Abstract
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) can improve the life quality of patients with advanced Parkinson disease (PD). However, previous studies have stemmed mainly from Western centers. Present study analyzed the 6-month outcomes of bilateral STN-DBS therapy that were observed during a 9-year period at a Taiwanese institute. We retrospectively reviewed 72 consecutive patients, whose mean disease history was 8 years when they underwent surgery. The median “drug-off” Hoehn and Yahr stage was 3. The STN was targeted using T2-weighted magnetic resonance imaging and electrophysiological guidance. The over-time mean differences in the Unified PD Rating Scale (UPDRS) scores and daily levodopa-equivalent dose (LED) were assessed using the repeated measurements ANOVA at 3 and 6 months relative to those of presurgical drug-off baseline. At 6 months postsurgery, the mean UPDRS total, Part II and Part III subscores significantly decreased by 27, 30 and 25 %, respectively, with clinically high effect size. Tremors were markedly (66 %) ameliorated. Moreover, problems of akinesia, rigidity, and locomotion were significantly improved by 20 %. The mean daily LED needs decreased by 25 %; thus, drug-induced dyskinesia was markedly (80 %) diminished. STN-DBS therapy could provide similarly effective impacts to Eastern and Western PD patients. Preoperative optimal selection of patients and postoperative delicate programming ensure a better surgical improvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic Parkinson disease (PD) is a complex, progressive neurodegenerative disorder manifested by many motor and non-motor features [1]. The current treatment of choice is mainly medical [2]; however, chronic dopaminergic therapy is limited by disease progression and development of drugs-related motor fluctuations or dyskinesia. Surgical interventions are strongly recommended for these patients [2–5]. Deep brain stimulation (DBS) of the subthalamic nucleus (STN) is preferred because most of its adverse effects are reversible, and it causes fewer complications than classic lesion surgery does [6, 7]. STN-DBS exhibited also more effective control of parkinsonian symptoms and reduction of dopaminergics than pallidal DBS does [3, 4, 8].
Previous studies [5, 6, 9–12] reported that 6-month clinical improvements after STN-DBS varied widely (20–75 %) compared to preoperative drug-off baseline. Most studies recruited only low numbers of patients, and stemmed mainly from Western centers [9, 12]. Search of the PubMed database up to July 2014, using the combined MeSH terms of “deep brain stimulation” and “Parkinson” yielded one Japanese (14 patients) [13], one Korean (9 patients) [14], and another Taiwanese (7 patients) [15] reports related to the 6-month efficacy of STN-DBS therapy. The prevalence and incidence rates of PD in Taiwan are closer to those in Western countries [16], thus the status of STN-DBS therapy for PD in Taiwanese populations must be clarified.
Materials and methods
Patients
Seventy-two consecutive PD patients underwent bilateral STN-DBS therapy between May 2004 and July 2013. Movement disorder specialists selected the surgical candidates according to the British PD Society Brain Bank criteria [1] and CAPSIT-PD criteria [17]. The patients were not eligible for surgery if they had substantial cognitive dysfunction, major depressive disorder or severe concomitant medical comorbidities. Their mean age was 61 years, mean illness duration was 8 years, and the median Hoehn and Yahr stage was 3 (Table 1). Preoperatively, the motor improved rate to single-dose anti-parkinsonian drugs was 32 %. After the levodopa (LD) challenge, using LD and benserazide of 1.5 times to daily divided levodopa-equivalent dose (LED), the motor improvements increased to 46 %. All patients provided written informed consent approved by a local ethics committee. This study was conducted in compliance with institutional guidelines and the regulations of the Declaration of Helsinki.
Surgical technique
All patients underwent a 2-stage operation performed using a standardized procedure. Dopaminergics were stopped overnight for at least 12 h prior to the surgery. The electrodes (Model 3389, Medtronic, Minneapolis, MN, USA) were implanted while the patient was awake. Five days later, an implantable pulse generator (IPG) (Kinetra) was placed in the left upper chest subcutaneous area of patient under general anesthesia.
The stereotactic coordinates of upper dorsolateral STN were initially defined by the indirect method based on the Schaltenbrand and Wahren atlas in relation to the anterior commissure and posterior commissure line [18], and modified afterwards using the red nucleus-based direct method [18], on high-resolution (1.5-T) T2-weighted magnetic resonance images (MRIs) in 2-mm-thick axial slices. We used the SurgiPlan software (version 2.11, Elekta Inc.) for surgical planning.
Intraoperatively, single-track microelectrode recordings (MERs) were routinely captured to identify the somatosensory region of the STN and its boundary to the substantia nigra, based on their characteristic neuronal firing patterns and responses to passive movements of the contralateral limbs [19, 20]. The length of trajectory should be at least 4.0 mm, otherwise, another entry will be tried.
The final trajectory for permanent lead was selected using macrostimulation based on the longest pass and the greatest amount of movement-driven activities without causing side effects [20]. The lead was positioned with its most distal contact at the inferior border of the STN span.
Programming
Programming was initiated approximately 3 weeks after the IPG was implanted, when the lesion effect had faded. The optimal combination of settings was that provides the greatest symptom control and the least adverse effects. Patients experiencing adverse effects in a narrow therapeutic window were administered a bipolar stimulation.
Clinical assessments
The primary endpoint was patients’ clinical outcomes at 3 and 6 months, according to the Unified PD Rating Scale (UPDRS) scores relative to the preoperative “drug-off” baseline. The patients were assessed after an at least 12-hour overnight withdrawal of anti-parkinsonian medication, initially in a “DBS-on” state and repeatedly after the “DBS-off” state began for at least 30 min [21]. The motor outcome measures were focused on the speech (Item 18), tremor (Items 20–21), rigidity (Item 22) and akinesia (Items 23–26). Locomotion was measured based on postural instability and gait disturbance (PIGD) (Items13–15 and 29–30). Dyskinesia complications (Items 32–35) following therapy were measured.
The secondary endpoint was a change in daily total LED. The conversion equation of LED was: 100 mg of standard LD is equivalent to 133 mg of controlled-release LD; 75 mg of LD and entacapone; 1 mg of pergolide, pramipexole, lisuride, or cabergoline; 5 mg of ropinirole; 10 mg of bromocriptine or apomorphine; and 20 mg of dihydroergocriptine [10].
Statistical methods
All data are expressed as the mean and 95 % confidence interval (CI). The Wilcoxon signed-rank test was used to compare the preoperative drug-on and drug-off states. The over-time differences in UPDRS scores and in LED requirements were analyzed using a repeated measurement ANOVA, based on the preoperative drug-off baseline. Sphericity assumption was evaluated by the Mauchly’s test, and adjusted using the Huynh–Feldt correction. Relevant differences in time-event changes were evaluated using a post hoc Bonferroni test. A 2-tailed P value less than 0.05 indicated statistical significance. The cut-off points for the clinical effect size (η 2) were defined as low (0.01), moderate (0.06) and high (0.14). All of the statistical analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Results
A total of 161 passes were performed with an average 1.3 ± 0.1 passes per lead implantation; nearly 80 % of the leads were successfully placed in the initial pass. The remaining leads were adjusted based on MERs. The final mean lateral (x), anteroposterior (y) and vertical (z) coordinates for the STN target were 11.9 ± 0.1, −3.4 ± 0.1 and −4.6 ± 0.2 mm relative to the midcommissural point, respectively. The mean STN span was 5.7 ± 0.2 mm. The mean parameters at 6-month for the right stimulator were: amplitude, 2.6 ± 0.1 V; pulse width, 65 ± 2.8 ms; frequency, 138 ± 3.4 Hz. For the left stimulator, the amplitude, pulse width, and frequency were 2.7 ± 0.1 V, 67 ± 3.2 ms and 138 ± 3.4 Hz, respectively. Monopolar stimulation was administered to 71 % (n = 51) of the patients. One patient was treated using a bipolar/monopolar combination.
Postoperative adverse effects
No surgical mortality or major adverse effects such as bleeding, dysarthria, visual defects, motor deficits, or hemiparesis occurred. Two patients experienced acute psychosis for 3–4 days, and one patient experienced a seizure the day after implantation. The right leads were malpositioned in two patients, and were relocated when the IPG was implanted. One diabetic patient experienced fluid accumulation in the chest IPG pocket within one week; therefore, the IPG was removed immediately, and reimplanted 4 months later. These six patients subsequently exhibited a smooth treatment course. No system failures occurred in this series.
Overall clinical improvements after surgery
Preoperatively, medication alone reduced the mean UPDRS total score, Part II (activities of daily living, ADL) and Part III (motor) subscores by 28 % (P < 0.001), 31 % (P < 0.001), and 32 % (P < 0.001), respectively (Tables 1, 2). Six months after STN-DBS therapy without medication, all patients experienced significant clinical improvements in mean UPDRS total scores (η 2 = 0.34, P < 0.001), ADL (η 2 = 0.31, P < 0.001) and motor (η 2 = 0.26, P < 0.001) subscores compared with the preoperative drug-off state. The improved rates were 27 % (P < 0.001) in total scores; 30 % (P < 0.001) in the ADL and 25 % (P < 0.001) in the motor subscores (Fig. 1; Table 2).
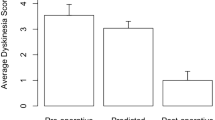
Before surgery, the mean Part IV subscore (drug-induced complications) was 4.1 ± 0.7 in 62 (86 %) patients. This score decreased to 1.7 ± 0.4, noted in 52 (72 %) patients, at 6 months post-DBS therapy (η 2 = 0.31, P < 0.001). Thirty-three (73 %) of 45 patients with drug-induced dyskinesia were completely free of this problem after DBS therapy, and another three (7 %) patients exhibited substantial improvement (η 2 = 0.22, P < 0.001). The total daily mean LED was significantly reduced (η 2 = 0.26, P < 0.001), from 663 ± 85 mg before surgery to 498 ± 59 mg (25 % reduction, p < 0.001) 6 months postoperation.
Clinical improvements in motor dysfunctions
The preoperative mean improvement after medication was 14 % (P = 0.01) in speech disturbance, 48 % (P < 0.001) in tremors, 35 % (P < 0.001) in rigidity, 27 % (P < 0.001) in akinesia and 33 % (P < 0.001) in locomotion (Table 1, 2). After 6 months DBS therapy, prominent improvement was particularly noted in tremors of 66 % (P < 0.001) with a high effect size (η 2 = 0.35, P < 0.001), followed by locomotion of 26 % (P < 0.001) (η 2 = 0.21, P < 0.001), by rigidity of 19 % (P = 0.008) (η 2 = 0.14, P < 0.001), and by akinesia of 17 % (P = 0.009) (η 2 = 0.09, P = 0.002) compared with the preoperative drug-off baseline values (Fig. 2; Table 2). Speech dysfunction was also mildly improved after STN-DBS alone (η 2 = 0.64, P < 0.01), and nine (13 %) patients complained of problems in verbal fluency after STN stimulation.
Discussion
In general, the surgical outcomes of DBS therapy depend closely on multiple factors such as patient selection [7, 22], disease progression [23], surgical techniques particularly accurate targeting [18, 24], postoperative programming strategies [25], rehabilitation [26], and rating techniques [21, 27]. Comparing published reports is thus not straightforward [9, 23].
Debates on surgical techniques
Accurate targeting is adversely influenced by (1) mechanical errors involving frame fixation [28] or fiducial markers [29], (2) distortion in the radio images [30] and (3) brain shifts attributable to cerebrospinal fluid loss or pneumocephalus [31]. A mismatch between the planned and real target coordinates is often observed; therefore, surgeons at most centers intraoperatively conduct some electrophysiological recordings, particularly MERs, to localize the STN [19, 20]. In the present study, we had to adjust approximately 20 % of the leads to the optimal trajectory according to the MERs for guidance.
Hariz [32] doubts that MERs might influence surgical outcomes, and emphasized the morbidity caused by MERs. In fact, age and hypertension were the most crucial factors related to an increased risk of hemorrhage [33]. No MERs-related neurologic deficits occurred during the surgery or subsequently in current series. We believe that the use of intraoperative MERs is safe. MERs help beginners improve the accuracy of targeting. Notably, an optimal MERs-defined position does not ensure the optimal therapeutic outcomes [19, 32]. Meticulous programming [7] or even reprogramming during the follow-up period [7, 25] is mandatory to obtain optimal clinical results.
Issues regarding therapeutic efficacy
Kleiner-Fisman et al. [9] estimated that the ratio of surgical mean improvement compared with the optimal preoperative mean LD response was approximately 80 % in a meta-analysis among 37 cohorts. Randomized controlled studies [10–12] and our study have verified their findings that the improved magnitude of UPDRS scores in the drug-off, DBS-on states was slightly inferior to that of preoperative LD responsiveness (Table 2). In current series, the symptomatic control rate was approximately 32 % after medication before the surgery. After DBS therapy, the mean UPDRS ADL and motor subscores significantly decreased by 25 to 30 % (η 2 = 0.26–0.34, P < 0.001).
Although our results and other Eastern reports [13–15] are qualitatively similar to results in previous meta-analysis [8, 9, 12], but the overall improved magnitude after DBS therapy is somewhat suboptimal and less than those reported for Western populations. At least two possible facts might account for this discrepancy. First, the preoperative LD response in our series was relatively inferior, 32 versus 60–65 % reported for Western populations [9]. Second, the setting of amplitude is relatively conservative in current series, 2.6 versus 2.9v in Western reports [5, 10, 12]. We observed that some patients did have achieved better improvement after reprogramming [25].
We stress a socioeconomic discrepancy existed between Eastern and Western populations. DBS therapy is currently expensive. In Taiwan, national health insurance does not cover this therapy. By contrast, the cost of medication is fully covered. If symptomatic improvement greater than 60 % could be achieved using medication alone, we experienced that persuading an elderly patient or his family to accept a mini-invasive but relatively aggressive intracranial procedure is quite difficult. Therefore, our patients undergo operation generally at a delayed stage with relatively lower LD response, which resulted in suboptimal surgical results [7, 9]. Reasonably, the other possibility of race-related differences in PD requires further investigation.
References
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376
Rascol O et al (2011) Milestones in Parkinson’s disease therapeutics. Mov Disord 26:1072–1082
Ferreira JJ et al (2013) Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol 20:5–15
Rodriguez-Oroz MC et al (2012) Long-term outcomes of surgical therapies for Parkinson’s disease. Mov Disord 27:1718–1728
Herzog J et al (2003) Two-year follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Mov Disord 18:1332–1337
Benabid AL et al (2009) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 8:67–81
Bronstein JM et al (2011) Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68:165–171
Weaver F et al (2005) Deep brain stimulation in Parkinson disease: a metaanalysis of patient outcomes. J Neurosurg 103:956–967
Kleiner-Fisman G et al (2006) Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord 21(Suppl 14):S290–S304
Deuschl G et al (2006) A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med 355:896–908
Weaver FM et al (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301:63–73
Hamani C et al (2005) Bilateral subthalamic nucleus stimulation for Parkinson’s disease: a systematic review of the clinical literature. Neurosurgery 56:1313–1321
Katayama Y et al (2001) Subthalamic nucleus stimulation for Parkinson disease: benefits observed in levodopa-intolerant patients. J Neurosurg 95:213–221
Chung SJ et al (2006) Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Eur Neurol 56:127–132
Chen CC et al (2003) Short-term effect of bilateral subthalamic stimulation for advanced Parkinson’s disease. Chang Gung Med J 26:344–351
Chen RC (2001) Prevalence, incidence, and mortality of PD: a door-to-door survey in Ilan county, Taiwan. Neurology 57:1679–1686
Defer GL et al (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 14:572–584
Andrade-Souza YM et al (2005) Comparison of three methods of targeting the subthalamic nucleus for chronic stimulation in Parkinson’s disease. Neurosurgery 56:360–368
Bour LJ (2010) Long-term experience with intraoperative microrecording during DBS neurosurgery in STN and GPi. Acta Neurochir (Wien) 152:2069–2077
Marceglia S et al (2010) Multicenter study report: electrophysiological monitoring procedures for subthalamic deep brain stimulation surgery in Parkinson’s disease. Neurol Sci 31:449–457
Temperli P et al (2003) How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 60:78–81
Morgante L et al (2007) How many parkinsonian patients are suitable candidates for deep brain stimulation of subthalamic nucleus? Results of a questionnaire. Parkinsonism Relat Disord 13:528–531
Post B et al (2007) Prognostic factors for the progression of Parkinson’s disease: a systematic review. Mov Disord 22:1839–1851
Wodarg F et al (2012) Stimulation site within the MRI-defined STN predicts postoperative motor outcome. Mov Disord 27:874–879
Moro E et al (2006) Subthalamic nucleus stimulation—improvements in outcome with reprogramming. Arch Neurol 63:1266–1272
Tassorelli C et al (2009) The role of rehabilitation in deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: a pilot study. Parkinsonism Relat Disord 15:675–681
Post B et al (2005) Unified Parkinson’s disease rating scale motor examination: are ratings of nurses, residents in neurology, and movement disorders specialists interchangeable? Mov Disord 20:1577–1584
Maciunas RJ et al (1994) The application accuracy of stereotactic frames. Neurosurgery 35:694–695
Mascott CR et al (2006) Quantification of true in vivo (application) accuracy in cranial image-guided surgery: influence of mode of patient registration. Neurosurgery 59:146–156
Menuel C et al (2005) Characterization and correction of distortions in stereotactic magnetic resonance imaging for bilateral subthalamic stimulation in Parkinson disease. J Neurosurg 103:256–266
Miyagi Y et al (2007) Brain shift: an error factor during implantation of deep brain stimulation electrodes. J Neurosurg 107:989–997
Hariz MI (2002) Safety and risk of microelectrode recording in surgery for movement disorders. Stereotact Funct Neurosurg 78:146–157
Zrinzo L et al (2012) Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg 116:84–94
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiou, SM., Lin, YC., Lu, MK. et al. Bilateral subthalamic stimulation for advanced Parkinson disease: early experience at an Eastern center. Neurol Sci 36, 515–520 (2015). https://doi.org/10.1007/s10072-014-2008-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-2008-x