we provide patient characteristics and their association with different CRP values using descriptive statistics (median, interquartile ranges) and Mann–Whitney U tests.
We performed an ordinal regression analysis to identify the best predictive model for outcome comparing the different CRP values (admission, within 24 h, and maximum value of 7-day follow-up-measures) including only those patients with all three measures available. We tested the proportion of correct classified patients with regard to functional outcome using McNemar Tests.
Receiver–operator curves (ROC) were developed for outcome (dependent, independent, and mortality). Areas under the curve (c-statistic) and 95 % CI were calculated as a measure of predictive ability.
The outcomes analyzed in multiple logistic regression models were sICH, dependent outcome, and mortality after 90 days, which were analyzed separately. Variables that showed relevant associations (p < 0.05) to the outcome variable in bivariate analyses were included as independent variables into regression models. All statistical analyses were performed using the SPSS software (Version 22.0). A significance level of α = 0.05 was used. No adjustment for multiple testing was applied.
Results
In total, 1242 stroke patients were registered in our local thrombolysis database. Baseline characteristics are listed in Table 2.
Admission CRP values (<5 mg/l) were normal in 1016 (78.6 %) patients. The median CRP value on (1) admission was 0.0 mg/l, (2) within 24 h after admission 7.4 mg/l, and (3) on follow-up 31.1 mg/l. The median hospital stay of all patients was 6 days. Twenty-two percent of patients developed an infection during hospital stay and 25 % had a large territorial infarction. Symptomatic hemorrhage after thrombolytic treatment occurred in 6.8 % of all patients and 7.1 % died within the first 24 h after thrombolytic treatment. After 90 days, 43 % had an independent outcome and 11 % had died.
Bivariate analyses revealed that coronary heart disease and infections were significantly associated with higher CRP values upon admission as well as with CRP within 24 h after admission, and follow-up CRP (Table 1). Older age (>65), presence of previous stroke, hypertension, diabetes, atrial fibrillation, large brain infarction, and higher stroke severity (NIHSS > 10) were significantly associated with higher CRP within 24 h after admission and follow-up (Table 1). No significant association was found between different CRP values and atherothrombotic stroke.
Stratifying the median follow-up CRP values of the stroke population into the five types of “infection” revealed that patients with pneumonia had the highest CRP values, followed by those with sepsis and those receiving antibiotics.
Patients with higher CRP values on admission, within 24 h after admission, and during follow-up were significantly more likely to have worse functional outcome (i.e., dependent outcome or death) than those with lower CRP values (p = 0.001, CRP upon admission; p < 0.001, CRP within 24 h after admission; p < 0.001, follow-up CRP; Table 3). Furthermore, bivariate analysis showed that age (p < 0.001), male gender (p < 0.001), diabetes (p < 0.001), atrial fibrillation (p < 0.001), infarct size (p < 0.001), infections (p < 0.001), and severity of stroke assessed by NIHSS (p < 0.001) were associated with poorer outcomes (i.e., dependence, death, sICH; Table 3).
There was a strong association between admission CRP values and mortality within 24 h after admission (p < 0.001).
Ordinal regression analysis revealed that follow-up CRP (compared to admission CRP and CRP within 24 h after admission) was the best predictor of poor outcomes (p < 0.001, follow-up versus CRP upon admission; p < 0.001, CRP follow-up versus CRP within 24 h after admission in McNemar test).
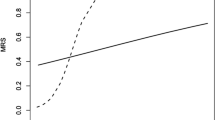
The ROC analysis for follow-up CRP showed predictive characteristics concerning dependent outcome [c-statistic 0.71 (95 % CI 0.67–0.75) p < 0.001] (Fig. 1) and mortality [c-statistic 0.70 (95 % CI 0.66–0.75) p < 0.001].
In multiple regression models, older age, severity of stroke assessed by the NIHSS, larger infarct, and higher values of follow-up CRP were significantly associated with dependent outcome after 90 days (Table 4). Furthermore, older age, higher severity of stroke assessed by the NIHSS, presence of sICH, larger infarct, higher values of follow-up CRP, and history of diabetes were independently associated with mortality after 90 days (Table 4).
Moreover, larger infarct and higher values of follow-up CRP were identified as significantly associated with sICH (Table 4).
Discussion
This study identified a strong independent association between CRP, functional outcome, and mortality in acute thrombolysed stroke patients. To date, this is the largest cohort study to specifically examine this relationship. Our results suggest that CRP levels assessed between days 2 and 7 post-stroke have the strongest association (positive) with 3-month follow-up mRS scores (compared to CRP on admission or CRP within 24 h after admission). Moreover, our data suggest that the predictive value of CRP increases with time (after stroke onset). As CRP is a marker of inflammation, higher CRP on follow-up may indicate ongoing or emerging infection or inflammation. Other independent predictors of 3-month functional outcome were age, infarct size, stroke severity, diabetes, and sICH.
Several authors have investigated the importance of CRP in stroke patients with conflicting results. The majority of these studies have leveraged data from heterogeneous sample populations and CRP assessment times. Moreover, important confounding variables with respect to CRP—such as infection and infarct size—have generally not been taken into account and the statistical power was low. However, the association of CRP (either measured on admission or later) and functional outcome or deaths was a robust finding [11, 14, 18, 20].
In our stroke population, 87 patients had elevated CRP values at follow-up, without suffering from an infection. Out of them, 30 patients had a large infarct in the MCA territory. So the majority of patients who had higher CRP values at follow-up were not attributable to the infarct size or the presence of infection.
In our cohort, mortality within the first 24 h of stroke onset was 7.1 % and associated with stroke severity, larger infarct size, and sICH. The same results were found for CRP values after 24 h. This confirms previous observations that CRP plays a prognostic role both at admission and within the first 24 h of stroke onset. Montaner et al. found a strong independent prediction of mortality with baseline CRP with an OR of 8.51 (95 % CI 2.16–33.5) [18].
Increased levels of CRP may also reflect a pre-existing degree of atherosclerosis or the presence of vascular risk factors. We found that patients with higher levels of CRP more frequently had hypertension, diabetes mellitus, atrial fibrillation, and infection, but we did not find an association between CRP values and atherothrombotic stroke.
Furthermore, cardioembolic stroke, larger strokes, and higher baseline and 24 h NIHSS were observed more often in patients with higher levels of CRP. Increased post-stroke CRP concentrations may be due to inflammation related to the pathophysiology of ischemic stroke and might reflect the extent of the ischemic area as described by Audebert et al. [21–23]. The results of our previous report also show post-stroke infections to be a strong independent predictor of outcome after 3 months [24]. To avoid bias, high CRP levels on admission (>5 mg/l) were an exclusion criteria in this previous analysis. One might argue that in our present study the CRP increase was at least partly due to present infections and that the observation of an independent association with outcome reflects secondary complications of stroke rather than a direct inflammatory response due to cerebral ischemia. Nonetheless, we found that higher follow-up CRP was significantly associated with mortality and that lower follow-up CRP was associated with independent outcome. By contrast, admission CRP values were strongly associated with mortality in the first 24 h but failed to independently predict outcome after 90 days.
It remains unclear whether post-stroke CRP elevation represents epiphenomena of an infection or the inflammatory response following cerebral ischemia or both. In our cohort, infections and infarct size were two important parameters in assessing outcomes. Follow-up CRP was associated with independent outcome even after corrections for those parameters, but failed to show significance for dependent outcome.
Therefore, follow-up CRP appears to be easy to test parameter indicating poor outcome after stroke.
To the best of our knowledge, this is the largest study concerning CRP values assessed at different time points that has focused on the thrombolysed stroke population. The reason to use this cohort for the present analysis was the homogeneous population with hyperacute strokes, robust outcome measures, and detailed information on confounders. We do not see any reason why our findings do not apply to non-thrombolysed patients as well. Nevertheless, some methodological limitations should be discussed. Though our data were collected in a prospective fashion and outcome parameters were assessed independently, data analysis has been done retrospectively including all known possible bias of such an approach. Because we took the maximum value of CRP between days 2–7, we cannot provide specific information on the most important time interval of CRP rise after stroke. Moreover, we did not determine high-sensitivity CRP values, which can lead to underestimation of any incipient infections, but as admission CRP was not an independent predictor of long-term outcome this should not affect our conclusions. An additional limitation of the study is the relatively large size of patients without CRP follow-up values. It must be assumed that the decision to repeatedly measure CRP in certain patients was not independent of the status of recovery after stroke, which might affect our estimates.
Conclusions
Despite the important relationship between infection, infarct size, stroke outcome, and CRP levels, we found that follow-up CRP, and not admission CRP, is an independent predictor of functional outcome and mortality after 90 days in thrombolysed acute stroke patients.
References
Tousoulis D, Kampoli AM, Papageorgiou N et al (2011) Pathophysiology of atherosclerosis: the role of inflammation. Curr Pharm Des 7:4089–4110
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Trans Med 7:97
Hasan N, McColgan P, Bentley P et al (2012) Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol 74:230–240
Whiteley W, Jackson C, Lewis S et al (2011) Association of circulating inflammatory markers with recurrent vascular events after stroke: a prospective cohort study. Stroke 42:10–16
Canoui-Poitrine F, Luc G, Mallat Z et al (2011) Systemic chemokine levels, coronary heart disease, and ischemic stroke events: the prime study. Neurology 77:1165–1173
Di Napoli M, Elkind MS, Godoy DA et al (2011) Role of c-reactive protein in cerebrovascular disease: a critical review. Expert Rev Cardiovasc Ther 9:1565–1584
Guasti L, Dentali F, Castiglioni L et al (2011) Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost 106:591–599
Clyne B, Olshaker JS (1999) The c-reactive protein. J Emerg Med 17:1019–1025
Ladenvall C, Jood K, Blomstrand C et al (2006) Serum c-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke 37:2018–2023
Eikelboom JW, Hankey GJ, Baker RI et al (2003) C-reactive protein in ischemic stroke and its etiologic subtypes. J Stroke Cerebrovasc Dis 12:74–81
Idicula TT, Brogger J, Naess H et al (2009) Admission c-reactive protein after acute ischemic stroke is associated with stroke severity and mortality: the ‘bergen stroke study’. BMC Neurol 9:18
Luo Y, Wang Z, Li J et al (2012) Serum crp concentrations and severity of ischemic stroke subtypes. Can J Neurol Sci 39:69–73
Seo WK, Seok HY, Kim JH et al (2012) C-reactive protein is a predictor of early neurologic deterioration in acute ischemic stroke. J Stroke Cerebrovasc Dis 21:181–186
den Hertog HM, van Rossum JA, van der Worp HB et al (2009) C-reactive protein in the very early phase of acute ischemic stroke: association with poor outcome and death. J Neurol 256:2003–2008
Winbeck K, Poppert H, Etgen T et al (2002) Prognostic relevance of early serial c-reactive protein measurements after first ischemic stroke. Stroke 33:2459–2464
Di Napoli M, Papa F, Bocola V (2001) C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke 32:917–924
Di Napoli M, Papa F, Bocola V (2001) Prognostic influence of increased c-reactive protein and fibrinogen levels in ischemic stroke. Stroke 32:133–138
Montaner J, Fernandez-Cadenas I, Molina CA et al (2006) Poststroke c-reactive protein is a powerful prognostic tool among candidates for thrombolysis. Stroke 37:1205–1210
Topakian R, Strasak AM, Nussbaumer K et al (2008) Prognostic value of admission c-reactive protein in stroke patients undergoing iv thrombolysis. J Neurol 255:1190–1196
den Hertog HM, van der Worp HB, van Gemert HM et al (2009) The paracetamol (acetaminophen) in stroke (pais) trial: a multicentre, randomised, placebo-controlled, phase iii trial. Lancet Neurol 8:434–440
Audebert HJ, Rott MM, Eck T et al (2004) Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke 35:2128–2133
Youn CS, Choi SP, Kim SH et al (2012) Serum highly selective c-reactive protein concentration is associated with the volume of ischemic tissue in acute ischemic stroke. Am J Emerg Med 30:124–128
Ormstad H, Aass HC, Lund-Sorensen N et al (2011) Serum levels of cytokines and c-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol 258:677–685
Rocco A, Fam G, Sykora M et al (2012) Poststroke infections are an independent risk factor for poor functional outcome after three-months in thrombolysed stroke patients. Int J Stroke 8:639–644
Conflict of interest
A. Rocco has received speaker honoraria and travel expenses from Bayer Health care and Ever Pharma.
P. Ringleb has received speaker honoraria and travel expenses from Boehringer Ingelheim (Manufacturer of Alteplase) and is National Coordinator of SITS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocco, A., Ringleb, P.A., Grittner, U. et al. Follow-up C-reactive protein level is more strongly associated with outcome in stroke patients than admission levels. Neurol Sci 36, 2235–2241 (2015). https://doi.org/10.1007/s10072-015-2342-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2342-7