Abstract
Exploration tendency, one of the most investigated animal personality traits, may be driven by either positive (when seeking interesting information) or negative (to reduce the uncertainty of the environment) affective/emotional profiles. To disentangle the valence of the affective state associated with exploration trait, we applied a judgment bias test to evaluate the animals’ responses in an ambiguous situation, allowing an assessment of their affective state or mood. Experiments were carried out in male house mice (Mus musculus) of wild origin. Individual differences in exploration tendency were assessed by repeated open field and novel object tests. To evaluate the animals’ judgment bias, we trained the subjects for 8 days in a 3-arm maze to discriminate between two extreme locations (outer arms: either positively reinforced with sugary water or less-positively reinforced with plain water), in terms of a shorter latency to approach the positively reinforced arm. After this learning criterion was reached, we repeatedly tested their responses to an ambiguous location (intermediate arm). The latencies to approach and consume the ambiguous reward were highly repeatable over the 3 days of testing; hence individuals expressed a stable judgment bias. Most importantly, more exploratory animals showed a more negative judgment bias, which supports the hypothesis that a higher exploration tendency was associated with a negative affective state. Further studies should investigate whether exploration in different situations might be due to distinct affective states.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal personality can be defined as consistent individual differences in behavior across time and/or context (Gosling and John 1999; Wolf and Weissing 2012). Personality traits, such as aggressiveness, boldness or sociability, can be considered to be based on individual differences in emotional reactivity (Boissy and Erhard 2014; Montag and Panksepp 2017). Exploration tendency is one of the most frequently studied personality traits (Careau et al. 2009; Carter et al. 2013; Duparcq et al. 2019; Réale et al. 2007; Rödel et al. 2015), but its interpretation in terms of underlying emotions remains debated. According to a model formulated by Wolf and co-workers (2007), exploration should be positively associated with boldness and aggressiveness, thus allowing more exploratory individuals to cope with unpredictable environments (Careau et al. 2009). In fact, such associations among these different personality traits are frequently found in various animal species (behavioral syndrome: Réale et al. 2007; Sih et al. 2004). From a psycho-biological point of view, exploration toward new information is generally associated with curiosity, which may be defined as a motivation to ‘know’, to ‘see’, or to ‘experience’, leading to information-seeking behavior (Berlyne 1960; Litman and Jimerson 2004). It has been suggested that on the one hand, an animal’s curiosity/exploration tendency could be related to positive affective states involved in the act of seeking out information of potential interest. But on the other hand, curiosity/exploration could be also related to a negative affective state leading the animals to search for information resolving the perception of environmental uncertainty (Litman 2007). In a given context, individuals might be more prone to express a specific affective state when exploring a novel environment or when confronted with a new situation. Therefore, we suggest that an increased, consistent exploration tendency, assessed through repeated open field and novel object tests, might be associated with a certain affective profile. We propose that investigating relationships between judgment bias and exploration trait could help to clarify whether the tendency to explore may be related to a higher tendency to express either positive or negative affective states.
Since recently, the judgment bias test (or “cognitive bias test”) has been increasingly used to assess affective (or emotional) states and moods using different animals of different taxa, mainly mammals and birds (Clegg 2018; Hales et al. 2014; Hintze et al. 2018; Roelofs et al. 2016). In such tests, each individual is first trained to discriminate between two highly distinct cues (e.g., two locations, tones, textures), one being associated with a positive outcome (food reward, access to the home cage, etc.) and the other with a negative, or less positive outcome (no or delayed food reward, air puff, etc.). In a second step, the subject is confronted with a novel, intermediate cue, for example a spatial cue located in the middle of the two previously learned ones, or an intermediate tone or texture. The response to this ambiguous stimulus is assumed to depend on the valence of the affective state of the subject; that is, an animal in a more negative affective state is expected to respond to the intermediate cue more similarly to the negatively, or less positively reinforced cue, e.g., with a higher latency to approach the intermediate cue (Mendl et al. 2009; Roelofs et al. 2016). Such a response pattern is typically interpreted as a more “pessimistic” response. On the other hand, when the individual shows similar responses for intermediate and positive cues (e.g., approaching faster both cues), it will be interpreted as an “optimistic” response, i.e., the expression of a positive affective state. A negative judgment bias can be due to a decreased expectation of a reward or an increased expectation of a punishment (Bateson and Nettle 2015). Judgment bias tests have been used to evaluate the changes in the valence of affective states after an experimental manipulation, mainly in relation to studies in the fields of psychopharmacology (Neville et al. 2019) or animal welfare (enrichment: Bethell et al. 2012; pain: Neave et al. 2013; stereotypic behavior: Novak et al. 2016). Some recent studies also showed that individual differences in judgment bias were consistent over time, at least over short time spans (bottlenose dolphins Tursiops truncatus: Clegg et al. 2017; calves Bos taurus: Lecorps et al. 2018b).
Yet other studies investigated the relationship between personality traits and individual differences in judgment bias, as the latter is assumed to reflect the valence of the subject’s affective state. They highlighted positive associations between optimism (as a proxy of a positive affective state of the animal) and different personality traits such as sociability (dogs Canis familiaris: Barnard et al. 2018; bottlenose dolphins: Clegg et al. 2017) or proactivity (domestic pigs Sus scrofa: Asher et al. 2016). More pessimistic individuals were also more fearful (calves: Lecorps et al. 2018b). In rodents, individual judgment bias has been shown to be related to individual differences in affective states. For example, more optimistic laboratory rats Rattus norvegicus were less vulnerable to stress-induced anhedonia (Rygula et al. 2013), were more motivated to obtain a reward (Rygula et al. 2015) and were less anxious in open field and elevated plus maze tests (Parker 2008). Yet associations between personality traits, especially exploration, and judgment bias remain sparsely studied in rodents. In particular, judgment bias is often used to assess an animal’s ability to react to and cope with stressful situations (e.g., pain: Lecorps et al. 2019; unpredictable housing: Parker 2008) or to study how the judgment bias can be influenced by different living conditions (e.g., environmental enrichment in laboratory rats: Brydges et al. 2011; Richter et al. 2012). To the best of our knowledge, judgment bias tests have never been used as a way to disentangle the emotional valence that may drive an animal’s exploration tendency.
Based on the assumption that exploration tendency (assessed through open field and novel object tests) is related to the tendency to show a certain affective state (Alcaro and Panksepp 2011; Montag and Panksepp 2017), this condition should affect the judgment bias of the individuals. A positive correlation between exploration tendency and a positive judgment bias (hypothesis 1) would support that high exploration is associated with a positive affective state (i.e., an increased interest for novelty in the environment: Berlyne 1967; Litman and Jimerson 2004). In contrast, a negative correlation (hypothesis 2) would support that high exploration is associated with a negative affective state, e.g., due to the tendency to reduce perceived environmental uncertainty for reassurance (Hebb 1955; Litman and Jimerson 2004).
Material and methods
Study animals and housing conditions
A total of 122 male house mice Mus musculus domesticus [mean litter size: 8.1, 95% CI (7.7, 8.4)] were tested for consistent individual differences in exploration behavior by repeated standard tests (see details in “Standard behavioral tests”). Animals were descendants of wild house mice caught around Lyon (France) and bred in the animal facilities of the Laboratoire d’Ethologie Expérimentale et Comparée (Université Sorbonne Paris Nord, France) for 9–10 generations. Among them, a subsample of 39 individuals [mean litter size: 7.9, 95% CI (7.3, 8.6)] was also used for the judgment bias test. Study animals were kept under constant conditions with a 14:10 light/dark cycle (light off at 09:00 am), a room temperature of 20 ± 2.0 °C, and with a humidity of approximatively 50%. The other males were used in other experiments, as phenotyping of exploration tendency was part of a larger project (not shown here).
At postnatal day 21, animals were weaned, and groups of males stemming from the same litter (3 groups of 2, 16 groups of 3 and 17 groups of 4 individuals) were formed. Females and surplus males were used for breeding and other experiments (not shown here). Groups were kept in polycarbonate cages (32.5 × 16.5 cm and 14.2 cm high, PLEXX, Elst, The Netherlands), with a bedding of wood shavings, four cotton balls (COMED, Strasbourg, France) per individual which the animals used as nest material, and two cardboard rolls as enrichment (7.5 × 3.8 cm of diameter). Food (rodent standard diet; Special Diet Services type M20, Witham, Essex, UK) and water were provided ad libitum.
At postnatal days 8, 11 and 35, each individual was marked with a permanent nontoxic hair dye to allow individual recognition within the group (Nyanzol-D, Greenville Colorants, Jersey City, NJ, USA). Animals were held by the experimenter and a unique symbol was rapidly and softly drawn on their back with a fine paint brush.
All experiments were conducted by the same experimenter (AV) under red light condition, corresponding to the activity period of the animals, in an experimental room maintained under the same light regime, temperature and humidity as the housing room. Mice are not completely insensitive to red light; therefore, it is unlikely that mice perceive red light as total darkness (Hawkins and Golledge 2018; Peirson et al. 2018), allowing them to navigate in all experimental apparatuses in a way expected to mimic natural night conditions. The latter (see description in “Standard behavioral tests” and “Judgment bias task”) were cleaned between testing of different individuals with water and non-perfumed soap (Colgate-Palmolive, New York, USA).
Standard behavioral tests
Prior to the judgment bias test, subjects were phenotyped for their exploration tendency (N = 122 subjects) by repeated (two test sessions) open field and novel object tests (Carter et al. 2009; Réale et al. 2007). Individuals were kept in sibling groups during the first test session (postnatal day 41, T1). Immediately after, they were isolated and placed into a new clean cage (same dimensions and content as the group cage, see “Study animals and housing conditions”) until the second test session (postnatal day 71, T2) and remained isolated until the end of the study. Behaviors were video recorded using a camera (T650sc, FLIR, Wilsonville, USA) mounted over the test apparatuses, and video footage was stored for later analysis.
Open field test
The open field consisted of a circular arena (diameter of 60 cm; area of 2827 cm2), surrounded by walls (69 cm high) made of white opaque polyethylene. A central circular area was defined (20 cm of diameter), representing one-ninth of the total area.
Subjects were placed close to the wall of the arena and the test started for 5 min once the animal was released. The video camera was mounted 140 cm above the center of the arena. We quantified the total distance covered in the open field and the distance covered in the central part of the arena (Lecorps et al. 2016; Mazza et al. 2018; Rangassamy et al. 2015; Yuen et al. 2017), using Ethovision XT10 (Noldus Information Technology, Wageningen, The Netherlands). The latter variable, which quantifies the activity of the animals in the central area, is a frequently used variable to assess individual differences in thigmotaxis (Hoy et al. 1999; Ohl 2003) or agoraphobia (Bourin et al. 2007; Prut and Belzung 2003).
Novel object test
The novel object test immediately followed the open field test: after the 5 min of the latter test, the individual was caught with a plastic box and placed again close to the wall of the arena. The object was positioned in the center of the arena, the individual was released and the test lasted for 5 min.
The object used on postnatal day 41 (T1) was a small oval metal box (length: 9.5 cm; height: 2.7 cm) and on postnatal day 71 (T2) the object was a round and opaque soft PVC toy (diameter of 8.5 cm and 4.5–5.0 cm high). The height of the objects allowed the animals to jump on them; due to their size and weight, objects could not be moved by the animals. We recorded the latency to approach and sniff the object for the first time and the number of explorations of the object, measured as the sum of occurrences of sniffing, touching (with mouth or forepaws) and staying on the top of the object (Duparcq et al. 2019; Mazza et al. 2018; Rangassamy et al. 2016). All behavioral measures were analyzed using the software BORIS 6.2.2 (Friard and Gamba 2016).
Judgment bias task
Description of the apparatus
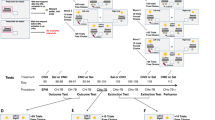
The apparatus (Fig. 1) was made of opaque white PVC (3 mm thick). The walls separating the arms from the central platform were removable, allowing the experimenter to place the appropriate walls (with or without a swinging door, that is, a door that can swing open in both directions) in front of the reference arms before each trial. The walls of the apparatus were 50 cm high to prevent the animals from jumping out of it. A video camera (FDR AX-100 4 K, Sony, UK) was mounted 150 cm above the center of the apparatus.
Schema of the judgment bias apparatus. It is composed of a start box, a corridor and a central platform leading to three same-sized arms placed at equal distance from the exit (4 × 4 cm) of the corridor. Dotted lines represent removable walls. Tested individuals could access the open arm through an opening (5 × 5 cm) with a squared swinging door. To avoid the individuals seeing which arm was opened before entering the central platform, a wall was placed 5 cm before the exit of the corridor with a rectangular opening (4 × 4 cm) at each side. See text for details on the experimental protocol
Part 1: training to the spatial discrimination task
The experiments started with a training phase during which individuals needed to learn the association between one location and one type of reward. The individuals (N = 39) were trained for 8 days, from postnatal day 75–82, and underwent one session of four trials per day. During the first day, only positively reinforced trials were conducted, as it has been shown to increase the speed of learning (Roelofs et al. 2016), although these data were not analyzed. During the next 7 days, two of the trials were positively reinforced and two were less-positively reinforced (see “Reinforcement of the reference arms”). The trials were pseudo-randomized, as the individuals were confronted with a different order each day. During each trial, only one arm (either the left or the right reference arm, Fig. 1) was made accessible.
Reinforcement of the reference arms
For one-half of the individuals (randomly chosen), the right reference arm (Fig. 1) was positively reinforced (hereafter: ‘positive arm’) and the left reference arm was less-positively reinforced (hereafter: ‘less-positive arm’), while the other half of the individuals were trained with the opposite location pattern. The positive reward consisted of a drop of water-diluted 10% sugar solution (sucrose, > 99% purity, Alfa Aesar—Thermo Fisher Scientific) and the less-positive reward of a drop of plain water. To allow video analysis of the consumption of the rewards, a blue food colorant (Vahiné, France) was added to the water for both positive and less-positive rewards. For each trial, the reward was deposited on the inner curved part of an open Petri dish (diameter: 3.5 cm) and fixed on the back wall of the arm, 1 cm above the floor, i.e., at the level of the animals’ head to allow them to consume in a quadrupedal position.
Sucrose is not volatile, hence often considered odorless (e.g., Neureither et al. 2017). However, olfaction has been shown to play a role in discriminating solutions containing different sucrose concentrations in laboratory mice (Zukerman et al. 2009). This discrimination is hypothesized to be based on impurities in the solution and/or oxidation products rather than the sucrose itself (Van Buskirk 1981; Ramirez 1993). To the best of our knowledge, no study investigated the minimal distance necessary for such discrimination. However, it is unlikely that mice can use odor cues from a drop of sucrose solution of low concentration, as used in our study, to differentiate between the rewards before entering the arm. Olfactory cues might have played a role after the individual closely approached the Petri dish. Yet individuals approached and consumed the ambiguous reward with an intermediate latency between the positive and less-positive rewards (see “Validation of the judgment bias test”), which validated our experimental protocol.
Experimental procedure
At the beginning of each daily test session, individuals were placed singly into the start box (Fig. 1) and then the first trial started. During the first 3 min when the animal remained in the start box, the appropriate reward was prepared as explained above (see “Reinforcement of the reference arms”) and the removable walls were placed to open or close the reference arms accordingly (Fig. 1). Then, the start box was opened and the individual was able to explore the apparatus for a maximum of 5 min. If during this period, the individual entered the reference arm, it was given 1 min to consume the reward before the trial was ended. At the end of this minute, or after 5 min in case the animal did not enter in the arm, the individual was gently guided by the hand of the experimenter to the start box and the second trial started as soon as the door of the start box was closed. At the end of the four trials, the individual was returned to its home cage and the apparatus was cleaned.
Definition of the learning criterion
For each trial, the latency to approach and consume the reward after the animal entered in the central platform was recorded. To assess if the individuals learned to discriminate between the two reference arms, we plotted the averaged latencies to approach and consume the positive and the less-positive rewards (in s) for each training day and visually compared the two curves. The learning criterion was defined as follows: individuals had to approach and consume the positive reward with a shorter latency than the less-positive reward for at least two consecutive days. Furthermore, the threshold for the difference between these two latencies (positive and less positive) was 5 s [mean difference among the individuals at the day they reached the learning criterion: 28.16 s, 95% CI (21.64, 34.68)]. All individuals were trained for 7 days, even if they reached the learning criterion (i.e., shorter latency to approach and consume the positive reward of at least 5 s during two consecutive days) before the last training day. Only individuals which maintained the criterion until the end of the training period were considered as learners. Four individuals reached the learning criterion during the last training day (that is, they approached and consumed the positive reward faster during the last two training days) and were also considered as having learned. In total, 25 out of 39 individuals (64%) reached the learning criterion and continued the test (see “Part 2: Judgment bias test”). Excluding these four ‘late learners’ from statistical analyses did not lead to different results from the ones presented below (see “Results”).
Part 2: judgment bias test
After the training, only the animals which reached the learning criterion were tested for their responses to an ambiguous location. During these trials, the centrally-located arm (hereafter: ‘the ambiguous arm’) was open while the two reference arms were closed. The test period lasted for 3 days, from postnatal day 83 to 85, with one session of three trials per day. The sessions were identical each day: a less-positive, a positive then an ambiguous trial. This allowed us to control for motivational effects that could influence the latency to reach the ambiguous reward depending on the valence of the trial preceding it. The ambiguous arm was also rewarded with plain (non-sugary) water to be able to measure the latency until consumption. The test sessions were performed following the same experimental procedure as the training sessions (see “Experimental procedure”).
Calculation of judgment bias index
For each individual successfully trained, we calculated a judgment bias index using the following formula, where L represents the latency to approach and start consuming the reward of the positively rewarded, the less-positively rewarded, or the ambiguous arm. The latency (with a resolution of 1 s) was measured from the time the animal introduced its head by the opening of the central platform until it started to consume the reward. Before the calculation of the index, the latencies of the positive and less-positive arms were averaged between the three testing days. We used the daily latency to consume the reward in the ambiguous arms to obtain one index per day.
For each day, we calculated a judgment bias index ranging from 0 (for a latency to consume the ambiguous reward similar to the latency to consume the less-positive one) to one (for a latency to consume the ambiguous reward similar to the latency to consume the positive one).
Statistical analysis
All statistical analyses were done with the software R, version 3.4.4 (R Core Team 2019). For all covariate effects, we calculated the marginal pseudoR2 using the package MuMIn (Bartoń 2018), which can be interpreted as the proportion of variation explained by the fixed effect (Nakagawa et al. 2017). Prior to analysis, the latency to approach and sniff the novel object was log[x + 1] transformed to adjust the data to a normal distribution. Moreover, we averaged the judgment bias index (calculated based on the latencies of positive, less-positive and ambiguous arms averaged between the three testing days) and log[x + 0.1] transformed the resulting values to increase the homogeneity of models residuals (Faraway 2006). All covariates were scaled for analysis and all P value calculations were based on 10,000 Monte-Carlo permutations.
First, we aimed to validate the protocol of the judgment bias test. Therefore, we tested for differences among the latencies to approach and consume the positive, less-positive and ambiguous rewards (averaged between the 3 days of testing) by running linear mixed-effects models (LMM) with litter identity as a random factor, using the R package nlme (Pinheiro et al. 2019). Then, for each latency separately and the judgment bias index, we tested for differences among the 3 days of testing using LMM with litter identity as a random factor. Finally, we applied two LMM to test for the associations between the individual judgment bias index (averaged and transformed; dependent variable, covariate) and (1) the individual speed of learning (i.e., the day at which the individual reached the learning criterion; independent variable, covariate), and (2) the time spent in the central platform before a first entry in the arm during ambiguous trials (averaged between the three testing days; independent variable, covariate).
To summarize the behavioral variables in a single score, we applied a principal component analysis (PCA; R package FactoMineR: Lê et al. 2008), using the behaviors quantified in the open field (total distance covered, distance covered in the central area) and the novel object tests (latency to sniff the object, number of explorations of the object), separately for each test session (T1, postnatal day 41; T2, postnatal day 71; Nindividuals = 122). We only used the first component of the PCA for later analysis as it had an eigenvalue > 1; that is, the first component accounted for more variance than any of the original variables of the standardized data. This component was interpreted as ‘exploration score’ for later analysis.
The repeatability of the exploration scores (Nindividuals = 122), as well as of the judgment bias index (Nindividuals = 25, that is, the number of individuals that reached the learning criterion, see “Definition of the learning criterion”), were calculated with intra-class correlations (RICC), using a LMM based on calculations of P values with 10,000 Monte Carlo permutations (package rptR: Stoffel et al. 2017), with individual identity as a random factor.
To test for associations between the exploration scores and the judgment bias index, we used the exploration scores of the individuals which successfully passed the judgment bias test (Nindividuals = 25), obtained from the PCA previously performed on the 122 individuals. Thus, we tested for the association between exploration scores (averaged between the two test sessions; independent variable, covariate) and individual judgment bias index (averaged and transformed; dependent variable, covariate) by running an LMM with litter identity as a random factor.
Results
Validation of the judgment bias test
During the 3 days of testing, the individuals approached and consumed the positive reward after on average 6.25 s, 95% CI [4.09, 8.41], the less-positive reward after 61.84 s, 95% CI [55.99, 67.68] and the ambiguous reward with an in-between latency of 45.84 s, 95% CI [37.91, 53.77] (see also Online Resource 1). The latencies differed significantly from each other (P < 0.001).
The latency to approach and consume the less-positive reward significantly increased on the third day compared to the first (P = 0.006) and second (P = 0.002) day of testing, although the two latter days did not differ significantly (P = 0.610). The latency to approach and consume the positive reward did not differ significantly among days (all P > 0.10).
Moreover, the latency to approach and consume the ambiguous reward, as well as the judgment bias index, did not differ significantly among the 3 days of testing (all P > 0.10). Therefore, it could be concluded that there was no notable effect of extinction attributed to learning that the ambiguous cue was not positively rewarded.
The individual speed of learning, defined as the day at which the individual reached the learning criterion, was not significantly associated with individual differences in judgment bias index (P = 0.192).
Finally, the individual time spent in the central platform before a first entry in the arm during ambiguous trials was not significantly associated with individual differences in judgment bias index (P = 0.998).
Consistent individual differences across time in exploration score
The first component of the PCA explained 47.7% (T1) and 54.8% (T2) of the variation of the data. During both times, higher scores indicated a greater total distance covered in the open field (loadings during T1: 0.685; loadings during T2: 0.797), a greater distance covered in the central area of the open field (T1: 0.558; T2: 0.760), a shorter latency to sniff the object for the first time (T1: -0.656; T2: -0.724) and a higher number of explorations of the object (T1: 0.834; T2: 0.674).
The exploration score (RICC = 0.564, P < 0.001, Fig. 2) was significantly repeatable across the two test sessions (N = 122). Thus, individuals showed consistent individual differences in exploration behavior.
Consistent individual differences in exploration scores across the two test sessions T1 and T2 (N = 122 adult males). Individual scores correspond to the first component of a PCA, using the averaged behaviors quantified in repeated open field and novel object tests (postnatal days 41 and 71). Higher scores indicate a higher exploration tendency (greater total distance covered in the open field and greater distance covered in the central part of the area, shorter latency to sniff and higher number of explorations of the object). Each circle represents the score of one individual. The association between the two test sessions was statistically significant and tested by intra-class correlations; see text for details
Consistent individual differences across time in judgment bias index
The judgment bias index was significantly repeatable at the individual level across the 3 days of testing (RICC = 0.711, P < 0.001, Fig. 3). Thus, individuals showed stable individual differences in the relative latencies to approach and consume the ambiguous reward.
Consistent individual differences in judgment bias index across 3 days of testing (N = 25 adult males). The calculation of the judgment bias index is detailed in the text. Judgment bias ranges from 0 (negative bias) to 1 (positive bias). Each circle represents the index of one individual. All associations shown were statistically significant and were tested by intra-class correlations; see text for details
Associations between individual differences in judgment bias index and exploration scores
Individual differences in judgment bias index were significantly and negatively associated with individual exploration scores (pseudoR2 = 0.285, P = 0.006, Fig. 4). That is, individuals which were more explorative during the open field and novel object tests showed a longer latency to approach and consume the ambiguous reward during the test situation (that is, they responded with a latency more similar to the one they showed when approaching the less-positive cue).
Association between judgment bias index and exploration score (N = 25 adult males). Judgment bias ranges from 0 (negative bias) to 1 (positive bias) and was averaged between the 3 days of testing. Higher exploration scores (averaged between the two test sessions, at postnatal days 41 and 71) indicate a higher exploration tendency (greater total distance covered in the open field and greater distance covered in the central part of the area, shorter latency to sniff and higher number of explorations of the object). Each circle represents the values of one individual. The association was statistically significant (details in text); analysis by LMM
Discussion
As expected, individual judgment bias was significantly associated with the animals’ exploration tendency. In accordance to our second hypothesis, more explorative subjects showed a more negative judgment of the ambiguous test situation.
Our results on the existence of consistent individual differences in exploration confirm the findings of previous studies in laboratory mice Mus musculus (Brust et al. 2015; Lewejohann et al. 2011; Rödel et al. 2012) and other rodent species of wild origin (mound-building mouse Mus spicilegus: Duparcq et al. 2019; Rangassamy et al. 2015; common vole Microtus arvalis: Herde and Eccard 2013; bank vole Myodes glareolus: Mazza et al. 2018; Eurasian harvest mouse Micromys minutus: Schuster et al. 2017). To the best of our knowledge, our study is the first to demonstrate consistent individual differences in exploration in house mice of wild origin.
We validated our judgment bias protocol based on positive and less-positive rewards, as our individuals responded to the presentation of the ambiguous cue with an intermediate latency between the positive and less-positive cues. Moreover, the individual judgment bias remained significantly stable across the 3 days of testing, indicating that our subjects did not learn that the ambiguous cue was not positively rewarded and the latter did not lose ambiguity, which can be a common issue in such tests (Roelofs et al. 2016). We also controlled for the motivation of mice to explore the central platform before first entering the ambiguous arm, which might explain the longer latencies to approach and consume the ambiguous reward interpreted as a negative judgment bias. However, such exploration was not significantly associated with individual judgment bias, although we do not exclude that mice left the arm to explore the maze after a first entrance. It is important to develop judgment bias tests which do not require the use of punishments, such as mild electric shocks (Enkel et al. 2010) or air puffs (Brajon et al. 2015), as they can directly modify the affective state of the subjects and lead them to avoid the ambiguous cue, hence exhibiting more pessimistic responses (Mendl et al. 2009). Others have already developed such tests using for example the presentation of one versus two food pellets in the laboratory rat (Parker et al. 2014) or of small versus large rewards in the domestic pig (Roelofs et al. 2017). An appropriate selection of the reinforcers becomes more crucial when the baseline judgment bias is assessed, as it has been done in our study. Furthermore, in accordance with studies in calves Bos taurus (Lecorps et al. 2018a, b), bottlenose dolphins (Clegg et al. 2017) and domestic pigs (Asher et al.2016), we demonstrated that wild-origin house mice displayed consistent individual differences in judgment bias over 3 days of testing.
Returning to the main goal of our study, we found that individuals expressing a more negative judgment bias were also the more explorative ones in open field and novel object tests. Exploration of novel situations (environment and objects) is often used as a proxy to assess anxiety and emotional reactivity (Harro 2018; Ohl 2003). Specifically, the behaviors we quantified (overall and central activity in the open field, number of explorations of the object and latency to approach the object) are likely to refer to the SEEKING system, a positive emotional system underlying explorative and approach behavior proposed by Panksepp and co-workers (Alcaro and Panksepp 2011; Montag and Panksepp 2017; Panksepp 2005). According to this approach, individuals with a more sensitive SEEKING system are more motivated to search for rewards and to explore new stimuli, which could greatly impact the responses when confronted with an ambiguous stimulus. In this context, we might expect that more explorative individuals would have positive expectations about the outcome of the ambiguous cue (i.e., positive judgment bias) and thus would faster approach and consume the ambiguous reward. Contradicting this prediction, our results show a correlation in the opposite direction, suggesting that more explorative individuals might have more negative expectations in an ambiguous situation.
We conclude that our results are consistent with others, suggesting that information-seeking behaviors may be mediated through curiosity reduction (curiosity-drive theory: Berlyne 1954, 1960), i.e., individuals are motivated to explore their environment to reduce uncomfortable states due to environmental uncertainty, or motivated by a lack of available information (curiosity as a feeling-of-deprivation: Litman and Jimerson 2004). These models, which involve a degree of negative affectivity during exploration, would imply that more explorative individuals have more difficulties to sustain the novelty of the environment in the open field and the novel object tests, pushing them to increase their explorative activities. These more explorative individuals might be characterized by a more pronounced tendency to express negative affective states (i.e., more negative affective profiles), leading them to engage in the exploration of novel and uncertain situations for reassurance. We suggest that findings obtained by judgment bias test indicate such negative affective profiles. Indeed, during the judgment bias test, individuals may have invested more time in exploratory activity, instead of consuming the reward, to reduce the negative affective state induced by the uncertainty of the novel, ambiguous cue, finally leading to a negative bias. Nevertheless, a spatial judgment bias task, as in our study, might have given them a greater opportunity to explore than if we have used non-spatial cues, hence leading to higher latencies to consume the ambiguous reward. Moreover, the association we reported here might remain highly dependent on the context we measured the exploration tendency. For future studies, we propose that associating a judgment bias test with the quantification of exploration in other experimental paradigms, such as ‘free exposure’ open field and novel object tests (Fonio et al. 2009; Griebel et al. 1993), would bring further insights into the association between individual differences in exploration tendency and affective profiles. Furthermore, some individuals showed an opposite pattern of association (Fig. 4), expressing both a higher exploration tendency and a more positive judgment bias. This highlights the variety of possible emotional profiles and the need to integrate individuality into the study of animal emotions. To better explain such variation, we suggest that future studies might evaluate emotional profiles by developing batteries of tests similar to cognition studies (Shaw and Schmelz 2017).
Finally, such explorative and proactive individuals (in the sense of Koolhaas et al. (1999); as fast exploration is a key component of proactivity), despite a higher novelty seeking, are also characterized by a lower executive control (de Boer et al. 2017). The ambiguity in spatial judgment bias tests might create a conflict between the tendency to explore and the behavioral inflexibility (i.e., low executive control) of proactive individuals. Proactive individuals are also less sensitive to changes in their environment and being more likely to form routines (Coppens et al. 2010; Sih and Del Giudice 2012). On the contrary, slow explorers take more time to gather more detailed information, which allow them to be more flexible when the environment changes. Such associations between individual differences in exploration types and cognitive abilities have already been highlighted by several studies (Guenther et al. 2014; Guillette et al. 2010; Mazza et al. 2018, 2019; Verbeek et al. 1994). For instance, proactive pigs were less successful in a reversal learning task, due to difficulties in inhibiting the behavioral patterns they previously learned (Bolhuis et al. 2004). In our study, consistently with a study in carpenter ants Camponotus aethiops (d’Ettorre et al. 2017), more explorative individuals showed longer latencies to reach the ambiguous reward. Hence, according to this hypothesis, the here observed relatively longer latencies in more exploratory animals might not be an expression of a negative affective state, but might rather be explained by a greater inflexibility in adjusting their behavior when confronted with a new situation, due to routines formed during the training period or possibly to conflict of motivation between exploration of the novel environment and the rapid approach and consumption of the reward (Coppens et al. 2010; Sih and Del Giudice 2012). This explanation would challenge the prevalent interpretation of a negative judgment bias as the expression of a negative affective state or mood. However, the association reported in the present study might only apply to spatial judgment bias tasks, as the behavioral inflexibility of proactive individuals might be strengthened when they are required to explore to find rewards (see also Asher et al. 2016). Although such inflexibility should also be expressed in non-spatial tasks (e.g., Guenther et al. 2014; Guillette et al. 2010), we suggest conducting tasks based on non-spatial cues (e.g., auditory or olfactory cues) to remove the need to explore. In association with a reversal learning task to phenotype individuals’ behavioral inflexibility, this would allow future studies to confirm or infirm this hypothesis.
In conclusion, our study is consistent with others showing associations between individual differences in judgment bias and a personality trait (here, exploration tendency). In particular, judgment bias and personality tests could complement each other to help determining the affective states underlying the classical personality traits, although the observed associations may remain highly dependent on the context of evaluation of the personality traits. Further studies should also investigate the potential confounding effects of behavioral inflexibility of proactive (and possibly more exploratory) individuals when aiming to assess their affective state through spatial judgment bias tests.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alcaro A, Panksepp J (2011) The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci Biobehav Rev 35:1805–1820. https://doi.org/10.1016/j.neubiorev.2011.03.002
Animal Behaviour (2018) Guidelines for the treatment of animals in behavioural research and teaching. Anim Behav. https://doi.org/10.1016/j.anbehav.2017.10.001
Asher L, Friel M, Griffin K, Collins LM (2016) Mood and personality interact to determine cognitive biases in pigs. Biol Lett 12:20160402. https://doi.org/10.1098/rsbl.2016.0402
Barnard S, Wells DL, Milligan ADS, Arnott G, Hepper PG (2018) Personality traits affecting judgment bias task performance in dogs (Canis familiaris). Sci Rep 8:1–8. https://doi.org/10.1038/s41598-018-25224-y
Bartoń K (2018) MuMIn: multi‐model inference. R package version 1.42.1. https://cran.r-project.org/package=MuMIn. Accessed 2 Apr 2019
Bateson M, Nettle D (2015) Development of a cognitive bias methodology for measuring low mood in chimpanzees. PeerJ 3(e998):1–25. https://doi.org/10.7717/peerj.998
Berlyne DE (1954) A theory of human curiosity. Br J Psychol 45:180–191. https://doi.org/10.1111/j.2044-8295.1954.tb01243.x
Berlyne DE (1960) Conflict, arousal, and curiosity. McGraw Hill, New York
Berlyne DE (1967) Arousal and reinforcement. In: Levine D (ed) Nebraska symposium on motivation. University of Nebraska Press, Lincoln, pp 1–110
Bethell EJ, Holmes A, MacLarnon A, Semple S (2012) Cognitive bias in a non-human primate: husbandry procedures influence cognitive indicators of psychological well-being in captive rhesus macaques. Anim Welf 21:185–195. https://doi.org/10.7120/09627286.21.2.185
Boissy A, Erhard HW (2014) How studying interactions between animal emotions, cognition, and personality can contribute to improve farm animal welfare. In: Grandin T, Deesing MJ (eds) Genetics and the behavior of domestic animals, 2nd edn. Elsevier Inc., Amsterdam 81–113. 10.1016/B978-0-12-394586-0.00003-2
Bolhuis JE, Schouten WGP, Leeuw JAD, Schrama JW, Wiegant VM (2004) Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behav Brain Res 152:351–360. https://doi.org/10.1016/j.Bbr.2003.10.024
Bourin M, Petit-Demoulière B, Nic Dhonnchadha B, Hascöet M (2007) Animal models of anxiety in mice. Fund Clin Pharmacol 21:567–574. https://doi.org/10.1111/j.1472-8206.2007.00526.x
Brajon S, Laforest JP, Schmitt O, Devillers N (2015) The way humans behave modulates the emotional state of piglets. PLoS ONE 10:1–17. https://doi.org/10.1371/journal.pone.0133408
Brust V, Schindler PM, Lewejohann L (2015) Lifetime development of behavioural phenotype in the house mouse (Mus musculus). Front Zool 12:S17. https://doi.org/10.1186/1742-9994-12-S1-S17
Brydges NM, Leach M, Nicol K, Wright R, Bateson M (2011) Environmental enrichment induces optimistic cognitive bias in rats. Anim Behav 81:169–175. https://doi.org/10.1016/j.anbehav.2010.09.030
Careau V, Bininda-Emonds ORP, Thomas DW, Réale D, Humphries MM (2009) Exploration strategies map along fast–slow metabolic and life-history continua in muroid rodents. Funct Ecol 23:150–156. https://doi.org/10.1111/j.1365-2435.2008.01468.x
Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R (2013) Animal personality: what are behavioural ecologists measuring? Biol Rev 88:465–475. https://doi.org/10.1111/brv.12007
Clegg ILK (2018) Cognitive bias in zoo animals: an optimistic outlook for welfare assessment. Animals 8:1–25. https://doi.org/10.3390/ani8070104
Clegg ILK, Rödel HG, Delfour F (2017) Bottlenose dolphins engaging in more social affiliative behaviour judge ambiguous cues more optimistically. Behav Brain Res 322:115–122. https://doi.org/10.1016/j.bbr.2017.01.026
Coppens CM, de Boer SF, Koolhaas JM (2010) Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc B Biol Sci 365:4021–4028. https://doi.org/10.1098/rstb.2010.0217
d’Ettorre P, Carere C, Demora L, Le Quinquis P, Signorotti L, Bovet D (2017) Individual differences in exploratory activity relate to cognitive judgment bias in carpenter ants. Behav Processes 134:63–69. https://doi.org/10.1016/j.beproc.2016.09.008
de Boer SF, Buwalda B, Koolhaas JM (2017) Untangling the neurobiology of coping styles in rodents: towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci Biobehav Rev 74:401–422. https://doi.org/10.1016/j.neubiorev.2016.07.008
Duparcq M, Jean O, Verjat A, Jaravel L, Jacquet D, Guerrero FR, Féron C, Rödel HG (2019) Differences in short-term tail temperature responses to handling in fast and slow explorer mice of wild origin. Behav Brain Res 376:112194. https://doi.org/10.1016/j.bbr.2019.112194
Enkel T, Gholizadeh D, von Bohlen und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, Gass P, Vollmayr B (2010) Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology 35:1008–1015. https://doi.org/10.1038/npp.2009.204
Faraway JJ (2006) Extending the linear model with R: Generalized linear, mixed effects and nonparametric regression models. Chapman and Hall/CRC, Boca Raton
Fonio E, Benjamini Y, Golani I (2009) Freedom of movement and the stability of its unfolding in free exploration of mice. Proc Natl Acad Sci USA 106:21335–21340. https://doi.org/10.1073/pnas.0812513106
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. https://doi.org/10.1111/2041-210X.12584
Gosling SD, John OP (1999) Personality dimensions in nonhuman animals: a cross-species review. Curr Dir Psychol Sci 8:69–75. https://doi.org/10.1111/1467-8721.00017
Griebel G, Belzung C, Misslin R, Vogel E (1993) The free-exploratory paradigm: an effective method for measuring neophobic behaviour in mice and testing potential neophobia-reducing drugs. Behav Pharmacol 4:637–644. https://doi.org/10.1097/00008877-199312000-00009
Guenther A, Brust V, Dersen M, Trillmich F (2014) Learning and personality types are related in cavies (Cavia aperea). J Comp Psychol 128:74–81. https://doi.org/10.1037/a0033678
Guillette LM, Reddon AR, Hoeschele M, Sturdy CB (2010) Sometimes slower is better: Slow exploring birds are more sensitive to changes in a vocal discrimination task. Proc R Soc Lond B 278:767–773. https://doi.org/10.1098/rspb.2010.1669
Hales CA, Stuart SA, Anderson MH, Robinson ESJ (2014) Modeling cognitive affective biases in major depressive disorder using rodents. Br J Pharmacol 171:4524–4538. https://doi.org/10.1111/bph.12603
Harro J (2018) Animals, anxiety, and anxiety disorders: how to measure anxiety in rodents and why. Behav Brain Res 352:81–93. https://doi.org/10.1016/j.bbr.2017.10.016
Hawkins P, Golledge HDR (2018) The 9 to 5 rodent: time for change? Scientific and animal welfare implications of circadian and light effects on laboratory mice and rats. J Neurosci Methods 300:20–25. https://doi.org/10.1016/j.jneumeth.2017.05.014
Hebb DO (1955) Drives and the C. N. S. (conceptual nervous system). Psychol Rev 62:243–254. https://doi.org/10.1037/h0041823
Herde A, Eccard JA (2013) Consistency in boldness, activity and exploration at different stages of life. BMC Ecol 13:1–10. https://doi.org/10.1186/1472-6785-13-49
Hintze S, Melotti L, Colosio S, Bailoo JD, Boada-Saña M, Würbel H, Murphy E (2018) A cross-species judgment bias task: integrating active trial initiation into a spatial Go/No-go task. Sci Rep 8:1–13. https://doi.org/10.1038/s41598-018-23459-3
Hoy JB, Cody BA, Karlix JL, Schmidt CJ, Tebbett IR, Toffollo S, van Haaren F, Wielbo D (1999) Pyridostigmine bromide alters locomotion and thigmotaxis of rats: gender effects. Pharmacol Biochem Behav 63:401–406. https://doi.org/10.1016/s0091-3057(99)00014-3
Koolhaas JM, Korte SM, de Boer SF, van Der Vegt BJ, van Reenen CG, Hopster H, De Jong I, Ruis MA, Blokhuis H (1999) Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23:925–935. https://doi.org/10.1016/S0149-7634(99)00026-3
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. J Stat Softw 25:1–18. https://doi.org/10.18637/jss.v025.i01
Lecorps B, Kappel S, Weary DM, von Keyserlingk MAG (2018a) Dairy calves’ personality traits predict social proximity and response to an emotional challenge. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-34281-2
Lecorps B, Ludwig BR, von Keyserlingk MAG, Weary DM (2019) Pain-induced pessimism and anhedonia: evidence from a novel probability-based judgment bias test. Front Behav Neurosci 13:1–7. https://doi.org/10.3389/fnbeh.2019.00054
Lecorps B, Rödel HG, Féron C (2016) Assessment of anxiety in open field and elevated plus maze using infrared thermography. Physiol Behav 157:209–216. https://doi.org/10.1016/j.physbeh.2016.02.014
Lecorps B, Weary DM, von Keyserlingk MAG (2018b) Pessimism and fearfulness in dairy calves. Sci Rep 8:1421. https://doi.org/10.1038/s41598-017-17214-3
Lewejohann L, Zipser B, Sachser N (2011) ‘‘Personality’’ in laboratory mice used for biomedical research: a way of understanding variability? Dev Psychobiol 53:624–630. https://doi.org/10.1002/dev.20553
Litman JA (2007) Curiosity as a feeling of interest and feeling of deprivation: the I/D model of curiosity. In: Zelick PR (ed) Issues in the psychology of motivation. Nova Science Publishers Inc., New York, pp 149–216
Litman JA, Jimerson TL (2004) The measurement of curiosity as a feeling of deprivation. J Pers Assess 82:147–157. https://doi.org/10.1207/s15327752jpa8202_3
Mazza V, Eccard JA, Zaccaroni M, Jacob J, Dammhahn M (2018) The fast and the flexible: cognitive style drives individual variation in cognition in a small mammal. Anim Behav 137:119–132. https://doi.org/10.1016/j.anbehav.2018.01.011
Mazza V, Jacob J, Dammhahn M, Zaccaroni M, Eccard JA (2019) Individual variation in cognitive style reflects foraging and anti-predator strategies in a small mammal. Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-46582-1
Mendl M, Burman OHP, Parker RMA, Paul ES (2009) Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl Anim Behav Sci 118:161–181. https://doi.org/10.1016/j.applanim.2009.02.023
Montag C, Panksepp J (2017) Primary emotional systems and personality: an evolutionary perspective. Front Psychol 8:1–15. https://doi.org/10.3389/fpsyg.2017.00464
Nakagawa S, Johnson PCD, Schielzeth H (2017) The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J R Soc Interface 14:1–11. https://doi.org/10.1098/rsif.2017.0213
Neave HW, Daros RR, Costa JHC, von Keyserlingk MAG, Weary DM (2013) Pain and pessimism: dairy calves exhibit negative judgment bias following hot-iron disbudding. PLoS ONE 8:8–13. https://doi.org/10.1371/journal.pone.0080556
Neureither F, Stowasser N, Frings S, Möhrlen F (2017) Tracking of unfamiliar odors is facilitated by signal amplification through anoctamin 2 chloride channels in mouse olfactory receptor neurons. Physiol Rep 5:e13373. https://doi.org/10.14814/phy2.13373
Novak J, Stojanovski K, Melotti L, Reichlin T, Palme R, Würbel H (2016) Effects of stereotypic behaviour and chronic mild stress on judgment bias in laboratory mice. Appl Anim Behav Sci 174:162–172. https://doi.org/10.1016/j.applanim.2015.10.004
Ohl F (2003) Testing for anxiety. Clin Neurosci Res 3:233–238
Panksepp J (2005) Affective consciousness: core emotional feelings in animals and humans. Conscious Cogn 14:30–80. https://doi.org/10.1016/j.concog.2004.10.004
Parker RMA (2008) Cognitive bias as an indicator of emotional state in animals. Doctoral dissertation, University of Bristol
Parker RMA, Paul ES, Burman OHP, Browne WJ, Mendl M (2014) Housing conditions affect rat responses to two types of ambiguity in a reward: reward discrimination cognitive bias task. Behav Brain Res 274:73–83. https://doi.org/10.1016/j.bbr.2014.07.048
Peirson SN, Brown LA, Pothecary CA, Benson LA, Fisk AS (2018) Light and the laboratory mouse. J Neurosci Methods 300:26–36. https://doi.org/10.1016/j.jneumeth.2017.04.007
Pinheiro J, Bates D, DebRoy S, Sarkar C, R Core Team (2019) nlme: linear and nonlinear mixed effects models. R package version 3.1–142. https://cran.r-project.org/package=nlme. Accessed 2 Apr 2019
Prut L, Belzung C (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol 463:3–33. https://doi.org/10.1016/s0014-2999(03)01272-x
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 2 Apr 2019
Ramirez I (1993) Role of olfaction in starch and oil preference. Am J Physiol 265:R1404–R1409. https://doi.org/10.1152/ajpregu.1993.265.6.R1404
Rangassamy M, Athari SK, Monclús R, Boissier M-C, Bessis N, Rödel HG (2016) Personality modulates proportions of CD4+ regulatory and effector T cells in response to socially induced stress in a rodent of wild origin. Physiol Behav 167:255–264. https://doi.org/10.1016/j.physbeh.2016.09.016
Rangassamy M, Dalmas M, Féron C, Gouat P, Rödel HG (2015) Similarity of personalities speeds up reproduction in pairs of a monogamous rodent. Anim Behav 103:7–15. https://doi.org/10.1016/j.anbehav.2015.02.007
Réale D, Dingemanse NJ, Reader SM, McDougall PT (2007) Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318. https://doi.org/10.1111/j.1469-185x.2007.00010.x
Richter SH, Schick A, Hoyer C, Lankisch K, Gass P, Vollmayr B (2012) A glass full of optimism: enrichment effects on cognitive bias in a rat model of depression. Cogn Affect Behav Neurosci 12:527–542. https://doi.org/10.3758/s13415-012-0101-2
Rödel HG, Hudson R, Rammler L, Sänger N, Schwarz L, Machnik P (2012) Lactation does not alter the long-term stability of individual differences in behavior on the elevated plus maze in laboratory mice. J Ethol 30:263–270. https://doi.org/10.1007/s10164-011-0320-y
Rödel HG, Zapka M, Talke S, Kornatz T, Bruchner B, Hedler C (2015) Survival costs of fast exploration during juvenile life in a small mammal. Behav Ecol Sociobiol 69:205–217. https://doi.org/10.1007/s00265-014-1833-5
Roelofs S, Boleij H, Nordquist RE, van der Staay FJ (2016) Making decisions under ambiguity: judgment bias tasks for assessing emotional state in animals. Front Behav Neurosci 10:1–17. https://doi.org/10.3389/fnbeh.2016.00119
Roelofs S, Nordquist RE, van der Staay JF (2017) Female and male pigs’ performance in a spatial holeboard and judgment bias task. Appl Anim Behav Sci 191:5–16. https://doi.org/10.1016/j.applanim.2017.01.016
Rygula R, Golebiowska J, Kregiel J, Kubik J, Popik P (2015) Effects of optimism on motivation in rats. Front Behav Neurosci 9:1–9. https://doi.org/10.3389/fnbeh.2015.00032
Rygula R, Papciak J, Popik P (2013) Trait pessimism predicts vulnerability to stress-induced anhedonia in rats. Neuropsychopharmacology 38:2188–2196. https://doi.org/10.1038/npp.2013.116
Shaw RC, Schmelz M (2017) Cognitive test batteries in animal cognition research: evaluating the past, present and future of comparative psychometrics. Anim Cogn 20:1003–1018. https://doi.org/10.1007/s10071-017-1135-1
Schuster AC, Carl T, Foerster K (2017) Repeatability and consistency of individual behaviour in juvenile and adult Eurasian harvest mice. Sci Nat 104:1–14. https://doi.org/10.1007/s00114-017-1430-3
Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19:372–378. https://doi.org/10.1016/j.tree.2004.04.009
Sih A, Del Giudice M (2012) Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc B Biol Sci 367:2762–2772. https://doi.org/10.1098/rstb.2012.0216
Stoffel MA, Nakagawa S, Schielzeth H (2017) rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol Evol 8:1639–1644. https://doi.org/10.1111/2041-210X.12797
Van Buskirk RL (1981) The role of odor in the maintenance of flavor aversion. Physiol Behav 27:189–193. https://doi.org/10.1016/0031-9384(81)90255-9
Verbeek MEM, Drent PJ, Wiepkema PR (1994) Consistent individual differences in early exploratory behavior of male great tits. Anim Behav 48:1113–1121. https://doi.org/10.1006/anbe.1994.1344
Wolf M, van Doorn GS, Leimar O, Weissing FJ (2007) Life-history trade-offs favour the evolution of animal personalities. Nature 447:581–584. https://doi.org/10.1038/nature05835
Wolf M, Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27:452–461. https://doi.org/10.1016/j.tree.2012.05.001
Yuen CH, Schoepf I, Schradin C, Pillay N (2017) Boldness: are open field and startle tests measuring the same personality trait? Anim Behav 128:143–151. https://doi.org/10.1007/s00265-015-1937-6
Zukerman S, Touzani K, Margolskee RF, Sclafani A (1976) Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses 34:685–694. https://doi.org/10.1093/chemse/bjp055
Acknowledgements
We are grateful to Ludivine Jaravel and Daphné Jacquet for their excellent animal care and to Céline Bocquet and Franco Robles for their assistance in video analysis.
Funding
Aurélie Verjat was funded by a PhD fellowship provided by the Ecole Doctorale Galilée, Université Sorbonne Paris Nord.
Author information
Authors and Affiliations
Contributions
Aurélie Verjat, Heiko G. Rödel and Christophe Féron conceived the study. Paul Devienne participated in the conception of the experimental method and constructed the apparatus. Aurélie Verjat planned and carried out the experiments, performed the behavioral and statistical analysis and wrote the first version of the manuscript. Heiko G. Rödel and Christophe Féron advised on the statistical analysis and participated in the writing of the manuscript. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors confirm that they do not have any conflict of interest.
Ethical approval
This study was conducted in accordance to the ‘Guidelines for the Treatment of Animals in Behavioural Research and Teaching’ (Animal Behaviour 2018) and to the ethics guidelines of France, where the project was conducted. All experimental procedures were approved by the French authority for animal care and use (APAFIS#7585–201610121409165) and by the institutional ethics committee (SBEA LEEC USPN). The individuals tested for their personality traits but not for their judgment bias were used for other experiments and for breeding. Animals which underwent the judgment bias test were euthanized at the end of the study, at postnatal day 87.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verjat, A., Devienne, P., Rödel, H.G. et al. More exploratory house mice judge an ambiguous situation more negatively. Anim Cogn 24, 53–64 (2021). https://doi.org/10.1007/s10071-020-01414-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-020-01414-y