Abstract
Learning by watching others can provide valuable information with adaptive consequences, such as identifying the presence of a predator or locating a food source. The extent to which nonhuman animals can gain information by reading the cues of others is often tested by evaluating responses to human gestures, such as a point, and less often evaluated by examining responses to conspecific cues. We tested whether ten brown capuchin monkeys (Cebus [Sapajus] apella) were able to use cues from monkeys and a pointing cue from a human to obtain hidden rewards. A monkey could gain access to a reward hidden in one of two locations by reading a cue from a conspecific (e.g., reaching) or a human pointing. We then tested whether they could transfer this skill from monkeys to humans, from humans to monkeys, and from one conspecific to another conspecific. One group of monkeys was trained and tested using a conspecific as the cue-giver and was then tested with a human cue-giver. The second group of monkeys was trained and tested with a human cue-giver and was then tested with a monkey cue-giver. Monkeys that were successful with a conspecific cue-giver were also tested with a novel conspecific cue-giver. Monkeys learned to use a human point and conspecific cues to obtain rewards. Monkeys that had learned to use the cues of a conspecific to obtain rewards performed significantly better than expected by chance when they were transferred to the cues of a novel conspecific. Monkeys that learned to use a human point to obtain rewards performed significantly better than expected by chance when tested while observing conspecific cues. Some evidence suggested that transferring between conspecific cue-givers occurred with more facility than transferring across species. Results may be explained by simple rules of association learning and stimulus generalization; however, spontaneous flexible use of gestures across conspecifics and between different species may indicate capuchins can generalize learned social cues within and partially across species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social learning, or learning by interacting with others, is adaptive. Animals attend to each other in their groups, using cues from conspecifics, such as location to a food source or fear behavior in the presence of predators, to learn novel responses. By monitoring others, an animal can learn which foods to eat, the location of food, and the location of predators. For example, Galef conducted classic experiments in which he demonstrated that rats learn what foods to eat by seeing the food choices of a rat in an adjacent enclosure, but only when olfactory cues were also available (e.g., Galef and Wigmore 1983). Lab-reared monkeys that were not afraid of snakes became fearful of them after watching a wild-reared conspecific fearing a snake, maintaining this fear over a three-month period after exposure (Mineka and Cook 1988). Such work promotes interest in the cues animals use to learn from others.
Much work on what cues animals use to modify their behavior comes from research on nonhuman animal responses to human gestures. For example, under controlled conditions a wide range of animals (e.g., apes, monkeys, canids, felids, bovids, pinnipeds, and cetaceans) can learn to use a human point as a cue to obtain hidden food or otherwise make a choice indicated by the experimenter (see review by Miklósi and Soproni 2006). More recently, pigs, Sus scrofa (Nawroth et al. 2013), elephants, Loxodonta africana (Smet and Byrne 2013), sea lions, Otaria byronia (Highfill et al. 2007) and birds (Clark’s nutcracker, Nucifraga columbiana; Tornick et al. 2011; Clary and Kelly 2013) have been added to the list. Typically, other cues such as eye gaze, head direction, body angle, indicator objects, and local enhancement, in which an animal moves to the location where it observed another animal, have also been examined to determine which cues, or combinations of cues, animals attend to and respond to when identifying the position of a hidden food item.
Tomasello et al. (1997) report a typical study in which chimpanzees (Pan troglodytes) and orangutans (Pongo abelii) were tested on their ability to use a cue to select hidden food from one of three unique containers. Only one of the nine subjects used the pointing cue effectively, and this was an enculturated orangutan that had already learned to understand the gesture. None of the apes without prior training used any of the cues successfully. Marsh (2012) conducted a similar study of five orangutans in a three-cup choice task using a human pointing cue (experimenter’s finger touching the baited cup) and a human local enhancement cue. Both cues were used by at least some subjects. Results of these two studies were typical in that some cues were used with more facility than others and there were large individual differences across subjects.
A study by Itakura et al. (1999) examining chimpanzees was unique because it is, perhaps, the only direct comparison between humans and conspecifics as cue-givers in primates. Chimpanzees were first tested with a conspecific providing a local enhancement cue and then a gaze/point cue and were then tested with a human providing the same two cues. No significant differences were found across type of informant for either cue, implying each type of informant was equally effective. However, all subjects received the conspecific informant first in this study, which may have influenced later performance with a human. To test for differences across type of informant more effectively, a counterbalanced design should be used, such as in a study on dogs (Canis familiaris) by Hare and Tomasello (1999). Dogs used a local enhancement cue and a gaze/point cue from a human and a conspecific. The authors found no order effects and no significant differences between the type of cue provided or the type of informant, concluding that all four types of cue were used equally well by the dogs. However, this counterbalanced design has not been applied within nonhuman primates.
The studies that make a direct comparison between human and conspecific informants are of interest because if animals can transfer the ability to read a cue from one species to another, then the process demonstrates some degree of flexibility in reading the cue. Flexibility is notable because a usual explanation for success in cueing studies is that animals are using associative learning of a discriminant stimulus to perform the tasks (Anderson et al. 1995). Using human pointing as an example, animals learn that a particular stimulus (a human hand near an object) is paired with a reward (food). Povinelli et al. (1999) refer to this as a “low-level” explanation for performance and contrast it with a “high-level” explanation in which the animal understands the internal mental state of the pointer, such as attention and knowledge. However, Call and Tomasello (Tomasello and Call 1997; Call 2001; Call and Tomasello 2005) propose “an explanation of a third kind” when applying these extremes to the study of cognitive abilities such as seeing and social cognition. They propose an intermediate explanation between learned behavioral contingencies and more mental abilities that imply knowing the mental states of others (i.e., theory of mind). They explain that primates are very skilled in the social domain and use their accumulated knowledge to form rules or predictions about the behavior of others. The application of this “knowledge” to solve novel physical and social problems does not rely on an animal’s understanding of the mental states of others. Using this more cognitive approach, if animals flexibly transfer the use of a cue from monkey to human or human to monkey, then it may indicate more than associative learning of a discriminative stimulus.
Another indicator of flexibility would be if animals could transfer between conspecific informants. Mason and Hollis (1962) tested for this in their study of local enhancement in rhesus macaques. Food was placed in one of four trays, hidden from the view of a subject monkey. An informant monkey could position itself behind one of four food trays and provide the subject monkey with a local enhancement cue to select the correct tray. Subject monkeys were paired with the same informant monkey until they reached a high level of successful performance using the informant as a local enhancement cue. When they switched the informant to a stranger rhesus monkey that also gave reliable local enhancement cues, there was immediate transfer to the new monkey. Interestingly, it took monkeys over a thousand trials to reach their plateau level of approximately 70% correct with their first informant. With the new strange partner, the monkeys reached approximately 70% correct in the first 24-trial block, indicating immediate transfer. Further, in the chimpanzee study in which one conspecific served as an effective local enhancement cue (Itakura et al. 1999), subjects were just as successful when the cue-giver was switched to a new conspecific. However, transfer between conspecifics has not been tested in capuchin monkeys.
We tested how flexibly brown capuchins would use cues of conspecifics and a human point to obtain hidden food in a choice task. Capuchins were excellent subjects for such a study, as they are known to use a human pointing gesture to obtain hidden food (Anderson et al. 1995; Itakura and Anderson 1996; Vick and Anderson 2000). Capuchins have also been shown to use the emotional expressions of conspecifics to an object in a box to make choices in a two-choice task (Morimoto and Fujita 2012), and show a preference for humans who match their behavior (Paukner et al. 2009). Capuchins also monitor the actions of conspecifics, and systematic tests have shown that they learn to solve food acquisition tasks by observing the actions of others (e.g., Dindo et al. 2008).
To counterbalance which species acted as a cue-giver first we tested half the subjects with a conspecific cue-giver first, followed by a novel conspecific cue-giver, followed by a human pointing. The other half of the monkeys were tested with a human pointing first, followed by a conspecific cue-giver, followed by a novel conspecific cue-giver. Since no direct comparisons have been conducted, we aimed to determine whether monkeys would learn differently from a conspecific than a human. We also aimed to test whether monkeys would transfer the ability to use a cue from (1) one conspecific cue-giver to another conspecific cue-giver, (2) from a conspecific cue-giver to a human, and (3) from a human to a conspecific. Based on similar studies of chimpanzees, macaques, and dogs (Mason and Hollis 1962; Hare and Tomasello 1999; Itakura et al. 1999), we predicted some degree of positive transfer, which would indicate flexible use of the cueing gestures.
Methods
Subjects
Subjects were ten brown capuchins from a group of 17 monkeys socially housed at the Bucknell University primate facility in Lewisburg, PA, USA (Table 1). The colony was established in 2000 from six monkeys acquired from Yerkes National Primate Research Center in Atlanta, GA. Monkeys were chosen to participate based on their willingness to voluntarily enter the apparatus and conduct trials seated across from another monkey. Five monkeys were randomly assigned to the group that received a conspecific cue-giver first, and five were assigned to the group that received the human cue-giver first (Table 1). None of these monkeys had prior experience testing with either a human or a conspecific providing gestural cues. Six monkeys had experience from prior experiments selecting between two visible objects (Judge and Bruno 2012; Judge and Essler 2013), but none had experience with a hidden object-choice task.
Six monkeys were used as cue-givers (Table 1). Again, these monkeys were selected for their willingness to enter the apparatus and provide some form of gestural cue toward a cup containing a reward. An attempt was made to randomly assign cue-giver monkeys to subject monkeys; however, some pairs proved incompatible in that the subject monkey would not remain in the testing chamber with some cue-givers and some cue-givers would not provide cues for particular subject monkeys. Cases of incompatibility were typically the result of monkeys being at the opposite ends of the dominance hierarchy. If monkeys would not work together, subject monkeys were randomly assigned another cue-giver.
Housing
The enclosure spanned three rooms and consisted of 17 subcompartments that were interconnected by doorways and overhead tunnels. The doorways could be closed, and the tunnels could be blocked with metal barriers to separate monkeys into separate compartments. Subcompartments averaged 1.5 w by 2.3 h by 2.3 l m and were made of stainless steel wiring and plastic paneling. All compartments were furnished with perches, platforms, poles, swings, and other climbing structures to allow for naturalistic movement. Floors were made of linoleum and covered with cedar chip shavings. Monkeys were fed a diet of monkey chow, cereals, nuts, grains, fruits, and vegetables, twice daily. Water was available ad libitum. Daily enrichment was provided for all monkeys. All experimental procedures were approved by Bucknell University’s Institutional Animal Care and Use Committee (Protocol# PGJ-07) and husbandry conformed to the guidelines within the Guide for the Care and Use of Laboratory Animals (Committee for the Update of the Guide for the Care and Use of Laboratory Animals 2011).
Procedures
Apparatus
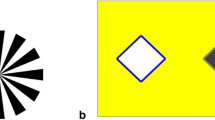
Monkeys were tested in three interconnected compartments that were a subsection of their home cage. The three compartments consisted of a 1.1 w by 2.3 h by 2.3 l m smaller center room, where the apparatus was placed, with 1.5 w by 2.3 h by 2.3 l m rooms to the left and right of the center room. The center room was connected to the others by a 0.6 w by 0.6 h m doorway on both walls, which was 0.8 m from the ground. The apparatus consisted of a rectangular steel frame measuring 0.6 w by 0.5 h by 1.1 l m. It was placed in the openings interconnecting the three rooms and spanned the length of the center room (Fig. 1). Wire caging partitioned the apparatus frame into three sections. Two outer sections allowed monkeys to enter into the center room from the side rooms and sit in the apparatus. The center section of the apparatus held a 43.2-cm metal bar that slid back and forth between the two side sections. On each end of the bar, 28 cm apart, was an inverted white cup with one side cut out that could rotate 360° on a swivel. After a cup was baited with a reward, experimenter(s) could move the bar toward a monkey allowing the monkey to select one of the cups. As the food reward was very light, it was unlikely that the weight of the cup turning would have been any indicator to the monkeys. The 5.0 w by 2.5 h cm (2 w by 1 h in.) holes in the caging wire entering into the center section of the apparatus were large enough for the monkeys to reach the length of their arm into the center section to access the cups.
The testing apparatus positioned between two subcompartments of the monkeys’ enclosure (a), a trial with a human experimenter providing a pointing cue on the left and the subject monkey’s correct choice on the right (b), and trial with a conspecific providing a reaching cue on the left and the subject monkey’s correct choice on the right (c)
Training
Training consisted of four phases that were meant to accustom the monkeys to the affordances of the apparatus and the nature of the experiment. Half of the subjects went through training with a conspecific cue-giver, whereas the other half went through training with a human cue-giver. In the following description, “cue-giver” was a monkey or a human, depending on the testing group to which the subject was assigned. In both training and testing phases, once the subjects made a choice, the bar was moved back to the middle of the apparatus so that the subject was only capable of making one choice. A cup choice was defined as the first cup that the monkey turned fully so that they could see whether a reward was in the cup.
Phase 1 trained the subjects and the cueing monkeys to properly turn the cups on the bar in the center of the apparatus to retrieve a reward (Fig. 2a). The monkey watched the experimenter bait one cup with a preferred food, the bar was moved toward the monkey, and the monkey was then allowed to choose one of the cups. The angles of the cups incrementally increased from 0° to 90°, 120°, and 180° away from the monkey. Monkeys were trained to consistently choose the baited cup to retrieve the reward even though they eventually could not see the reward at the 180° angle, requiring them to attend to the baiting process. Monkeys were given five trials at each angle for a total of 20 trials. The side on which the reward was placed alternated between trials.
Diagrams of the training phases and a test trial. Food is represented by a red dot, and cups are represented by semicircles. Arrows indicate which monkey (subject or cue-giver) chose a cup in that particular phase. In phase 1 (a), the subject monkey witnessed the baiting with the cups facing away. In phase 2 (b), the subject monkey and a cue-giving monkey witnessed the baiting with the cups facing the subject monkey. In phase 3 (c), the subject monkey and a cue-giving monkey witnessed the baiting with the cups facing the cue-giving monkey. In phase 4 (d), the subject monkey did not witness the baiting process, which was blocked by an occluder, and the cups faced the cue-giving monkey. In a test trial (e), the baiting was blocked from the view of both monkeys with occluders and the cups faced the cueing monkey. To conduct a trial, the occluders were removed, the cups were moved toward the cueing monkey to elicit a cue, and the cups were then moved to the subject monkey for a choice (color figure online)
Phase 2 allowed the subject monkey to learn that the cue-giver was able to retrieve a reward from the cups (Fig. 2b). The cups were oriented so that the open side faced the subject monkey. Both the subject and cue-giver watched the baiting process, and the cups were then moved toward the cue-giver. Subject monkeys observed the cue-giver turn the cups to retrieve the reward. Twenty trials were conducted in this phase in two 10-trial sessions in which the baiting switched between the left cup and the right cup, respective to the subject monkey. In this phase and in all subsequent training and testing sessions, the cups were baited in a pseudo-randomized order for a set of 20 trials, such that 10 trials were on the left, 10 trials were on the right, and there were no more than two trials in a row on the same side.
Phase 3 allowed the subject to learn that the cue-giver could see and retrieve the reward when the subject could not see the reward in the cup (Fig. 2c). Both the subject and cue-giver watched the baiting process with the cups oriented so that the open side faced the cue-giver. The cups were then moved toward the cue-giver, who retrieved the reward. Thus, even though the subject could not see the reward in the cup, it could see that the cue-giver was informed of its location and able to retrieve it. Subjects were given two blocks of ten trials in this phase.
Phase 4 allowed the subject to learn that the cue-giver was able to see and retrieve the reward when the subject did not witness the baiting process, and was therefore still informed about the location of the food (Fig. 2d). The cups were oriented so that the open side faced the cue-giver and the baiting process and partner were hidden from the subject by a 0.7 w by 0.4 h m wooden board occluder. When the board was removed, the cups were moved toward the cue-giver who retrieved the reward. Though the subject did not see the baiting process, it could see that the cue-giver was informed about the location of the reward. Subjects were given four blocks of ten trials at this phase, resulting in 40 total trials. More trials were used in this phase than earlier training phases to familiarize the subjects to the novel occluder. All of the subject monkeys in each condition progressed through every phase of training.
After the cue-giver received the reward in each training trial, a reward would be placed in the same cup and moved to the subject monkey in order to maintain its interest in participating. When two experimenters were present with the apparatus, baiting was alternated between the two to avoid bias toward the side of one experimenter. When only one experimenter was present with the apparatus, the experimenter spent the first five trials on one side of the apparatus and the next five trials on the other side of the apparatus. The side on which the single experimenter started was alternated at every block of trials, so the position of the experimenter could not systematically bias cup choices.
Testing: conspecific cue-giver
In the conspecific cue-giver condition, the subject monkey would sit opposite another monkey and use any cues given by a cue-giver monkey to choose a cup. First, an occluder was placed in front of each monkey so that neither could observe the baiting process. One of the experimenters then baited one of the two cups. Even though the baiting process was obstructed by occluders, as a precaution, the baiting experimenter touched both cups at the same time when baiting, so that the subject monkey could not determine which cup was baited by using subtle body gestures or sounds made by the experimenter. Further, both experimenters faced forward, to reduce the chance the subject used inadvertent facial cues from either experimenter. The experimenters then removed the occluders, and the cueing monkey was able to see which of the two cups was baited. Once the cueing monkey and subject monkey faced the cups for three consecutive seconds, the experimenters moved the bar toward the cueing monkey to promote natural cueing behavior toward the reward (Fig. 2e). Cues included extending the entire arm through the cage wiring, extending the hand to the wrist through the cage wiring, extending a hand toward a cup but not through the wire, sitting in front of a cup, and looking toward a cup. Once a cue was provided toward the baited cup, the bar was moved within reach of the subject monkey. After the subject monkey rotated a cup, regardless of the outcome, the bar was immediately moved back to center and out of range of both monkeys. If the subject monkey selected the unbaited cup, the experimenter rotated the baited cup to show that the other cup contained a reward. Between each testing trial, there was a trial dedicated to rewarding the cueing monkey to maintain its interest in testing. Both occluders were placed in front of the monkeys for baiting, the cups were turned to face the cueing monkey, and one cup was baited. The occluder in front of the cueing monkey was then removed, and the cups were moved toward the cueing monkey so that it could retrieve the reward.
Testing: human cue-giver
Trials with a human cue-giver were conducted much the same as those with a conspecific cue-giver. One or two experimenters would bait the cups and operate the occluders from the sides of the apparatus while a human sat across from the subject monkey as a cue-giver. The human cue-giver remained constant for each subject monkey. Aspects of a testing trial that were necessary when a conspecific was the cue-giver were maintained with a human pointer even though they were not necessary. For example, two occluders continued to be used during the baiting process and the human pointer was “rewarded” between trials by putting in both occluders, baiting one of the cups, removing the occluder on the human’s side, and sliding the cups toward the human. Continuing these steps assured that the procedures and timing of the trials were the same across the conspecific and human cueing conditions. During a human pointing trial, the human pointer would slowly lift the arm on the same side of the baited cup and extend the arm forward, protruding her index finger through the caging toward the baited cup (Fig. 1b). The human pointer would alternate her gaze between the subject and the baited cup. Thus, all human cue-givers used a “point and gaze” cue.
Testing: control trials
Control trials were conducted without a cue-giver to assure that successful subjects were not using aspects of the testing procedures (e.g., cues from the experimenters standing next to the apparatus, sounds from the baiting process, the smell of the reward) to successfully obtain rewards. Control trials were identical to those using a conspecific cue-giver or human cue-giver except that the compartment in the apparatus across from the subject monkey was empty. Each subject received 100 control trials (10 sessions of 10 trials).
Testing schedules
Subjects were given 10-trial testing sessions once per day. Sessions lasted about 5–7 min per monkey. In the group that started with a conspecific cueing partner, each subject monkey was paired with a female cueing partner. We selected females and a juvenile male as cue-giving partners because we thought that subject monkeys might be intimidated by an adult male cue-giver, which would interfere with testing. After at least 100 trials, or five 20-trial blocks, with their first partner, the subject monkeys were paired with a second cueing partner. For subject monkeys that were successful reading the cues of their first conspecific cueing partner, changing partners would test for transfer of the ability to different conspecific partners. Our criterion for success for all testing was 17 out of 20 (85%) baited cup selections by the subject monkey in two consecutive 20-trial blocks. If subject monkeys were not successful reading the cues of their first conspecific cueing partner after five 20-trial blocks, they were given a new female cueing partner for five 20-trial blocks in the event that having a new partner might improve performance. After subjects had been tested with two partners, they were then tested with a human cue-giver for at least five 20-trial blocks. If subject monkeys had been successful reading a conspecific cue-giver at this point, using a human cue-giver would test for transfer from a conspecific to a human. If subject monkeys were unsuccessful reading a conspecific cue-giver at this point, success with a human cue-giver might indicate that a human-given cue was more salient than a conspecific cue. If subject monkeys were unsuccessful with a human pointing cue at that point, we added a vocalization to the pointing cue. Call et al. (2000) showed that the use of vocalizations in an object-choice task helped increase the performance of some chimpanzees. A vocalization cue can be helpful because it can draw the attention of the subject to the cue-giver and because many primates use vocalizations in the context of finding and eating food (Call et al. 2000; Itakura et al. 1999). The point with vocalization cue was identical to the human pointing cue except the experimenter would say “This one” when pointing to the baited cup. If monkeys became successful at the human point with vocalization cue, we retested them using a point without vocalization cue. If monkeys were unsuccessful with a conspecific but were successful with a human pointer, we retested them with a conspecific cue-giver to test for transfer from a human to a conspecific.
The group of subject monkeys that were first tested with a human cue-giver received at least five 20-trial blocks with a human pointing. If subject monkeys were successful with the human cue-giver, they were then tested with a conspecific cue-giver to test for transfer from a human to a conspecific. If subject monkeys were not successful with the human cue-giver, they were still tested with a conspecific cue-giver to determine whether cues from a conspecific were more salient cues to the subject monkey. If monkeys were unsuccessful with a human cue-giver but subsequently were successful with a conspecific, they were retested with a human cue-giver. If monkeys were unsuccessful with a human and then unsuccessful with a conspecific cue-giver, they were retested with a human cue-giver using a point with vocalization. If monkeys became successful after the added vocalization, they were retested with just the human point cue without vocalization. If monkeys were then successful with just the human point, they were retested with a conspecific.
Data analysis
Video cameras were placed facing each side opening to simultaneously film the choices of the subject monkey and the cues of the cueing monkey on each trial (Fig. 1). We combined 10-trial test sessions into 20-trial blocks and tallied the number of times a subject monkey selected the baited cup. Assuming a 50% probability of obtaining a reward on the two-choice task, a performance of 15 out of 20 (75%) would be statistically significant according to a binomial distribution (p = .041, two-tailed). We adopted the more conservative criterion of 17 out of 20 (85%) baited cup choices as successful performance. Monkeys had to perform at this level in two consecutive 20-trial blocks to be considered successful at the task. If a monkey was not at or near criterion after five 20-trial blocks, we considered performance unsuccessful and moved on to the next scheduled testing condition. If the unsuccessful performance was the monkey’s last scheduled condition, we discontinued testing.
Our planned test to determine whether monkeys learned differently from a conspecific cue-giver than a human pointing was to use an independent-group t test to compare the mean number of twenty-trial blocks to criterion with a conspecific cue-giver to that of a human pointing. To test for transfer between cue-givers, we used one-sample t tests to compare the mean performance on the first 20-trial block after a switch of cue-giver to the hypothesized mean of 10 correct if monkeys were performing randomly. If some degree of transfer were occurring, we would expect the mean with the new cue-givers to be significantly higher than chance. To test for differences in responding when monkeys switched conspecific cue-givers, we conducted a paired sample t tests to compare the number of correct trials in the last 20-trial block before switching conspecific cue-givers to the first 20-trial block after switching to novel conspecific cue-givers. To test for differences in performance when monkeys switched species of cue-giver, we conducted a two-way repeated measures ANOVA with time (before and after species switch) as a repeated measure variable and order of switch (conspecific to experimenter and experimenter to conspecific) as a between subjects variable. For the dependent variable, we used the number of correct trials in the last 20-trial block before the switch and the number of correct trials in the first 20-trial block after the switch. We used a one-sample t test to determine whether performance in control trials was different than chance. All tests were run at two-tailed alpha = .05.
Results
Cues provided
We examined 300 cueing episodes given by the six cueing monkeys to determine the reliability of the cues provided. Five 10-trial cueing sessions were selected for each cueing monkey. The 10-trial cueing sessions selected were distributed across the subject monkeys for each cueing monkey and randomly selected from the sessions for that pair. In 98.3% of cases, the cueing monkey reached toward the baited cup either by extending its entire arm through the wire caging (78.3%), extending its arm through the caging up to the wrist (14.0%), or moving a hand toward the cup without protruding it through the caging wire (6.0%). In the remaining 1.7% of cases, the cueing monkey did not provide a reaching cue and either sat on the side with the baited cup (1.3%) or looked toward the baited cup (0.33%). On most occasions when the cueing monkey extended its entire arm toward the baited cup, the monkey was attempting to grab the baited cup and sometimes made contact with the cup. Also, by default, when a monkey reached toward a cup, it also provided a local enhancement cue. Thus, cueing monkeys provided subject monkeys with salient, reliable cues. We did not analyze vocalizations made by cueing monkeys due to the fact that it would have been unreliable given the filming angle and the presence of multiple other monkeys in the same room or adjacent room (also possibly vocalizing) during testing periods.
Individual performances
Individual performances were quite varied (Fig. 3). In the group of five monkeys that started with a conspecific cue-giver, two of the five monkeys (Socrates and Newton) gradually reached our criterion for success with their first conspecific cue-giver, transferred to a novel conspecific cue-giver, and transferred to a human point (Fig. 3a, b). Two monkeys in this group (Schroeder and Niko) were unsuccessful with two conspecific cue-givers, were successful with a human pointing with a vocalization, and transferred to a conspecific (Fig. 3c, d). The fifth monkey in this group (Monet) was not successful with any cue-giver (Fig. 3e). In the group of five monkeys that started with a human cue-giver, two of the five monkeys (Stella and Nye) gradually became successful with the human cue-giver and transferred to two conspecifics (Fig. 3f, g). One monkey in this group (Smithson) was initially unsuccessful with a human, was successful with two conspecifics, and transferred to a human pointing (Fig. 3h). Another monkey in this group (DaVinci) was unsuccessful with both a human and a conspecific, successful with a human pointing and vocalizing and unsuccessful with a conspecific (Fig. 3i). The fifth monkey in this group (Sheba) was not successful with any type of cue-giver (Fig. 3j). For a more detailed description of individual performances, see the supplementary online material.
Percent of trials correct for each subject. Each point represents a block of 20 trials (two ten-trial sessions). The dashed line indicates the criterion for success: 85% or 17 out of 20 correct. Monkeys were considered successful if they reached this criterion in two consecutive 20-trial blocks. Vertical lines indicate changes across types of cue-givers. Two-letter codes with upper then lower case indicate the identity of a cueing monkey (See Table 1). “E” indicates a human experimenter pointing/gazing. “EV” indicates a human experimenter pointing/gazing and vocalizing
Group-wide performance
The idiosyncratic patterns with which the monkeys progressed through their testing schedules precluded a formal statistical test of whether they could learn to use a human or conspecific cue more quickly; however, outcomes with each type of cue-giver were identical. Two of five monkeys in the conspecific cue-giver group initially learned to use the conspecific cues, and two of five monkeys in the human cue-giver group initially learned to use the human cue. All four of these monkeys took six 20-trial blocks (120 trials) to reach their first 20-trial block performing at or above our 85% correct criterion.
Regarding transfer between conspecific cue-givers, five monkeys who were successful with one conspecific cue-giver were then tested with a novel conspecific cue-giver. All five monkeys were successful with the novel conspecific, and the mean number of correct trials in their first 20-trial block with the new cue-giver (Mean = 17.80, SD 1.64) was significantly higher than chance, t(4) = 10.61, p < .001, 95% CI [15.76, 19.84], indicating some degree of positive transfer. Further, there was no change in performance across conspecific cue-givers as the mean number correct in the last 20-trial block with the first conspecific cue-giver (Mean = 18.40, SD 1.34) was not significantly different than the mean number correct in the first 20-trial block with the novel conspecific cue-giver (Mean = 17.80, SD 1.64), t(4) = 0.88, p = .43, 95% CI of the mean difference [−1.28, 2.48].
Regarding transfer between species of cue-givers, five monkeys who were successful with a human cue-giver were then tested with a conspecific cue-giver. The mean number of correct trials in the first 20-trial block with the conspecific cue-giver (Mean = 14.20, SD 2.95) was significantly higher than chance, t(4) = 3.18, p < .05, 95% CI [10.54, 17.86], indicating successful transfer. Only three monkeys transferred from a conspecific to a human cue-giver and, although the mean number of correct trials in their first 20-trial block with the human cue-giver was greater than the mean of ten expected if monkeys were performing randomly (Mean = 15.33, SD 4.04), the difference was not statistically significant, t(2) = 2.29, p = .15, 95% CI [5.29, 25.37], indicating a lack of transfer. However, with only two degrees of freedom, the test was not very powerful and violated the need to have a sample size of five or more to conduct a t test. Nevertheless, one of these three monkeys, Smithson, selected 20 out of 20 correct in his first 20-trial block with a human pointing cue after reaching criterion with a conspecific (Fig. 3h), indicating capuchins have the cognitive ability to make this transition.
The two-way repeated measures ANOVA to test for changes in performance across species of cue-giver found a main effect of cue-giver transition (before and after switching species of cue-giver), F(1,6) = 13.65, p = .010, partial η 2 = .69, no main effect for order of switching (human to conspecific versus conspecific to human), F(1,6) = 0.12, p = .74, and no interaction effect, F(1,6) = 0.35, p = .58 (Fig. 4). Every monkey that was successful with a first species and tested with a second species was used in the analysis regardless of performance with the second species. Concerning the main effect of cue-giver transition, the mean number of correct trials in the first 20-trial block with the second species of cue-giver (Mean = 14.77, SD 3.45) was significantly lower than the mean number of correct trials in the last 20-trial block with the first species (Mean = 18.73, SD 0.99), indicating that there was a decrement in performance when transferring across species.
Average number of correct trials in the last 20-trial block before transferring to a new species and the first 20-trial block after transferring to a new species. Separate lines indicate whether the transfer was from a conspecific to an experimenter (CS to EX) or from an experimenter to a conspecific (EX to CS)
Control trials
Eight monkeys showed success with at least one type of cue-giver and were tested with 100 control trials in which no cue-giver was present. A binomial test indicated that no individual monkeys performed over chance (lowest number correct = 43; highest number correct = 57) and the mean number correct across subjects (Mean = 49.12, SD 4.67) was not significantly different from chance, one-sample t test t(7) = −0.53, p = .61, 95% CI [45.22, 53.04]. Thus, monkeys were not using inadvertent cues provided by the experimenters to make correct selections.
Discussion
Our study is common to many similar studies in that there was considerable individual variation in performance across subjects. Eight of ten capuchin monkeys were able to read either the cues of a conspecific monkey or a human point to solve the hidden object-choice task. Two monkeys maintained persistent side biases and were unable to learn any of the cues provided (conspecific cues, human pointing, or human pointing with vocalization). A main novel finding was that seven of the capuchin monkeys used conspecific cues to locate the hidden reward. Three of the seven learned first from a conspecific and the remaining four learned to use conspecific cues after they had already learned to use a human pointing cue. As seen in Anderson et al. (1995), results confirm that capuchin monkeys can learn to make a choice based on information drawn from the body movements of others. Use of conspecific cues to find hidden food has also been found in rhesus macaques (Mason and Hollis 1962), chimpanzees (Itakura et al. 1999), dogs (Hare and Tomasello 1999), and another study of capuchins (Morimoto and Fujita 2012).
We could not test for a difference in acquisition between the human point group and the conspecific cueing group, but outcomes did not indicate a difference. Two of five monkeys were initially successful in each cueing group, and the two successful monkeys in each group took the same number of trials (N = 120) to attain our criterion of 85% correct. Hare and Tomasello (1999) found similar results in dogs in that there was no difference in performance on an object-choice task when the same cue was provided by a human or another dog. Itakura et al. (1999) also found that chimpanzees showed no difference in performance on an object-choice task when a local enhancement cue and a gaze/point cue were provided by a conspecific or a human. With only three studies making direct comparisons, no firm conclusions should be drawn, but it appears that animals are attending to the relevant aspects of the cues rather than the species providing them.
The capuchin monkeys’ performance using the human point and gaze cue appeared to be superior to a previous study much like our own (Anderson et al. 1995). Three capuchins were given a human point and gaze cue in a two-choice hidden reward task (Experiment 3). Monkeys were tested in 30-trial blocks, and although all three performed significantly above baseline trials in which no cue was given, they rarely reached our 85% correct criterion. Our monkeys achieved a sustained high performance more quickly, perhaps because Anderson et al. (1995) interspersed baseline trials between each test trial, which may have affected learning of the cues. Although successful performance with a human point and gaze was not spontaneous, results reflect the relatively quick speed at which capuchins were capable of learning to use a human cue to their advantage. However, it must be mentioned that three of the five monkeys in our human pointing group did not initially learn to use the cue.
The question arises as to how the monkeys regarded the cue-giver and the cues provided. The training phases were designed such that, if the subject monkey could understand what the cueing monkey or experimenter could see, it should be able to begin making correct selections on the first test trials. Hare et al. (2003) have shown that capuchin monkeys most probably do not understand what other conspecifics can see, however. At another level, the training would allow the subject monkey to use local enhancement cues from the experimenter or cueing monkeys as discriminative stimuli to make correct selections spontaneously when testing began. The training did not appear to have an influence as most monkeys started test trials with a sustained side preference (six of ten monkeys). The four that did not maintain side preferences did not begin testing by spontaneously making correct selections but learned to read the cues as testing progressed. All had gradually increasing learning curves (Fig. 3a, b, f, g), implying that the monkeys did not learn from the training that “the cup with the food is indicated by the act of another” but learned once testing began that some aspect of the cueing monkey or experimenter was acting as a discriminative stimulus.
We cannot suggest what the monkeys were attending to during acquisition. The monkeys may have passively learned a stimulus–response association and picked the same side as the cue. The monkeys may have thought the monkey (or experimenter) was attempting to obtain a piece of food. Monkeys may have been mentally representing a piece of food in the cup, associating the cueing stimulus with an unseen object. Or they may have understood what the cue-giver saw, although that was unlikely (Hare et al. 2003). One way to test for a simple stimulus–response association in future experiments would be to initially test some monkeys with an object or some other marker that indicates the cup containing food. We did not have enough subjects to initially test some monkeys with a stimulus other than a monkey or a human, but Mason and Hollis (1962) tested this in their local enhancement study of rhesus macaques. Monkeys that were already capable of reading a conspecific local enhancement cue were tested with a strange new monkey proficient at providing local enhancement cues and a monkey puppet or a plaque positioned at the correct selection containing food. Monkeys showed spontaneous transfer to the stranger monkey could not use the plaque as a discriminative stimulus and, although these experienced monkeys started off at chance levels with the puppet, they gradually learned to use the puppet as a cue. Performance with the puppet was below that of the stranger monkey, however. Marsh (2012) showed that orangutans could use paper markers as cues to select a baited cup in a choice task, but they learned significantly faster using experimenter-given cues (pointing and local enhancement) than the markers. Tomasello et al. (1997) found that apes would not use markers as cues unless they were previously trained to do so. We assume that the monkeys used the conspecific and experimenter-given cues as local enhancement. Cueing monkeys provided a local enhancement cue when they moved to the baited side and reached. The experimenter did not move to the baited side, but her arm, hand, and finger may have provided a local enhancement cue (Fig. 1b).
Positive transfer occurred across conspecifics and across species in that monkeys performed above chance immediately after switching partners. The transfer could have been a simple case of stimulus generalization. Monkeys transferred more smoothly from a conspecific to a novel conspecific than between species, possibly because cues provided by conspecifics (reaching) were more similar than the transition between conspecifics and experimenter, whose cues were rather different (Fig. 1b vs. c). For example, no significant decrement in performance occurred when monkeys transferred between conspecifics, but, despite positive transfer, there was a significant group-wise decrement in performance when transferring between species (conspecific to experimenter and experimenter to conspecific), indicating that the monkeys were not conceptualizing the two types of cues as the same. The number of cases of spontaneous transfer between cue-givers, in which monkeys remained above the 85% correct criterion in their first two 20-trial blocks after a transfer, reflect the results of the statistical tests: Transfer from a conspecific to a new conspecific appeared to occur more smoothly than transfer between species. Four of five monkeys spontaneously transferred from one conspecific to another conspecific. Such spontaneous transfer from conspecific to conspecific has been observed in other studies (Mason and Hollis 1962; Itakura et al. 1999). Transitions were not quite as smooth between a human and a monkey. Only two of five monkeys spontaneously transferred from a human to a monkey, and one of the five did not transfer at all. Only one of three monkeys spontaneously transferred from a monkey to a human. Stimulus generalization of the cues provided might explain these differences.
Even if simple stimulus generalization were occurring, we suggest it was quite a feat for a monkey to take a human pointing gesture and spontaneously understand that a monkey reaching toward a cup was an equivalent signal, yet two monkeys (Fig. 3c and 3f) spontaneously transferred from a human point to a conspecific. Again, the transition between the two types of cues was very different (Fig. 1b versus 1c), and the monkeys would need to immediately understand that the reaching of a monkey was equivalent to a human hand pointing.
One explanation for positive transfer from conspecific partner to conspecific partner and across species may have been because monkeys were simply gaining more experience with the apparatus and the testing situation. That may be the case for some monkeys, but the general decline in performance when cue-givers switched species suggests that simple experience with the apparatus cannot account for sustained performance. For example, Nye had a precipitous decline in performance when he was transferred from an experimenter’s point to conspecific cues (Fig. 3g).
Three monkeys that were unable to use a human point or conspecific cues (Fig. 3c, d, i) became successful when a vocalization was added to the experimenter’s pointing cue. All three of these monkeys had acute side biases that were interfering with learning, but providing a vocalization allowed them break their pattern of behavior. Call et al. (2000) and Itakura et al. (1999) improved the performance of chimpanzees on an object-choice task by adding vocalizations and other noises to human gaze cues. In the capuchins, perhaps the human vocalization called the monkeys’ attention away from their automatic side preference selection so that they could attend to other aspects of the environment, including the cue. Once our three monkeys learned to use the human pointing cue when it was accompanied by a vocalization, all three spontaneously transferred to an experimenter pointing without a vocalization. Interestingly, two of these three monkeys went on to learn the cues of the conspecific with which they were previously unsuccessful. For one monkey, Schroeder, this transition was spontaneous (Fig. 3c).
Perhaps coincidentally, the two monkeys that did not learn to use either type of cue-giver were the alpha male and alpha female in the group (Fig. 3e, j). They persisted with left side preferences throughout their testing. Four other monkeys exhibited acute side preferences from the beginning, but they were able to overcome them and learn to use the cues provided. Gazes et al. (2013), in a preliminary analysis of rhesus macaques, found no difference in performance on a battery of cognitive tasks across dominance ranks, although none of the tasks required social learning. In social learning tasks involving a conspecific demonstrator, low-ranking animals appear to outperform high-ranking animals in horses (Equus caballus, Krueger et al. 2013), black-capped chickadees (Poecile atricapillus, An et al. 2011), and dogs (Pongrácz et al. 2008). However, dominant dogs learned better than subordinate dogs from a human demonstrator (Pongrácz et al. 2012). Pongrácz et al. (2008) suggested that dominant dogs do not need to learn by observing subordinate dogs because they can take whatever they want from a subordinate. On the other hand, a subordinate dog must pay attention to another dog’s actions, which might improve performance on observational tasks. Perhaps our high-ranking capuchin monkeys learned not to attend to the behavior of others during feeding situations because they always obtained the food they wanted and did not need to develop feeding strategies by observing others. Results are suggestive that social rank may be related to observational learning, but few studies have examined the association and future research would be needed to draw conclusions.
A final question is whether our results qualify as “an explanation of a third kind” as proposed by Call and Tomasello (Tomasello and Call 1997; Call 2001; Call and Tomasello 2005). Were results simply associative learning of a discriminative stimulus during acquisition followed by stimulus generalization during transfer? Results possibly support this extreme of learned behavioral contingencies. We would certainly not advocate for the opposite mental extreme that the monkeys understood that the experimenter was trying to provide them with information or that they thought the cueing monkey was showing them the location of the reward. But, did monkeys’ flexible use of the cues provide evidence for an intermediate explanation indicating more advanced cognition than behavioral contingencies? Use of cues was certainly flexible. Monkeys transferred from conspecific cue-givers to novel conspecific cue-givers and across species of cue-giver, often spontaneously. The cases of spontaneous transfer across species, when the cues were very different from one another, are perhaps the best evidence for application of prior knowledge to solve a novel physical problem. The cases where individuals showed sudden solutions to the task, sometimes after 100 unsuccessful trials, may also indicate that more may have been occurring than association from repeated stimulus–response pairings. However, more evidence is needed, particularly direct comparisons with nonsocial cues (e.g., a light or marker) to determine whether social cues indeed transfer more effectively than nonsocial cues.
Although Morimoto and Fujita (2012) showed that brown capuchin monkeys could use conspecific emotional expressions to obtain hidden food, ours was the first study to show that brown capuchins could also use body cues from conspecifics to solve a hidden object-choice task. The study was also the first to show that capuchins could transfer this skill from monkeys to humans and from humans to monkeys. Results demonstrate capuchins’ ability to read various cues from conspecifics and perhaps even other species of animals. In the wild, capuchin monkeys would benefit from the ability to read the body language of conspecifics in order to find food, or in other situations, such as learning the location of possible predators. However, the fairly long time it took for our monkeys to succeed at the task, over 100 trials, might imply that under more naturalistic social conditions, some monkeys may not be able to learn to read indicator cues such as reaching, but long-lived animals in long-term social groups would have hundreds and hundreds of interactions with which to learn. The results here add to the growing literature on nonhuman animals’ abilities to read the human pointing cue, and more specifically, how the ability to recognize (and use) cues from conspecifics and humans may be linked.
References
An YS, Buddhamas K, Newman AE, MacDougall-Shackleton EA, MacDougall-Shackleton SA (2011) Social rank, neophobia and observational learning in black-capped chickadees. Behaviour 148:55–69. doi:10.1163/000579510X545829
Anderson JR, Sallaberry P, Barbier H (1995) Use of experimenter-given cues during object-choice tasks by capuchin monkeys. Anim Behav 49:201–208. doi:10.1016/0003-3472(95)80168-5
Call J (2001) Chimpanzee social cognition. Trends Cogn Sci 5:388–393. doi:10.1016/S1364-6613(00)01728-9
Call J, Tomasello M (2005) What chimpanzees know about seeing, revisited: an explanation of the third kind. In: Eilan N, Hoerl C, McCormack T, Roessler J (eds) Joint attention: communication and other minds: issues in philosophy and psychology. Oxford University Press, Oxford, pp 46–64
Call J, Agnetta B, Tomasello M (2000) Cues that chimpanzees do and do not use to find hidden objects. Anim Cogn 3:23–34
Clary D, Kelly DM (2013) Are Clark’s nutcrackers (Nucifraga columbiana) able to discriminate knowledge states of human experimenters during an object-choice task? Evol Psychol 11:628–646
Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington, DC
Dindo M, Thierry B, Whiten A (2008) Social diffusion of novel foraging methods in brown capuchin monkeys (Cebus apella). Proc Biol Sci 275:187–193. doi:10.1098/rspb.2007.1318
Galef BG, Wigmore SW (1983) Transfer of information concerning distant foods: a laboratory investigation of the “information-centre” hypothesis. Anim Behav 31:748–758. doi:10.1016/S0003-3472(83)80232-2
Gazes RP, Brown EK, Basile BM, Hampton RR (2013) Automated cognitive testing of monkeys in social groups yields results comparable to individual laboratory based testing. Anim Cogn 16:445–458. doi:10.1007/s10071-012-0585-8
Hare B, Tomasello M (1999) Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. J Comp Psychol 113:173–177
Hare B, Addessi E, Call J et al (2003) Do capuchin monkeys, Cebus apella, know what conspecifics do and do not see? Anim Behav 65:131–142. doi:10.1006/anbe.2002.2017
Highfill LE, Schwammer H, Kuczaj SA (2007) A brief report: the use of experimenter-given cues by South American sea lions. Int J Comp Psychol 20:368–373
Itakura S, Anderson JR (1996) Learning to use experimenter-given cues during an object-choice task by a capuchin monkey. Cah Psychol Cogn/Curr Psychol Cogn 15:103–112
Itakura S, Agnetta B, Hare B, Tomasello M (1999) Chimpanzee use of human and conspecific social cues to locate hidden food. Dev Sci 2:448–456
Judge PG, Bruno S (2012) Transport of functionally appropriate tools by capuchin monkeys (Cebus apella). Am J Primatol 74:199–209. doi:10.1002/ajp.21987
Judge PG, Essler JL (2013) Capuchin monkeys exercise self-control by choosing token exchange over an immediate reward. Int J Comp Psychol 26:256–266
Krueger K, Farmer K, Heinze J (2013) The effects of age, rank and neophobia on social learning in horses. Anim Cogn 17:645–655. doi:10.1007/s10071-013-0696-x
Marsh HL (2012) Orangutans’ use of contiguous versus distal social and non-social cues in an object-choice task. Int J Comp Psychol 25:299–308. doi:10.5811/westjem.2011.5.6700
Mason WA, Hollis JH (1962) Communication between young rhesus monkeys. Anim Behav 10:211–221. doi:10.1016/0003-3472(62)90040-4
Miklósi A, Soproni K (2006) A comparative analysis of animals’ understanding of the human pointing gesture. Anim Cogn 9:81–93. doi:10.1007/s10071-005-0008-1
Mineka S, Cook M (1988) Social learning and the acquisition of snake fear in monkeys. In: Zentall TR, Galef BG (eds) Social learning: psychological and biological perspectives. Lawrence Erlbaum Associates Inc., Publishers, Mahwah, NJ, pp 51–74
Morimoto Y, Fujita K (2012) Capuchin monkeys (Cebus apella) use conspecifics’ emotional expressions to evaluate emotional valence of objects. Anim Cogn 15:341–347. doi:10.1007/s10071-011-0458-6
Nawroth C, Ebersbach M, von Borell E (2013) Juvenile domestic pigs (Sus scrofa domestica) use human-given cues in an object choice task. Anim Cogn 17:1–13. doi:10.1007/s10071-013-0702-3
Paukner A, Suomi S, Visalberghi E, Ferrari PF (2009) Capuchin monkeys display affiliation toward humans who imitate them. Science 325:880–883. doi:10.1126/science.1176269
Pongrácz P, Vida V, Bánhegyi P, Miklósi Á (2008) How does dominance rank status affect individual and social learning performance in the dog (Canis familiaris)? Anim Cogn 11:75–82. doi:10.1007/s10071-007-0090-7
Pongrácz P, Bánhegyi P, Miklósi Á (2012) When rank counts—dominant dogs learn better from a human demonstrator in a two-action test. Behaviour 149:111–132. doi:10.1163/156853912X629148
Povinelli DJ, Bierschwale DT, Čech CG, Cech CG (1999) Comprehension of seeing as a referential act in young children, but not juvenile chimpanzees. Br J Dev Psychol 17:37–60. doi:10.1348/026151099165140
Smet AF, Byrne RW (2013) African elephants can use human pointing cues to find hidden food. Curr Biol 23:2033–2037. doi:10.1016/j.cub.2013.08.037
Tomasello M, Call J (1997) Primate cognition. Oxford University Press, New York
Tomasello M, Call J, Gluckman A (1997) Comprehension of novel communicative signs by apes and human children. Child Dev 68:1067–1080
Tornick JK, Gibson BM, Kispert D, Wilkinson M (2011) Clark’s nutcrackers (Nucifraga columbiana) use gestures to identify the location of hidden food. Anim Cogn 14:117–125. doi:10.1007/s10071-010-0349-2
Vick SJ, Anderson JR (2000) Learning and limits of use of eye gaze by capuchin monkeys (Cebus apella) in an object-choice task. J Comp Psychol 114:200–207. doi:10.1037/0735-7036.114.2.200
Acknowledgements
The primate facility was supported by Bucknell University. Mary Gavitt and Gretchen Long provided animal care and technical support. Kate Albertini, Chelsea Burleson, Stephanie Casino, Morgan Ramos, Meg Rash, Evan Sloan, Mackenzie Smith, Amari Suskin-Sperry and Eden Wondra assisted with data collection. Jeremy Cain provided graphical and art support on the manuscript. Jennifer Essler was supported by a Bucknell University Graduate Summer Research Fellowship. Lindsay Schwartz was supported by a Bucknell University Undergraduate Summer Research Fellowship. Mattea Rossettie was supported by a Bucknell Geisinger Research Initiative grant: “Social–Cognitive Ability and the Autism Spectrum: Functional Imaging, EEG and Genetic Variation” and by the Bucknell Program for Undergraduate Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical standard
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Essler, J.L., Schwartz, L.P., Rossettie, M.S. et al. Capuchin monkeys’ use of human and conspecific cues to solve a hidden object-choice task. Anim Cogn 20, 985–998 (2017). https://doi.org/10.1007/s10071-017-1118-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-017-1118-2