Abstract
Spatial learning is an important cognitive function found across a multitude of species. Natural selection can enhance specific cognitive abilities depending on species ecology but, under certain conditions, spatial learning is also known to vary between sexes according to reproductive status. Despite abundant studies on spatial learning across animal taxa, those focusing on sexually dimorphic spatial learning have been largely limited to rodents. Here, we found that spatial cognition varies between the sexes in an intertidal goby, and this difference fluctuates across seasons. Males and females demonstrated similar cognitive abilities when solving a simple maze during all seasons except spring, when males were significantly less successful than females. Spring marks the beginning of the breeding season for this species, when females move between nests to choose a suitable mate, while males guard their nest and forego foraging excursions. We suggest that the reduction in male cognitive ability reduces metabolic costs at a time of reduced need. This study presents the first evidence for sexually dimorphic spatial learning in fish driven by differences in reproductive behaviour as dictated by the mating system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spatial learning is the process through which individuals collect information about the layout of their environment to relocate required resources efficiently (Floresco 2014). Animals benefit from direct and purposeful movements between resources while minimising energy expenditure (Odling-Smee et al. 2006), so it is advantageous to learn the spatial layout of their habitat (Healy 1998; Giraldeau 1997). Owing to the obvious fitness advantages, spatial learning is widely observed in varied capacities across the animal kingdom (e.g. Garber 1989; Krebs 1990; Lacreuse et al. 1999; Broglio et al. 2003; Jozet-Alves et al. 2008; Noble et al. 2012; Carazo et al. 2014) and is a useful tool for probing the cognitive ability of animals within a comparative framework.
Just as interspecific variations in spatial learning ability can be predicted by the demands of the animal’s environment (Healy and Jones 2002), so too can the evolution of intraspecific variation, specifically between sexes, be understood in this context. Males and females commonly demonstrate different cognitive abilities (Halpern 1991; Kimura 1999), with male spatial learning skills often exceeding those of females, as demonstrated in several mammalian species (e.g. Kavaliers et al. 1996, 1998; Spritzer et al. 2011). Several hypotheses have attempted to explain sex-biased variation in mammalian spatial ability (reviewed in Jones et al. 2003). Gray and Buffery (1971) proposed that mating systems are responsible for the variation, arguing that males of polygynous species show greater spatial ability than females as a result of moving across large areas to mate with multiple females and maximise their reproductive success (Gaulin 1995) (e.g. meadow vole, Microtus pennsylvanicus); (Gaulin and Fitzgerald 1986, 1989). In monogamous species, where both parents tend to be tied to single nest locations, there are no differences in spatial learning between the sexes (e.g. prairie vole, Microtus ochrogaster); (Gaulin and Fitzgerald 1986, 1989). This hypothesis is supported by a substantial number of mammalian studies, as well as others in reptiles (Noble et al. 2012; Carazo et al. 2014) and birds (Astié et al. 1998; González-Gómez et al. 2014), which attribute sexually dimorphic spatial learning ability to selective pressures from mating systems. Another hypothesis predicts that decreased spatial learning ability in females during reproductive periods is correlated to their reduced mobility while nesting and changes in hormones during weaning (parental care hypothesis; Sherry and Hampson 1997). For instance, female deer mice (Peromyscus maniculatus) show decreased spatial learning compared to males during the breeding season only, suggesting that hormone changes associated with reproduction can influence spatial learning skills in females (Galea et al. 1994).
Fish are often used as models to understand the evolution of spatial cognition in vertebrates (Odling-Smee and Braithwaite 2003; Odling-Smee et al. 2011) as well as divergent cognitive abilities between sexes (Cummings 2018). Despite the many and varied mating systems in fish, surprisingly few studies have investigated sexually dimorphic spatial learning in the context of breeding season (Costa et al. 2011; Lucon-Xiccato and Bisazza 2017). Sovrano et al. (2003) reported slightly superior male performance in the redtail splitfin (Xenotoca eiseni) when tested in a reorientation task. Male freshwater blennies (Salaria fluviatilis) defend nest territories and remain sedentary in sole parenting duties until the eggs hatch, while females are the more mobile sex (Wickler 1957; Vinyoles and Sostoa 2007). When tested, however, males learned a two-choice maze faster than females, despite having smaller home ranges of the two sexes (Costa et al. 2011; Fabre et al. 2014). More recently, male and female zebrafish (Danio rerio) were reported to solve a spatial task in similar time frames, although males made fewer errors than females (Roy and Bhat 2017). Thus, support for the hypothesis that mating systems influence variation in spatial learning in fishes remains equivocal.
Gobies encompass a large part of fish diversity (Thacker 2009) and engage in various mating systems ranging from monogamy (e.g. Hernaman and Munday 2007) to polygamy (e.g. Mazzoldi et al. 2005). Such a broad range of mating systems provides a useful comparative framework to investigate sexually dimorphic spatial learning. Cognitive functioning such as spatial learning has been extensively researched in gobies, particularly rockpool species which display heightened awareness of geographic position learned during foraging excursions at high tide (Aronson 1951; Gibson 1967, 1999; Jorge et al. 2012). Moreover, gobies acquire spatial information in new environments rapidly (Markel 1994), return to their home rockpools following displacement (Griffiths 2003; White and Brown 2014a) and use cognitive maps to navigate (Aronson 1951, 1971). Despite such interest in the general cognitive abilities of gobies, spatial learning ability between males and females remains unexplored.
Here, we tested seasonal variation in spatial learning ability in both sexes of the intertidal Cocos Frillgoby, Bathygobius cocosensis (Bleeker, 1854), using a two-choice spatial task. Breeding in Bathygobius occurs in spring and is comprised of male-male competition for nest sites and female choice (Taru et al. 2002; Thia et al. 2018). Females deposit their eggs in a nest established and guarded by a single male that protects the eggs until hatching. In B. cocosensis, therefore, we might expect females to retain their spatial learning ability throughout the breeding season because they move between locations evaluating the quality of the males and their nests. On the other hand, males are tied to their nest location and forego foraging excursions at high tide, so it is expected their spatial cognition ability is lessened during spring. Thus, we expected to observe a difference in spatial learning skills between males and females during the breeding season but not in other seasons.
Methods
Study animals
Bathygobius cocosensis is commonly found along the rock pools and reefs in the intertidal zone along the east coast of New South Wales, Australia. While its breeding system has not yet been formally described, our observations suggest that it conforms with other Bathygobius species (Taru et al. 2002). Throughout 2015–2016, adult individuals were collected from Dee Why (33.7502° S, 151.2991° E) during the first week of the middle month of each season (i.e. January, April, July and October). All individuals (n = 61) were collected during low tide using hand-held nets then transported to the Seawater Facility at Macquarie University. Once there, the gobies were separated into two groups according to size (< 3.5 cm, > 3.5 cm) to minimise aggressive behaviour and housed in opaque 70L white plastic tubs (64.5 × 41.3 × 27.6 cm). The water temperature in the flow-through system was maintained between 19 and 23 °C, depending on the season (mean 21 °C). The water was distributed to the holding tubs through 13-mm valves (5 L/min) and depth was maintained at 25 cm. Lighting was kept to 10 h daily under full UV spectrum lights. To mimic the gobies’ natural environment, the substrate in the housing tubs was a combination of fine sand and larger shell grit pieces. Each tub also had several artificial shelters (12 cm halves of 25 mm white, non-reflective PVC) to enable the gobies to seek protection. The gobies were housed in these tanks for 5 days to acclimate and were introduced to frozen Artemia for food.
Tagging
Following the settlement period, gobies were lightly anaesthetised in a 1L bath of buffered sea water with 50 mg of dissolved MS222 for between 30 and 60 s until sedated. They were then weighed, measured and tagged with a Visible Implant Fluorescent Elastomer tag (VIE: Marine Technology, Inc. 2008) for unique identification (White and Brown 2013). The process took less than 2 min per fish, and all gobies recovered within 5 min before they were returned to the holding tubs for another 5 days to recover.
Test apparatus
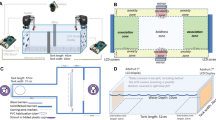
The test apparatus was adapted from a plus maze from White and Brown (2014b) that could be modified to a t-maze (Fig. 1). Two duplicate mazes (total L × W 50 cm) made of 3-mm PVC plastic were submerged in identical rectangular tubs (L100 × W50 × D18cm) of aerated sea water. The water level was approximately 10 cm deep and the bases lined with fine sand. The beginning of each arm had a clear, glass dish (3 cm in diameter × 1.5 cm deep) in which a food reward could be placed, and a shelter identical to those in the holding tanks. All arms were identical except for an inaccessible shelter in the incorrect arm, which was fitted with a clear plastic film on both ends to prevent entry. The water temperature in the maze was adjusted for each test group to match the temperature of the home tank, and experiments were run under ambient light from fluorescent lights only. A video camera was mounted above both mazes for observation.
Procedure
Prior to commencing trials, each group of gobies was introduced into the maze for a familiarity period of 24 h (Brown 2003). The maze was completely open during this time with no partitions, and all start boxes accessible with open shelters. After 8 h, the food dishes were filled with Artemia to encourage foraging behaviour. After the familiarity session, all gobies were returned to their housing tanks for another 24 h to maximise hunger while minimising loss of familiarity with the test environment. To avoid possible lateralisation bias (Brown and Braithwaite 2004), gobies were randomised into either the right- or left-hand training group. Each goby was tested individually for three consecutive trials per day, and the location of the start box was randomised each day.
For each trial, one individual was gently netted from the home tank and introduced to the test apparatus. After a settlement period (5 min), the temporary partition was removed and the goby was free to explore the maze, the objective being to locate the reward arm. The exit time (s) from the start box in the maze was recorded when half of the fish’s body emerged from the start box and this time was used as an indication of how motivated the gobies were to engage in the assay. A small rock was placed off-centre in the junction of the maze as a landmark to guide the gobies to the correct arm; individuals had to turn away from the rock if they were to choose the correct direction. If the test goby chose the correct arm, they were rewarded with shelter and food; 2 Artemia delivered from a clear 3-ml pipette into the food dish. To minimise chemical cues in the maze, food was only given after the task was completed and removed with a 50-ml syringe if not eaten before the next trial to remove some of the surrounding water. If a goby chose the incorrect arm, the escape door was closed, and the goby held inside for 3 min without a food reward or access to the shelter, before being gently ushered into the correct arm. Gobies were given a 5 min rest interval between trials (White and Brown 2014b), after which the maze was reset; the reward arm then became the start box, the landmark was shifted to the new layout and the inaccessible shelter was moved to the incorrect arm. After three trials, each goby was returned to their home tank and 10L of water were removed from the mazes and replenished with clean salt water before the next trial began.
Each goby was trialled three times per day until they achieved three correct turns each day for 5 consecutive days, after which training ceased. If an individual chose incorrectly in one trial, the count would be re-started from the trials the following day. During the first 5 days of the trial period, if gobies took longer than 5 min to leave the start box, they were given a ceiling value of maximum trial time (s). Further, if they chose the wrong side, they were given a food reward after they were encouraged into the correct side. After the 5th day, gobies were encouraged out of the start box after 5 min and received no food reward if they made an incorrect choice. To avoid observer induced bias, trials were recorded from over-head cameras and several behaviours were noted including emergence time, side chosen, completion time and whether the individual returned to the start box.
All gobies remained housed in the laboratory until all season trials concluded, after which they were released at the original site of capture.
Statistical analysis
Days to reach criteria
In all cases, data were normally distributed and analysed using parametric tests. We used two-way ANOVA to investigate effects of seasonality on spatial learning performance, using the number of days to reach criteria as the dependent variable with season and sex as fixed factors. Pair-wise analyses applying a Bonferroni correction were used to determine the differences between gobies tested during each season.
Daily scores
Daily score was based on the number of correct choices out of three trials per day, then converted to a daily percentage. These daily percentage scores were then averaged across all individuals per season for an average daily score. Gobies that had reached criteria were included in the data set, even though they no longer participated in the trials, and were given a score of 100%. This was necessary because of the variation in the number of days taken to reach criteria. We used a repeated measures ANOVA with mean daily score on increment days of 5 between days 1 and 25 (i.e. days 1, 5, 10, 15, 20 and 25) per treatment group as the dependent variable with season and sex as fixed factors.
Motivation and learning
Emergence time was averaged for each goby from three trials per day for a daily mean. The daily mean for days 1, 5, 10, 15, 20 and 25 were then analysed using a repeated measures ANOVA with season and sex as fixed factors. Total trial time was calculated from the moment subjects chose a side subtracted from their emergence time and was similarly averaged per individual from three trials per day. We used total trial time averages as an indicator for learning rate, and these were analysed using the same techniques as average emergence times. All analyses were performed using StatView Version 232 5·0·1 (SAS Institute Inc. 1998).
Results
Days to reach criteria
There was a significant effect of season in the average number of days to reach criteria (ANOVA: F3,53 = 12.211, P = < 0.001) with gobies completing trials faster in summer and autumn compared to winter and spring (Bonferroni: P < 0.001 in all cases). There was no significant effect of sex on the number of days to reach criteria (P > 0.05), however there was a significant interaction between season and sex (ANOVA: F1,3 = 3.568, P = 0.020; Fig. 2). Pair-wise comparisons revealed a significant difference between males and females only in spring (Bonferroni: P = 0.029) with females reaching criteria faster than males.
Daily scores
Overall, there was a significant effect of season on daily score (ANOVA: F3,53 = 8.634, P = < 0.001) which were higher in summer and autumn than winter and spring. Females had higher scores than males (ANOVA: F1,53 = 6.081, P = 0.017). There was also a significant effect of trial day; in general, fish improved their scores as training went on (ANOVA: F5,265 = 10.832, P = < 0.001). There was a significant interaction between season and sex (ANOVA: F3,53 = 4.211, P = 0.010; Fig. 3) with the difference between males and females being most marked in winter (P = 0.040) and spring (P = 0.014).
The combined mean (± SE) daily performance scores (%) across days 1–25 for males and females in each season. Daily performance was calculated as a percentage score based on a correct/incorrect choice out of three trials. Significant differences between sexes within treatment groups are marked by (*)
Within sexes, male daily performances varied significantly between seasons; they scored much higher in summer and autumn compared to winter and spring (ANOVA: F3,35 = 16.344, P < 0.001), while females showed little seasonal differences in their performance (F3,18 = 1.915, P = 0.163). We found a trial day and season interaction (ANOVA: F15,265 = 2.747, P < 0.001; Fig. 4), as performance generally improved over time during summer and autumn, but not in winter and spring. All other interactions were non-significant.
The mean (± SE) combined daily performance scores (%) of all gobies in each treatment group (filled diamond: summer, filled square: autumn, filled triangle: winter, times: spring), shown for days incremented by 5. Note: for analysis purposes, gobies that had reached criteria before the 25th day were assigned a score of 100% for consecutive days
Motivation
We used daily mean exit time to gauge the gobies’ motivation to engage with the learning assay. There was a significant effect of season (ANOVA: F3,53 = 3.208, P = 0.030) and males were less motivated than females (ANOVA: F1,53 = 4.106, P = 0.048). Gobies were particularly poorly motivated to commence the task in winter, however, individuals emerged from the start box faster as trial days went on, suggesting increased motivation as they learned the task (ANOVA: F5,265 = 17.721 = P < 0.001). There were no significant interactions.
Trial time
We analysed average total trial time based on the time each goby left the start box to the time it took for them to enter either the correct or incorrect box. There was a significant effect of season with trial time being particularly long in winter (ANOVA: F3,53 = 16.435, P < 0.001). There were no differences between the sexes, nor was there a significant interaction between season and sex (P > 0.05 in both cases). Total trial time decreased with increasing trial number (ANOVA: F5,265 = 11.554, P = < 0.001) and there was also a significant interaction between trial number and season (ANOVA: F15,265 = 3.932, P = < 0.001) with the greatest improvement over time observed in winter.
Discussion
Our study found that cognitive function demonstrated through spatial learning performance in gobies varied between seasons. Males and females performed similarly to each other in summer and autumn, but not in winter and spring. The significant difference in cognitive performance between sexes in spring suggests a reflection of male and female reproductive behaviour dictated by their mating system of nest guarding and female choice, respectively. Males demonstrated decreased spatial learning performance in spring, while females performed in spring as they did during summer and autumn trials. These results suggest that sexually dimorphic spatial learning ability corresponds to a time when males are confined to their nests and females visit multiple nests to choose a suitable male to fertilise their eggs, whilst also foraging and avoiding predators. This variation in life-history priorities perhaps favours a reduction in male cognitive performance likely achieved through phenotypic plasticity under hormonal control. To our knowledge, however, correlations between cognitive function and hormones in fish have only been found in non-spatial contexts, such as lateralisation (Schaafsma and Groothuis 2011).
Spatial learning is ubiquitous across vertebrate taxa, and many mammalian models illustrate that it is a cognitive function influenced by mating systems (e.g. Gaulin and Fitzgerald 1986, 1989; Galea et al. 1994; Kavaliers et al. 1996, 1998), in turn driving variation in spatial learning between the sexes. Studies have shown sexually dimorphic spatial learning ability can fluctuate between seasons, a trend seemingly tied to hormonal changes (Galea et al. 1994, 1996). Research using mammalian models suggests hormonal changes during reproduction and weaning impact spatial awareness in females (e.g. Galea et al. 1994), while other studies report a similar trend accompanied by reduced volume of specific brain regions (Yaskin 1984; Smith et al. 1997; Tramontin et al. 1998; Tramontin and Brenowitz 2000). As brain functions require more energy per mass than any other tissue, and responses such as a reduction in mass could lower energetic costs, brain and behavioural plasticity are expected to be advantageous (Jacobs 1996). Given the energetic demands of cognition, it makes sense to reduce metabolic costs if cognitive requirements are lessened, which may explain why spatial performance in nest-bound male B. cocosensis was significantly worse than females during spring.
Sexually dimorphic spatial learning ability in fishes is somewhat equivocal, although male guppies (Poecilia reticulata), redtail splitfins (Xenotoca eiseni), zebrafish (Danio rerio) and freshwater blennies (Salaria fluviatilis) reportedly outperform females in spatial tasks (Sovrano et al. 2003; Fabre et al. 2014; Lucon-Xiccato and Bisazza 2017; Roy and Bhat 2017). Males of the first three species tend to disperse further than females and chase multiple mating opportunities, behaviours somewhat reminiscent of a typical mammalian system (Silverman and Eals 1992). In contrast, female Azorean rockpool blennies (Parablennius parvicornis) move greater distances relative to males during the breeding season, and thus have a greater demand for spatial cognition to recall multiple nest locations. Correspondingly, females have larger lateral palliums compared to males (Carneiro et al. 2001).
While the mating system of B. cocosensis remains undescribed, other Bathygobius species are known to engage in male competition and nest-holding disputes, while females are the choosy sex (e.g. Tavolga 1954; Taru et al. 2002; Kong and Chen 2013). In premating rituals, females actively search for potential mates, while males remain in their chosen nest site, alternatively cleaning the site and courting passing females. Once spawning occurs, females will return to their home range (Taru et al. 2002) while males guard the eggs until hatching. Given that breeding in B. cocosensis primarily occurs in spring (Thia et al. 2018), we suggest that reduced cognitive ability in males during this time is because males are site attached to their nest, so their need for neurologically expensive spatial ability presumably decreases, leading to reduced calorific intake during this time as well reduced foraging opportunities. Here we found that males required significantly more days to reach criteria and had lower daily scores in spring compared to females, corresponding to their nest-holding and egg-guarding behaviour during this time. It should be noted that water temperature at the collection site is similar in autumn and spring (Carbia and Brown, unpublished data), however males performed similarly to females only in autumn, suggesting there is another factor in play, other than temperature, affecting spatial performance in males. In contrast, female performance in spring reflected summer/autumn patterns, suggesting no apparent change to their spatial learning capabilities in the breeding season when, in addition to their regular activities, they are also moving between nests to choose a suitable mate.
Both males and females demonstrated slower exit times in winter compared to other seasons. Although females were faster to leave the start box on average compared to males, the overall increased exit time may be interpreted as reduced motivation to complete the task due to lowered metabolic rate. Given that females invest heavily in egg production, it may be that they are slightly more motivated than males to search for food because of their enhanced energy requirements. Total trial time was also significantly higher in winter compared to other seasons, but improved as trial days went on. This is likely a reflection of the fact that fish were adjusting their behaviour to the routine of the maze and securing the reward more quickly. It should be noted, however, that daily scores of both sexes remained stable throughout winter, suggesting a general reduction in spatial learning ability when metabolic demands are lower.
To conclude, this study presents the first evidence that spatial learning in fish varies between seasons and may be influenced by a mating system where males and females play contrasting roles to those previously explored in mammalian models. Future studies should consider the underlying physiological mechanisms behind this phenomenon which may include hormonal influences on brain plasticity.
References
Aronson LR (1951) Orientation and jumping behaviour in the gobiid fish Bathygobius soporator. Am Mus Novit 1286:1–22
Aronson LR (1971) Further studies on orientation and jumping behavior in the gobiid fish, Bathygobius soporator. Ann N Y Acad Sci 188(1):378–392
Astié AA, Kacelnik A, Reboreda JC (1998) Sexual differences in memory in shiny cowbirds. Anim Cogn 1(2):77–82
Broglio C, Rodriguez F, Salas C (2003) Spatial cognition and its neural basis in teleost fishes. Fish Fish 4:247–255
Brown C (2003) Habitat–predator association and avoidance in rainbowfish (Melanotaenia spp.). Ecol Freshw Fish 12:118–126
Brown C, Braithwaite VA (2004) Size matters: a test of boldness in eight populations of the poeciliid Brachyraphis episcopi. Anim Behav 68:1325–1329
Carazo P, Noble DW, Chandrasoma D, Whiting MJ (2014) Sex and boldness explain individual differences in spatial learning in a lizard. Proc R Soc B 281(1782):20133275
Carneiro LA, Andrade RP, Oliveira RF, Kotrschal K (2001) Sex differences in home range and dorso-lateral telencephalon in the Azorean rock-pool blenny. Soc. Neurosci, Abs, p 27
Costa SS, Andrade R, Carneiro LA, Gonçalves EJ, Kotrschal K, Oliveira RF (2011) Sex differences in the dorsolateral telencephalon correlate with home range size in Blenniid fish. Brain Behav Evol 77:55–64
Cummings ME (2018) Sexual conflict and sexually dimorphic cognition—reviewing their relationship in poeciliid fishes. Behav Ecol Sociobiol 72(4):73
Fabre N, García-Galea E, Vinyoles D (2014) Spatial learning based on visual landmarks in the freshwater blenny Salaria fluviatilis (Asso, 1801). Learn Motiv 48:47–54
Floresco SB (2014) Spatial learning in animals. In: Encyclopedia of psychopharmacology. Springer, Berlin, pp 1620–1623
Galea LAM, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL (1994) Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res 635(1–2):18–26
Galea LA, Kavaliers MARTIN, Ossenkopp KP (1996) Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J Exp Biol 199(1):195–200
Garber PA (1989) Role of spatial memory in primate foraging patterns: Saguinus mystax and Saguinus fuscicollis. Am J Primatol 19(4):203–216
Gaulin SJ, Fitzgerald RW (1986) Sex differences in spatial ability: an evolutionary hypothesis and test. Am Nat 127(1):74–88
Gaulin SJ, Fitzgerald RW (1989) Sexual selection for spatial-learning ability. Anim Behav 37:322–331
Gaulin SJC (1995) Does evolutionary theory predict sex differences in the brain? In: Gazzaniga MS (ed) The cognitive neurosciences. MIT, Cambridge, pp 1211–1224
Gibson RN (1967) The agonistic behaviour of juvenile Blennius pholis L. (Teleostei). Behaviour 30(2):192–217
Gibson RN (1999) Movement and homing in intertidal fishes. In: Horn MH, Martina KLM, Chotkowski MA (eds) Intertidal fishes: life in two worlds. Academic Press, San Diego, pp 97–125
Giraldeau LA (1997) The ecology of information use. Behav Ecol Evol Approach 4:42–68
González-Gómez PL, Madrid-Lopez N, Salazar JE, Suárez R, Razeto-Barry P, Mpodozis J, Bozinovic F, Vásquez RA (2014) Cognitive ecology in hummingbirds: the role of sexual dimorphism and its anatomical correlates on memory. PLoS ONE 9(3):e90165
Gray JA, Buffery AW (1971) Sex differences in emotional and cognitive behaviour in mammals including man: Adaptive and neural bases. Acta Physiol (Oxf) 35(2):89–111
Griffiths SP (2003) Homing behaviour of intertidal rockpool fishes in south-eastern New South Wales, Australia. Aust J Zool 51(4):387–398
Halpern DF (1991) Sex differences in cognitive ability, 2nd edn. Erlbaum, Hillsdale
Healy S (ed) (1998) Spatial representation in animals. Oxford University Press, New York
Healy SD, Jones CM (2002) Animal learning and memory: and integration of cognition and ecology. Zoology 105:321–327
Hernaman V, Munday PL (2007) Evolution of mating systems in coral reef gobies and constraints on mating system plasticity. Coral Reefs 26(3):585–595
Jacobs LF (1996) The economy of winter: phenotypic plasticity in behavior and brain structure. Biol Bull 191(1):92–100
Jones CM, Braithwaite VA, Healy SD (2003) The evolution of sex differences in spatial ability. Behav Neurosci 117(3):403
Jorge PE, Almada F, Gonçalves AR, Duarte-Coelho P, Almada VC (2012) Homing in rocky intertidal fish. Are Lipophrys pholis L. able to perform true navigation? Anim Cognit 15(6):1173–1181
Jozet-Alves C, Modéran J, Dickel L (2008) Sex differences in spatial cognition in an invertebrate: the cuttlefish. Proc R Soc Lond B Biol Sci 275(1646):2049–2054
Kavaliers M, Ossenkopp KP, Prato FS, Innes DGL, Galea LAM, Kinsella DM, Perrot-Sinal TS (1996) Spatial learning in deer mice: sex differences and the effects of endogenous opioids and 60 Hz magnetic fields. J Comp Physiol A 179(5):715–724
Kavaliers M, Ossenkopp KP, Galea LA, Kolb B (1998) Sex differences in spatial learning and prefrontal and parietal cortical dendritic morphology in the meadow vole Microtus pennsylvanicus. Brain Res 810(1–2):41–47
Kimura D (1999) Sex and cognition. MIT, Cambridge
Kong YH, Chen IS (2013) Reproductive biology of intertidal frillfin goby, Bathygobius fuscus in Keelung, Taiwan. J Mar Sci Technol 21:213–215
Krebs JR (1990) Food-storing birds: adaptive specialization in brain and behaviour? Philos Trans R Soc Lond B 329(1253):153–160
Lacreuse A, Herndon JG, Killiany RJ, Rosene DL, Moss MB (1999) Spatial cognition in rhesus monkeys: male superiority declines with age. Horm Behav 36(1):70–76
Lucon-Xiccato T, Bisazza A (2017) Sex differences in spatial abilities and cognitive flexibility in the guppy. Anim Behav 123:53–60
Markel RW (1994) An adaptive value of spatial learning and memory in the blackeye goby Coryphopterus nicholsi. Anim Behav 47(6):1462–1464
Mazzoldi C, Petersen CW, Rasotto MB (2005) The influence of mating system on seminal vesicle variability among gobies (Teleostei, Gobiidae). J Zool Syst Evol Res 43(4):307–314
Noble DW, Carazo P, Whiting MJ (2012) Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol Let 8(6):946–948
Odling-Smee L, Braithwaite VA (2003) The role of learning in fish orientation. Fish Fish 4(3):235–246
Odling-Smee L, Simpson SD, Braithwaite VA (2006) The role of learning in fish orientation. In: Brown C, Laland KN, Krause J (eds) Fish cognition and behaviour. Blackwell, Cambridge, pp 119–138
Odling-Smee L, Simpson SD, Braithwaite VA (2011) The role of learning in fish orientation. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior. Wiley-Blackwell, Chichester, pp 166–185
Roy T, Bhat A (2017) Divergences in learning and memory among wild zebrafish: do sex and body size play a role. Learn Behav 46:124–133
Schaafsma SM, Groothuis TG (2011) Sex-specific effects of postnatal testosterone on lateralization in cichlid fish. Anim Behav 81(1):283–288
Sherry DF, Hampson E (1997) Evolution and the hormonal control of sexually-dimorphic spatial abilities in humans. Trends Cogn Sci 1(2):50–56
Silverman I, Eals M (1992) Sex differences in spatial abilities: evolutionary theory and data. In: Portions of this paper were presented at the meetings of the International Society for Human Ethology in Binghamton, NY, Jun 1990, the Human Behavior and Evolution Society in Los Angeles, CA, Aug 1990, and the European Sociobiological Society in Prague, Czechoslovakia, Aug 1991. Oxford University Press, Oxford
Smith GT, Brenowitz EA, Wingfield JC (1997) Seasonal changes in the size of the avian song control nucleus HVC defined by multiple histological markers. J Comp Neurol 381:253–261
Sovrano VA, Bisazza A, Vallortigara G (2003) Modularity as a fish (Xenotoca eiseni) views it: conjoining geometric and nongeometric information for spatial reorientation. J Exp Psychol Anim Behav Process 29(3):199–210
Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN (2011) Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav 59(4):484–496
Taru M, Kanda T, Sunobe T (2002) Alternative mating tactics of the gobiid fish Bathygobius fuscus. J Ethol 20:9–12
Tavolga WN (1954) Reproductive behaviour in the gobiid fish Bathygobius sporator. Bull Am Mus Nat Hist 104:427–460
Thacker CE (2009) Phylogeny of Gobioidei and placement within Acanthomorpha, with a new classification and investigation of diversification and character evolution. Copeia 2009:93–104
Thia JA, Riginos C, Liggins L, Figueira WF, McGuigan K (2018) Larval traits show temporally consistent constraints, but are decoupled from postsettlement juvenile growth, in an intertidal fish. J Anim Ecol 87(5):1353–1363
Tramontin AD, Smith GT, Breuner CW, Brenowitz EA (1998) Seasonal plasticity and sexual dimorphism in the avian song control system: stereological measurement of neuron density and number. J Comp Neurol 396:186–192
Tramontin AD, Brenowitz EA (2000) Seasonal plasticity in the adult brain. Trends Neurosci 23:251–258
Vinyoles D, De Sostoa A (2007) Life-history traits of the endangered river blenny Salaria fluviatilis (Asso) and their implications for conservation. J Fish Biol 70(4):1088–1108
White GE, Brown C (2013) Site fidelity and homing behaviour in intertidal fishes. Mar Biol 160(6):1365–1372
White GE, Brown C (2014a) A comparison of spatial learning and memory capabilities in intertidal gobies. Behav Ecol Sociobiol 68(9):1393–1401
White GE, Brown C (2014b) Cue choice and spatial learning ability are affected by habitat complexity in intertidal gobies. Behav Ecol 26(1):178–184
Wickler W (1957) Vergleichende Verhaltensstudien an Grundfischen I. Beiträge zur Biologie, besonders zur Ethologie von Blennius fluviatilis Asso im Vergleich zu einigen anderen Bodenfischen. Ethology 14(4):393–428
Yaskin VA (1984) Seasonal changes in brain morphology in small mammals. In: Advances in the biology of shrews. Carnegie Museum of Natural History Special Publication
Acknowledgements
This research was funded by the Department of Biological Sciences at Macquarie University, and P. Carbia as supported by an MQ10 PhD Scholarship. The authors wish to thank the SWF technician of Macquarie University, Josh Aldridge, for assistance in animal husbandry.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Gobies were caught in compliance with NSW Fisheries (Permit No. P08/0010-3.0). Husbandry and experimental conditions were approved by the Macquarie University Ethics Committee (ARA 2014/003). All individuals were kept in the Sea Water Facility of Macquarie University for the full duration of the experiment to prevent re-capture of individuals. Upon conclusion of research, all gobies were released at the site of capture.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carbia, P.S., Brown, C. Seasonal variation of sexually dimorphic spatial learning implicates mating system in the intertidal Cocos Frillgoby (Bathygobius cocosensis). Anim Cogn 23, 621–628 (2020). https://doi.org/10.1007/s10071-020-01366-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-020-01366-3