Abstract
Prior research with terrestrial toads (Rhinella arenarum) in a water-reinforced instrumental situation indicated a direct relationship between acquisition rate and reward magnitude. However, a reward downshift produced a gradual adjustment of instrumental performance and a rapid adjustment of consummatory performance, rather than the abrupt and transient deterioration of behavior typical of a successive negative contrast effect. In Experiment 1, using a two-chamber box, a downshift from deionized water (which supports maximal rehydration) to 250-mM sodium chloride solution (which supports a lower rehydration), also yielded a gradual adjustment of instrumental behavior. In this experiment, animals received one trial per day and were allowed 300 s of access to the reward in the goal box. Experiment 2 used the same procedure, except that animals were allowed access to the solution in the goal box for 600 s. Under these conditions, reward downshift led to longer latencies (instrumental) and lower rehydration levels (consummatory) than those of unshifted controls, providing evidence for successive negative contrast. Unlike in similar experiments with mammals, the effect was not transient, but persisted relatively unmodified over twelve daily postshift trials. In this case, the possibility of adaptation of the peripheral mechanisms for water uptake is considered. The comparative relevance of these results is discussed in terms of habit formation versus expectancy-guided behavior in vertebrate learning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphibians are thought to express learned responses on the basis of the strength of antecedent stimuli (i.e., stimulus–response associations, S-R), rather than being guided by reward expectancies (Muzio et al. 2011). The best evidence for this view comes from experiments involving shifts in reward magnitude, which have been extensively explored from a comparative perspective (Papini 2014). The procedure used in the present experiments involves a downshift in reward magnitude from a large to a small reward, under widely spaced-training conditions that minimize stimulus carryover effects across trials, and compared to a condition that always received access to the small reward. Exposed to this training protocol, mammalian species typically exhibit a transient deterioration of behavior (whether instrumental or consummatory) following the reward downshift, in comparison to unshifted controls (Flaherty 1996). This phenomenon is known as successive negative contrast (SNC). Unlike in mammals, toads exposed to a similar situation typically exhibit the same behavioral profile in the acquisition directly proportional to the reward magnitudes, but a gradual postshift adjustment of instrumental performance (Muzio et al. 2011; Papini et al. 1995). This effect, known as reversed SNC, lacks the exaggerated rejection of the downshifted reward typically observed in rats.
A reversed SNC effect is consistent with habit formation via S-R associations, an associative structure that does not require reward encoding. The hallmark of habit formation is that the strength of behavior is directly related to reward frequency and magnitude (e.g., Dickinson 1985; Hull 1943; Rescorla and Wagner 1972). Incentive learning, however, involves encoding some aspects of the incentive event that can then be anticipated, thus inducing emotional reactions when the expectation is violated, as in reward downshift situations (Amsel 1992; Flaherty 1996; Papini 2003).
SNC does not always occur in experiments with rats. For example, rats reinforced with sucrose solutions for running down a runway exhibited no evidence of SNC in their instrumental behavior (no iSNC effect), but they rejected the downshifted sucrose solution in the goal box, thus showing evidence of contrast in their consummatory behavior (cSNC effect; e.g., Sastre et al. 2005). Results such as these suggest that cSNC may be more sensitive or easily triggered than iSNC. To accommodate this distinction, Papini and Pellegrini (2006) argued that whereas cSNC is based on recognition memory (i.e., detecting the disparity between the current, downshifted solution and the solution received during preshift trials), iSNC is based on cued-recall memory (i.e., anticipatory reactivation of the preshift incentive memory). This issue could be relevant to reconsidering the results of previous studies developed in our laboratory to explore the adjustment of toads to a reward downshift manipulation, using saline solutions of several concentrations as reinforcers (Muzio et al. 2011).
Previous studies showed that the salinity of the solution determines the extent to which water-deprived toads can hydrate (Daneri et al. 2007; Loza Coll and Muzio in preparation; Muzio et al. 2011). Toads rehydrate by absorption through a specialized area of vascularized ventral skin located between the hind legs, known as pelvic patch, hence referred to as water uptake (Christensen 1974), a process under the control of beta-adrenergic control (Reboreda et al. 1991). Additional work has shown that toads have a plasma concentration of about 245 mOsm/kg, which is isotonic to a 115 mM sodium chloride (NaCl) solution (Reboreda et al. 1991; Schmajuk and Segura 1982). Thus, hypotonic and slightly hypertonic NaCl solutions (0–250 mM) yield weight gain and support goal approach learning because they correct a fluid imbalance caused by dehydration. However, intermediate hypertonic solutions (around 300 mM) do not easily yield weight gain or loss, thus tending to be motivationally neutral. On the other hand, highly hypertonic solutions (350–1000 mM) lead to a loss of weight and, consequently, support goal avoidance. Using the properties of hypertonic saline solutions, Muzio et al. (2011, Experiment 4) explored a cSNC situation in toads comparing the performance of groups of animals exposed to a downshift from deionized water to either 225, 212, or 200 mM NaCl solutions. The performance of downshifted groups was compared to that of an unshifted control that had received only the corresponding hypertonic solution throughout training. Although the results failed to statistically detect a cSNC in terms of weight variation, there was a tendency for downshifted animals to gain less weight relative to unshifted controls (see Fig. 11 in Muzio et al. 2011).
The goal of the present experiments was to explore the effects of reward downshifts on both instrumental and consummatory behaviors using a modified training protocol that introduced novel testing conditions relative to previous experiments (Muzio et al. 2011). First, toads had access to a 250 mM NaCl solution, a more hypertonic solution than those tested previously (Muzio et al. 2011), although still only slightly hypertonic. This was intended to increase the disparity between preshift and postshift rewards, a procedure that, at least in rats, tends to increase the size of SNC effects (Di Lollo and Beez 1966; Papini and Pellegrini 2006). Second, two access times to the reward solutions were implemented, 300 s (Experiment 1; as in previous experiments) and 600 s (Experiment 2). Longer times of access to water reward tend to strengthen acquisition of runway performance in toads (Muzio et al. 1992), so it was hypothesized that this procedural change might also increase the chance of observing SNC effects. The present experiments, therefore, were designed to determine whether a reward downshift manipulation could lead to iSNC and/or cSNC effects using a modified training procedure relative to previous experiments.
Experiment 1
Method
Subjects
The subjects were 16 experimentally naive, adult, male toads (Rhinella arenarum) captured in ponds around Buenos Aires, Argentina. This species is not listed as threatened (IUCN 2014). Animals were maintained according to the National Institutes of Health Guide for Care and Use of Laboratory Animals (National Research Council 2011). Testing procedures adhered to regulations set forth by the Institutional Animal Care and Use Committee (IACUC protocol 035/2016 IBYME-CONICET, Argentina). Toads were placed in group cages with running tap water during at least two weeks following their arrival in the laboratory. Subjects were fed with insectivorous bird meal (Cédé Insect). The vivarium was kept at a constant temperature (21–23 °C) and under a 16:8 h light: dark cycle (light from 03:00 to 19:00 h). Before the start of the experiment, animals were transferred to individual cages with ad libitum deionized water. The standard body weight (weight of the fully hydrated animal after the urinary bladder has been emptied; Ruibal 1962) was assessed for each toad. The mean (± SEMs) standard weights for Groups DW-250 (300) and 250–250 (300) were 101.53 g (± 8.53) and 100.78 g (± 11.02); the difference was not significant, F < 1.
Apparatus
Training took place in a two-chamber black Plexiglas box (each chamber being 15 × 15 × 20 cm, L × W × H; the same apparatus used in Muzio et al. 2011, Experiment 4). One compartment was the start box and the other was the goal box (Fig. 1). The goal box was connected to a hydraulic system that allowed for the presentation and draining of the appropriate solution during the trial. The chambers were separated by a guillotine door and a barrier (15 × 3 cm, L × H). Toads were required to cross over the barrier with the four legs, moving from the start compartment to the goal compartment. The chambers were covered with translucent Plexiglas lids; a mirror located above the lids allowed for direct observation of the animal. The experimenter recorded the response latency with a manual stopwatch. Response latency was defined as the time elapsing from the start of the trial until the moment the animal was completely out of the start box and into the goal box with its four legs.
A schematic diagram of the training chamber used in both experiments (see also Muzio et al. 2011, Experiment 4; further details in the text)
Procedure
All toads received two 5-min pre-training trials (one per day). On pre-training trials, animals were free to move about in the experimental chambers in the absence of rewards (i.e., no water or saline solutions present). Training started the following day. Each toad received 12 preshift trials, followed by 12 postshift trials. Training involved one trial per day, 7 days per week, at about the same time each day (from 10:00 to 16:00 h). Toads were randomly assigned to one of two groups (n = 8). Animals in Group DW-250 (300) had access to deionized water in the goal box on preshift trials 1–12 and then access to a 250-mM NaCl solution on postshift trials 13–24. Thus, these toads received a reward downshifted from deionized water (DW) to 250 mM saline solution. Animals in Group 250–250 (300) received constant access to 250-mM NaCl solution on trials 1–24. The number in parentheses (300) refers to the time (s) of access to the fluid in each trial.
Two dependent variables were recorded. (1) Latency of response was recorded manually in seconds (in 0.01-s units) and transformed to the log10 to improve normality and allow for the use of parametric statistics. (2) Weight variation (g/100 g) was the weight (g) before and after each trial to assess the amount of water uptake that occurred during the trial. The difference between these two weights was divided by the standard weight computed before the first pre-training trial and multiplied by 100 to provide a relative measure of water uptake corrected for individual differences in body weight (Muzio et al. 1992).
Prior to the two pre-training tests, toads were placed in dehydration boxes, where they gradually reached a weight close to 80% of their standard weight. Every day after the trial, animals were transferred to dry cages where they remained until the next day. Toads were between 79 and 81% of the standard weight at the start of each training trial. Each trial started with the animal being placed in the start box for 30 s, after which the guillotine door was raised. Each animal was allowed a maximum of 300 s to leave the start box. On incomplete trials, the animal was gently guided to the goal box where it received the outcome scheduled for that particular trial. A latency of 300 s was assigned on guided trials. Analysis of variance (ANOVA) with trials as a repeated-measure factor whenever applicable, followed by pairwise comparisons of groups based on the Least Significant Difference (LSD) test were applied to all the data reported in this article. The alpha value was set to the 0.05 level for all statistical tests. Effect size is indicated for significant factors in terms of the partial eta square (ηp2) index. All statistics were computed with the IBM SPSS Statistics 24 package.
Results
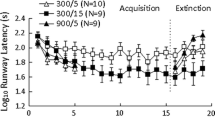
Figure 2, top panel, shows the response latencies for each group in Experiment 1. Group (DW-250, 250–250) by Trial (1–12) analyses were computed separately for preshift and postshift trials. The analysis of preshift instrumental behavior (trials 1–12) showed a significant acquisition effect, F(11, 154) = 3.79, p < 0.001, ηp2 = 0.21, and a significant difference between groups, F(1, 14) = 19.67, p < 0.001, ηp2 = 0.58. The group by trial interaction was nonsignificant, F(11, 154) = 1.61, p > 0.10. Postshift response latencies (trials 13–24) provided no indication of an iSNC effect. Rather, latencies in Group DW-250 (300) gradually met the level of latencies in Group 250–250 (300). The overall analysis of postshift trials indicated that none of the factors was significant, Fs < 2.17, ps > 0.10.
Log10 latency of response (s) of toads during preshift (1–12) and postshift trials (13–24) in Experiment 1 (top panel). Animals were reinforced by access to either deionized water (DW) or a 250 mM NaCl solution (250) during 300 s during preshift trials. Group DW-250 (300) was downshifted from DW to 250 mM NaCl solution, whereas Group 250–250 (300) was exposed to the low reward during all training trials. In a given trial, latencies varied between 2 and 180 s. Weight variation (g/100 g) in each of the two groups of Experiment 1 (bottom panel). Means (± SEMs) are plotted
Figure 2, bottom panel, shows the weight variation for each group across the 24 trials. Preshift consummatory behavior (trials 1–12) showed there were significant effects for trials, F(11, 154) = 10.89, p < 0.001, ηp2 = 0.44, and groups, F(1, 14) = 46.96, p < 0.001, ηp2 = 0.77; their interaction was not significant, F(11, 154) = 1.06, p > 0.39. However, the postshift results (trials 13–24) showed only a significant effect of trials, F(11, 154) = 18.63, p < 0.001, ηp2 = 0.57. There were nonsignificant effects for groups and for the group by trial interaction, Fs < 1.29, ps > 0.23.
Experiment 2
Experiment 2 attempted to enhance the effects of the same reward downshift experience by extending the time of exposure to the solutions from 300 s in Experiment 1 to 600 s. Lengthening access to the solution would allow animals more extensive exposure to the relatively low levels of rehydration afforded by the 250-mM NaCl reward, thus potentially enhancing the disparity between the preshift and postshift rewards.
Methods
Subjects and apparatus
A total of 15 experimentally naive, adult, male toads served as subjects. They were obtained from the same source and maintained as described in Experiment 1. Toads received training in the same experimental chamber described in Experiment 1, Fig. 1. The mean (± SEMs) standard weights for Groups DW-250 (600) and 250–250 (600) were 101.44 g (± 10.17) and 100.34 g (± 7.41); the difference was not significant, F < 1.
Procedure
Toads were randomly assigned to one of two groups: DW-250 (600) (n = 7), and 250–250 (600) (n = 8). The only difference was that animals were left for 600 s per trial in the goal box (instead of 300 s in Experiment 1). All other aspects of the training procedure, including depriving toads to an 80% of their standard weight before each trial, the dependent variables, and statistical treatment of data were as described in Experiment 1.
Results
Figure 3, top panel, shows response latencies for each group in Experiment 2. Both groups exhibited improved instrumental behavior during preshift trials 1–12. There was a significant group by trial interaction, F(11, 143) = 2.51, p < 0.007, ηp2 = 0.16, as well as significant differences across groups, F(1, 13) = 7.51, p < 0.02, ηp2 = 0.34, and across trials, F(11, 143) = 3.82, p < 0.001, ηp2 = 0.23. Interestingly, reward downshift led to a consistently higher mean response latency in Group DW-250 (600) relative to the unshifted control during postshift trials 13–24. This difference was captured by a significant group effect, F(1, 13) = 5.23, p < 0.05, ηp2 = 0.29. However, the interaction and trial effects were nonsignificant, Fs < 1.22, ps > 0.28.
Log10 latency of response (s) of toads during preshift (1–12) and postshift trials (13–24) in Experiment 2 (top panel). The experimental treatments were the same as in Experiment 1, except that animals were allowed 600 s per trial of access to the solutions in the goal box. In a given trial, latencies varied between 1 and 180 s. Weight variation (g/100 g) in each of the two groups of Experiment 2 (bottom panel). Means (± SEMs) are plotted
Figure 3, bottom panel, shows the weight variation for these toads. Preshift consummatory behavior (trials 1–12) showed significant differences between groups, F(1, 13) = 42.26, p < 0.001, ηp2 = 0.77, and across trials, F(11, 143) = 30.68, p < 0.001, ηp2 = 0.70. The interaction was not significant, F(11, 143) = 1.24, p > 0.26. In accordance with response latency data, weight variation during postshift trials 13–24 was consistently lower for downshifted toads compared to unshifted controls. The group, F(1, 13) = 11.34, p < 0.006, ηp2 = 0.77, and trial effects were significant, F(11, 143) = 25.48, p < 0.001, ηp2 = 0.66. The interaction was not significant, F < 1.
Discussion
Experiment 1 replicated previous results obtained with a less concentrated solution and with a 300-s reward exposure (see Muzio et al. 2011, Experiment 4) using a downshift from DW, the large reward, to a 250-mM NaCl solution, the small reward. Importantly, Experiment 2 provided the first ever evidence of SNC in toads using the same training parameters as in Experiment 1, but extending the reward exposure from 300 to 600 s. Such evidence was observed in terms of both instrumental and consummatory responses (Fig. 3). That toads exhibit the same behavioral phenomena also described in mammals, both iSNC and cSNC (see Papini 2014), does not automatically demonstrate that the underlying mechanisms are the same. The following discussion centers on three topics: (1) recovery from SNC, (2) negative emotion associated with SNC, and (3) the covariation between SNC and effects involving unexpected reward omissions.
Recovery from SNC
A hallmark of SNC effects in mammals is their transient nature. Both iSNC and cSNC effects are usually maximal during the initial trials after a downshift and subsequently are reduced such that the performance of downshifted and unshifted groups converges to a similar level (Flaherty 1996). A study of recovery profiles from the cSNC effect in rats, using latent growth mixture modeling, determined that 83% of a large data sample (217 out of 262 animals) recovered their consummatory behavior to the level of unshifted controls within three daily downshift trials (Papini et al. 2014). In that sample, 11% (30/262) showed no evidence of recovery within five trials and 6% (15/262) showed no cSNC effect whatsoever. Unlike in the rat SNC effects, the current effect showed no hint of being transient even after 12 daily trials. This transient property of the rat SNC suggests that animals are comparing the current reward with the memory of previous rewards—hence the word “contrast” to label this effect. As the animal experiences the new, downshifted reward, a memory update process would register this new magnitude and thus reduce the discrepancy between obtained and expected rewards (Ortega et al. 2017; Papini 2003). Such a comparison mechanism was either absent or weakened in the present Experiment 2, in which both instrumental and consummatory behaviors showed no clear evidence of recovery. Rather, downshifted toads behaved as if prior experience with DW had modified their ability to rehydrate in a rather rigid manner. If this change is yet another expression of the toad’s tendency to acquire information in a habitual, S-R fashion then presumably more extensive training might result in a convergence of instrumental and consummatory responses with the level of unshifted controls. Both responses are gradually modified by experience, as indicated by changes in the preshift performance for both response latency and weight variation (see Figs. 2, 3). It has traditionally been assumed that S-R associations are modified relatively slowly in the absence of emotional modulation (Amsel 1992).
Another possibility is that extensive exposure to the rehydration procedure with DW during preshift trials modified the peripheral mechanisms for water uptake. Expression of aquaporin water channel proteins, present in the pelvic skin of toads and other amphibians (Suzuki et al. 2007; Suzuki and Tanaka 2009; Titon et al. 2010), may be reduced after extensive hydration exposure, thus interfering with transepithelial water movement when hydration conditions are reduced, as during a change from DW to 250 mM NaCl solution. Plasticity for water uptake in the opposite direction has been observed in the current experiments in terms of increased absorption in animals repeatedly exposed to 250 mM NaCl during preshift trials. Moreover, salt-acclimated toads, both in vivo and in isolated skin, showed increased water absorption (Katz 1987). These effects may be mediated by the enhanced expression of aquaporin proteins. Unlike in these cases, repeated exposure to DW may reduce aquaporin expression in the SNC situation, especially when such exposure is extensive (600 s vs. 300 s per trial). Such a mechanism would predict a similar “SNC effect” even if the current protocol were applied to isolated pelvic skin patches. This possibility remains to be investigated.
Negative emotion associated to SNC
Another characteristic of SNC in rats is its emotional correlates (Papini et al. 2015). This statement is consistent with the modulation of SNC by benzodiazepine anxiolytics (Flaherty et al. 1986), ethanol (Kamenetzky et al. 2008), and opioids (Daniel et al. 2009); the correlated increased in stress hormones during reward downshift (Pecoraro et al. 2009); the enhancement of contrast by posttrial administration of corticosterone (Bentosela et al. 2006; Ruetti et al. 2009); the hypoalgesia response (Jiménez-García et al. 2016; Mustaca and Papini 2005) and fever (Pecoraro et al. 2007) triggered by reward downshift; and the modulation of SNC by lesions in the amygdala, anterior cingulate cortex, insular cortex, and ventrolateral orbital cortex (Kawasaki et al. 2017; Kawasaki et al. 2015; Liao and Chuang 2003; Lin et al. 2009; Ortega et al. 2013, 2011), all areas known to participate in other emotional behaviors. We are clearly far from having a similarly detailed information of emotional correlates of reward downshift in toads. There is, nonetheless, some evidence consistent with emotional responses in toads. For example, toads rapidly learn to avoid a compartment that gives them access to a highly hypertonic solution (800 mM NaCl solution) that dehydrates them (Daneri et al. 2007). Moreover, there is evidence of amygdala activation in toads after passive avoidance training in a dehydration procedure (Puddington et al. 2016). Because dehydration is life-threatening for an amphibian, these avoidance responses are consistent with the acquisition of a fear-like emotional response. The procedure used successfully in Experiment 2 to produce evidence of SNC opens the way to testing some of the factors affecting the behavior and physiology of rats exposed to reward downshifts to determine whether they also influence toads.
Covariation between SNC and effects involving unexpected reward omissions
SNC covaries with other spaced-training effects involving reward downshifts, including the partial reinforcement extinction effect (PREE) and the magnitude of reinforcement extinction effect (MREE). In rats trained under spaced-trial conditions, acquisition under partial reinforcement or small rewards leads to slower extinction than after continuous reinforcement or large rewards—the PREE and MREE (see Amsel 1992; Flaherty 1996; Papini 2014). In toads, prior research has shown reversed PREE and MREE, but, would training under the same conditions used in Experiment 2 lead to regular PREEs and MREEs in toads?
There is at least one case in which these effects do not covary. Spaced-trial runway training with pigeons has produced, within the same experiment, a PREE and a reversed MREE (Thomas and Papini 2003). This dissociation was also consistent with contrasting drug effects on the pigeon PREE. For example, treatment with the dopamine D2-receptor antagonist haloperidol, which had been shown to have no measurable effect on the PREE in rats (Feldon et al. 1988; Feldon and Weiner 1991), actually eliminated the PREE in pigeons (Thomas and Papini 2003). Similar drug dissociation effects between rats and pigeons were observed with two other drugs: chlordiazepoxide (an allosteric GABA agonist acting on the benzodiazepine site of GABA receptors) and nicotine (an agonist of the acetylcholine receptor). These results suggest the possibility that similar behavioral effects in rats and toads may be based on different underlying neurochemical mechanisms. A similar approach could be implemented to identify the mechanisms underlying the SNC effects observed in Experiment 2 with toads.
Final remarks
The present results are the first in a long series of experiments to provide evidence of instrumental and consummatory contrast in toads. These effects are similar to those described in mammals, but their apparent permanence is unlike what is regularly observed in analogous experiments with mammals. Still, the method and results reported here open new questions concerning the evolution of vertebrate mechanisms underlying the response to environmental change in resources vital for survival, such as water for amphibians. Understanding the processes underlying the effects reported here would shed light on the question of the homology vs. homoplasy of learning mechanisms of SNC among vertebrates.
References
Amsel A (1992) Frustration theory. Cambridge University Press, Cambridge
Bentosela M, Ruetti E, Muzio RN, Mustaca AE, Papini MR (2006) Administration of corticosterone alter the first downshift trial enhances consummatory successive contrast. Behav Neurosci 120(2):371–376. https://doi.org/10.1037/0735-7044.120.2.371
Christensen CU (1974) Adaptation in water economy of some anuran Amphibia. Comp Biochem Physiol 47A:1035–1049
Daneri F, Papini MR, Muzio RN (2007) Common toads (Bufo arenarum) learn to anticipate and avoid hypertonic saline solutions. J Comp Psychol 121(4):419–427. https://doi.org/10.1037/0735-7036.121.4.419
Daniel AM, Ortega LA, Papini MR (2009) Role of the opioid system in incentive downshift situations. Neurobiol Learn Mem 92:439–450. https://doi.org/10.1016/j.nlm.2009.06.003
Di Lollo V, Beez V (1966) Negative contrast effect as a function of magnitude of reward decrement. Psychonomic Sci 5:99–100. https://doi.org/10.3758/BF03328300
Dickinson A (1985) Actions and habits: the development of behavioural autonomy. Philos Trans R Soc Lond B 308:67–78. https://doi.org/10.1098/rstb.1985.0010
Feldon J, Weiner J (1991) Effects of haloperidol on the multitrial partial reinforcement extinction effect (PREE): evidence for neuroleptic drug action on nonreinforcement but not on reinforcement. Psychopharmacology 105:407–414. https://doi.org/10.1007/BF02244437
Feldon J, Katz Y, Weiner J (1988) The effects of haloperidol on the partial reinforcement extinction effect (PREE): implications for neuroleptic drug action on reinforcement and nonreinforcement. Psychopharmacology 95:528–533. https://doi.org/10.1007/BF00172968
Flaherty CF (1996) Incentive relativity. Cambridge University Press, Cambridge
Flaherty CF, Grigson PS, Rowan GA (1986) Chlordiazepoxide and the determinants of contrast. Anim Learn Behav 14:315–321. https://doi.org/10.3758/BF03200073
Hull CL (1943) Principles of behavior. Appleton-Century, Nueva York
IUCN (2014) International union for conservation of nature and natural resources red list of threatened species. Version 2014.3. IUCN, Cambridge
Jiménez-García AM, Ruiz-Leyva L, Cendán CM, Torres C, Papini MR, Morón I (2016) Hypoalgesia induced by reward devaluation in rats. PLoS One 11:1–15. https://doi.org/10.1371/journal.pone.0164331
Kamenetzky GV, Mustaca AE, Papini MR (2008) An analysis of the anxiolytic effects of ethanol on consummatory successive negative contrast. Adv Latin Am Psychol 26:135–144
Katz U (1987) The effect of salt acclimation of the water uptake and osmotic permeability of the skin of the toad (Bufo viridis, L.). J Physiol 82:183–187
Kawasaki K, Glueck AC, Annicchiarico I, Papini MR (2015) Function of the centromedial amygdala in reward devaluation and open-field activity. Neuroscience 303:73–81. https://doi.org/10.1016/j.neuroscience.2015.06.053
Kawasaki K, Annicchiarico I, Glueck AC, Moron I, Papini MR (2017) Reward loss and the basolateral amygdala: a function in reward comparisons. Behav Brain Res 331:205–213. https://doi.org/10.1016/j.bbr.2017.05.036
Liao RM, Chuang FJ (2003) Differential effects of diazepam infused into the amygdala and hippocampus on negative contrast. Pharmacol Biochem Behav 74:953–960. https://doi.org/10.1016/S0091-3057(03)00023-6
Lin J, Roman C, Reilly S (2009) Insular cortex and consummatory successive negative contrast in the rat. Behav Neurosci 123:810–814. https://doi.org/10.1037/a0016460
Loza Coll MA, Muzio RN (in preparation) Hypertonic NaCl solutions as aversive stimuli in terrestrial toads
Mustaca AE and Papini MR (2005) Consummatory successive negative contrast induces hypoalgesia. Int J Compar Psycholo 18:255–262. https://escholarship.org/uc/item/0wk482rp
Muzio RN, Segura ET, Papini MR (1992) Effect of schedule and magnitude of reinforcement on instrumental acquisition and extinction in the toad, Bufo arenarum. Learn Motiv 23:406–429. https://doi.org/10.1016/0023-9690(92)90004-6
Muzio RN, Pistone Creydt V, Iurman M, Rinaldi M, Sirani B, Papini MR (2011) Incentive or habit learning in amphibians? PLoS ONE 6(11):e25798. https://doi.org/10.1371/journal.pone.0025798 (1–12)
National Research Council (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington
Ortega LA, Uhelski M, Fuchs PN, Papini MR (2011) Impairment of recovery from incentive downshift after lesions of the anterior cingulate cortex: Emotional or cognitive deficits? Behav Neurosci 125:988–995. https://doi.org/10.1037/a0025769
Ortega LA, Glueck AC, Uhelski M, Fuchs PN, Papini MR (2013) Role of the ventrolateral orbital cortex and medial prefrontal cortx in incentive downshift situations. Behav Brain Res 244:120–129. https://doi.org/10.1016/j.bbr.2013.01.029
Ortega LA, Solano JL, Torres C, Papini MR (2017) Reward loss and addiction: opportunities for cross-pollination. Pharmacol Biochem Behav 154:39–52. https://doi.org/10.1016/j.pbb.2017.02.001
Papini MR (2003) Comparative psychology of surprising nonreward. Brain Behav Evol 62:83–95. https://doi.org/10.1159/000072439
Papini MR, Pellegrini S (2006) Scaling relative incentive value in consummatory behavior. Learn Motiv 37:357–378. https://doi.org/10.1016/j.lmot.2006.01.001
Papini MR, Muzio RN, Segura ET (1995) Instrumental learning in toads (Bufo arenarum): reinforcer magnitude and the medial pallium. Brain Behav Evol 46:61–71. https://doi.org/10.1159/000113259
Papini MR (2014) Diversity of adjustments to reward downshift in vertebrates. Int J Compar Psychol 27:420–445. https://escholarship.org/uc/item/4db381nz
Papini S, Galatzer-Levy IR, Papini MR (2014) Identifying profiles of recovery from reward devaluation in rats. Behav Brain Res 275:212–218. https://doi.org/10.1016/j.bbr.2014.09.006
Papini MR, Fuchs PN, Torres C (2015) Behavioral neuroscience of psychological pain. Neurosci Biobehav Rev 48:53–69. https://doi.org/10.1016/j.neubiorev.2014.11.012
Pecoraro N, Ginsberg AB, Akana SF, Dallman MF (2007) Temperature and activity responses to sucrose concentration reductions occur on the 1st but not the 2nd day of concentration shifts, and are blocked by low, constant glucocorticoids. Behav Neurosci 121:764–778. https://doi.org/10.1037/0735-7044.121.4.764
Pecoraro N, de Jong H, Dallman MF (2009) An unexpected reduction in sucrose concentration activates the HPA axis on successive post shift days without attenuation by discriminative contextual stimuli. Physiol Behav 96:651–661. https://doi.org/10.1016/j.physbeh.2008.12.018
Puddington MM, Daneri MF, Papini MR, Muzio RN (2016) Telencephalic neural activation after passive avoidance learning in the terrestrial toad Rhinella arenarum. Behav Brain Res 315:75–82. https://doi.org/10.1016/j.bbr.2016.08.003
Reboreda JC, Muzio RN, Viñas MC, Segura ET (1991) β-adrenergic control of the water permeability of the skin during rehydration in the toad Bufo arenarum. Comp Biochem Physiol 100C:433–437
Rescorla RA, Wagner AR (1972) A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF (eds) Classical conditioning II: current research and theory. Appleton, New York, pp 64–99
Ruetti E, Justel N, Mustaca AE, Papini MR (2009) Postsession corticosterone administration enhances the effects of incentive downshift: exploring the boundaries of this effect. Behav Neurosci 123:127–144. https://doi.org/10.1037/a0013805
Ruibal R (1962) The adaptive value of bladder water in the toad (Bufo cognatus). Physiol Zool 35:218–223. https://doi.org/10.1086/physzool.35.3.30152806
Sastre A, Lin J-Y and Reilly S (2005) Failure to obtain instrumental successive negative contrast in tasks that support consummatory successive negative contrast. Int J Compar Psychol 18:307–319. https://escholarship.org/uc/item/8r04f6hc
Schmajuk N, Segura ET (1982) Behavioural regulation of water balance in the toad Bufo arenarum. Herpetologica 38:296–301
Suzuki M, Tanaka S (2009) Molecular and cellular regulation of water homeostasis in anuran amphibians by aquaporins. Compar Biochem Physiol A 153:231–241
Suzuki M, Hasegawa T, Ogushi Y, Tanaka S (2007) Amphibian aquaporins and adaptation to terrestrial environments: a review. Compar Biochem Physiol A 148:72–81
Thomas BL, Papini MR (2003) Mechanisms of spaced-trial runway extinction in pigeons. Learn Motiv 34:104–126. https://doi.org/10.1016/S0023-9690(02)00506-4
Titon B Jr, Navas CA, Jim J, Gomes FR (2010) Water balance and locomotor performance in threespecies of neotropical toads that differ in geographical distribution. Compar Biochem Physiol A 156:129–135
Funding
This research was funded in part by Grant PICT 4300 (FONCYT), by Grant PIP 0893 (CONICET), and by Grant UBACYT P0068BA (Universidad de Buenos Aires), Argentina, all to RNM. Animal procedures were authorized under Institutional Animal Care and Use Committee (IACUC) protocol 035/2016 IBYME-CONICET, Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muzio, R.N., Yohena, A. & Papini, M.R. Evidence of successive negative contrast in terrestrial toads (Rhinella arenarum): central or peripheral effect?. Anim Cogn 25, 1453–1460 (2022). https://doi.org/10.1007/s10071-022-01626-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-022-01626-4