Abstract
Humans often strategically manipulate the informational access of others to their own advantage. Although chimpanzees know what others can and cannot see, it is unclear whether they can strategically manipulate others’ visual access. In this study, chimpanzees were given the opportunity to save food for themselves by concealing it from a human competitor and also to get more food for themselves by revealing it to a human cooperator. When knowing that a competitor was approaching, chimpanzees kept more food hidden (left it covered) than when expecting a cooperator to approach. When the experimenter was already at the location of the hidden food, they actively revealed less food to the competitor than to the cooperator. They did not actively hide food (cover up food in the open) from the competitor, however. Chimpanzees thus strategically manipulated what another could see in order to maximize their payoffs and showed their ability to plan for future situations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humans can withhold information from competitors or highlight information for cooperators—something that one can already see in children as young as 2.5 years (who probably have played competitive and cooperative games with peers and adults; Flavell et al. 1978). By 4 years of age, children flexibly adjust their strategies for competitors and cooperators and are even capable of creating false information like creating false trails to mislead competitors (Sodian et al. 1991). Both withholding information and misleading competitors are behaviors considered as tactical deception (see Whiten and Byrne 1988).
Although non-human primates (henceforth: primates) can infer what others can and cannot see and what they have seen in the past (for reviews, see Call and Tomasello 2008; Tomasello et al. 2003; Whiten 2013), little is known about whether they can use this information to strategically manipulate others. Most evidence stems from research on deceptive behaviors. Experimental results suggest that primates conceal information from competitors by hiding themselves (de Waal 1998; Gygax 2000; Kummer et al. 1996) or by refraining from doing certain actions while being observed. For instance, subdominant chimpanzees (Hirata and Matsuzawa 2001; Menzel 1974) and mangabeys (Coussi-Korbel 1994) who know the location of hidden food avoid accessing it when the dominant can see them, and rhesus macaques and ringtailed lemurs avoid approaching food near a human competitor who was looking at the food over one who could not see it (Flombaum and Santos 2005; Sandel et al. 2011). Other studies show that chimpanzees prefer the hidden route to food over the visible route when interacting with a human competitor, and a quiet route over a noisy one (Hare et al. 2006; Melis et al. 2006).
Yet, some studies also investigated apes’ manipulative strategies in cooperative contexts. For instance, great apes are able to draw the cooperator’s attention to hidden food (Call and Tomasello 1994; Leavens et al. 2004; Menzel 1999; Roberts et al. 2014) or tools (Call and Tomasello 1994; Gómez 1998; Zimmermann et al. 2009) that they cannot reach themselves.
However, none of these studies systematically investigated whether chimpanzees use their manipulative strategies flexibly, i.e., adopt them to intentions of their counterpart (competitive/cooperative) in the same setting. This is surprising, given that flexibility is one of the cornerstones of intentional deception (Whiten and Byrne 1988). There is a set of studies that originally aimed at investigating intentional communication, in which primates could inform, not inform, or mislead an either cooperative or competitive experimenter about the location of hidden food, which they could not reach themselves (Anderson et al. 2001; Mitchell and Anderson 1997; Woodruff and Premack 1979). In the original study by Woodruff and Premack (1979), four chimpanzees were trained to point to the baited of two containers to receive the covered food; after they reliably pointed to the baited container, they then faced either a competitive or a cooperative trainer. If they pointed correctly in the presence of the collaborator, they received the food. In contrast, in the presence of the competitor they would not receive the food if they pointed to the baited container, but to the unbaited one. While chimpanzees were able to guide the cooperator’s attention to the baited container from the beginning, they did not behave differently with the competitive trainer within the first 24 trials, and it took between 60 and 120 trials until they discriminated between the trainers. Over the course of the study, the apes increasingly withheld behavioral cues about the food’s location from the competitor, and two subjects progressed to actively (deceptively?) pointing to the unbaited container, but only after more than 200 training trials.
Similar studies with capuchin monkeys (Mitchell and Anderson 1997) and squirrel monkeys (Anderson et al. 2001) required even longer training. Anderson et al. (2001) explicitly framed their task as a training task, but even after 400 trials, only one of the three squirrel monkeys reliably indicated the baited container to the cooperator, and only one withheld information from the competitor (while not succeeding in the cooperative situation). Two of the three studied capuchin monkeys showed improvement in performance over time, but there was no reliable difference between the conditions before 450/650 trials (Mitchell and Anderson 1997).
There are two major problems when interpreting these studies in terms of deceptive strategies. First, all subjects experienced a helpful trainer in a pretest for a considerable number of trials (24 for chimpanzees, 80–110 for capuchin monkeys, and 500–600 for the squirrel monkeys), which might have made it harder for them to switch to a competitive mode. Second, the test itself was the first situation in which subjects could learn about the differing roles of the trainers, and with a vast number of trials, there was a substantial amount of learning opportunities for simple rules such as “If the cooperator approaches, point to the baited cup; if the competitor approaches, point to the unbaited cup.” This renders it impossible to exclude conditional discrimination learning as an explanation (Anderson et al. 2001; Heyes 1993, 1998). Thus, while these results indicate that primates can learn to withhold information from competitors, they do not provide evidence for deceptive, flexible strategies.
The first question in our study therefore was whether chimpanzees use their manipulative strategies flexibly, i.e., do they adapt them in the same setting depending on the intentions of their counterpart and do they so without previous learning experience. Second, we asked whether chimpanzees are able to actively hide objects from a competitor. Despite the positive findings on primates keeping objects or acts hidden from competitors, there is surprisingly little known about their ability to actively hide objects from others—a skill that is present in human children already by the age of 2.5 years (Flavell et al. 1978). A broad range of other species actively hide objects such as their nests, eggs, food, or offspring (Caro 2005; Vander Wall 1990), but for primates, there are only some anecdotes (Whiten and Byrne 1988) that indicate that they hide body parts from dominants, e.g., their erected penis or their fear grimace (de Waal 1986, 1998). Clearly, the hiding behaviors of many species are low-level, hard-wired responses largely devoid of cognitive content (Mitchell and Thompson 1986), but for primates, there is a lack of experimental evidence for active hiding of objects, even in its most basic form.
We thus tested chimpanzees’ hiding skills and their flexibility in a feeding context with a clear separation of the learning and the test phase. In an initial training, chimpanzees could passively learn about the role of the experimenter (cooperative/competitive). In the test, chimpanzees could then actively hide food (which they could access later) from the competitor who would otherwise steal it from them. They could also passively keep covered food hidden. In the other condition, they could actively reveal hidden food to the collaborator who would give it to them or passively keep visible food uncovered.
We measured whether chimpanzees hid or revealed food depending on the type of experimenter they faced (cooperative/competitive) both before and after the experimenter’s arrival (anticipation/reaction phase). If chimpanzees hid food from a competitor and revealed it to a collaborator, this would demonstrate flexible manipulation of others’ perceptual states. Moreover, if they acted prior to the experimenter’s arrival by hiding food only when anticipating a competitor, but not when anticipating a collaborator, this would be evidence of strategic future planning—a skill that is present in food-caching birds (Cheke and Clayton 2012; Correia et al. 2007; Raby et al. 2007), but despite some positive findings both from the wild (Janmaat et al. 2014; van Schaik et al. 2013) and from experimental studies (Dufour and Sterck 2008; Mulcahy and Call 2006; Osvath and Osvath 2008) still under debate in great apes (Roberts 2002; Shettleworth 2010; Suddendorf et al. 2009). To our knowledge, this is the first study investigating whether chimpanzees can actively hide objects from others (and not just passively keep them hidden).
Method
Subjects
Twenty-four chimpanzees (Pan troglodytes; 50 % females) participated in this study, all living at Ngamba Island Sanctuary (www.ngambaisland.com), Uganda (mean age 17.4 years, range 12–28). All apes came to the sanctuary as orphans as a result of the illegal bushmeat trade and were then raised by humans together with peers and later often adopted by conspecific foster mothers. They all lived in social groups at the time of testing and could move freely in a 100-acre rainforest during the day. All of them had experience with experimental testing due to previous research at the sanctuary, but had never participated in studies on hiding. Subjects were fed according to their regular diet and never food or water deprived.
Materials
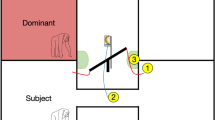
The apparatus consisted of a green box (69 × 31 cm) with four food trays outside the chimpanzees’ enclosure (Fig. 1). Subjects could access one side of the box and push each tray to the left or right with their fingers. They could not access the rewards (banana slices) on the trays. Half-opaque, half-transparent Plexiglas covers topped each tray. Two of the covers were opaque on the right and two on the left. By moving a tray, the subject could hide the food underneath the opaque cover side or make it visible underneath the transparent side. The four covers could be interchanged for counterbalancing. From the two possibilities to hide the food—moving an occluder in front of it or moving it behind the occluder, we chose the latter as this is the easier action for human infants (Flavell et al. 1978; McGuigan and Doherty 2002).
Experimental setup (online version in color). The big arrow indicates how the transparent Plexiglas panel can be opened to give the subject access to the apparatus through the wholes in the bars above the apparatus. Each of the four trays with banana could be moved by the subject to underneath the opaque cover side and back
The experimenter could slide open the covers from her side and access the rewards. Above the apparatus was a Plexiglas panel with four holes, each corresponding to one tray. They were usually shut by a transparent trap door on the experimenter’s side, but the experimenter could open it to give the subject access to the rewards.
Design
We used a between-subject design with 12 chimpanzees per condition, counterbalanced for age and sex. Each subject first had to pass an apparatus familiarization before she could move on to training (three sessions) and test (three sessions). We administered four trials per daily session, summing up to 12 training and 12 test trials.
Procedure
Apparatus familiarization
This ensured that subjects could manipulate the position of the trays. First, the apparatus (without covers) was out of reach of the subject and baited with banana. The experimenter demonstrated how to move the trays. She then installed the apparatus within reach of the subject. To pass the criterion, subjects had to move each tray at least twice to each side. The experimenter randomly fed the subject peanuts to keep up their attention. The session ended when the subject reached criterion or after 25 min. On average, a familiarization session took 20 min (95 % CI [17.6; 22.4]). Three subjects needed a second session to pass the criterion (Namukisa, Nani, Pasa).
Training
This served to acquaint chimpanzees with the role of the experimenter (competitive/cooperative) and the course of events. The subject could see the apparatus, but could not reach it. First, a keeper baited all trays and arranged the apparatus according to a predefined, counterbalanced scheme (0/50/100 % of rewards visible). The keeper then left, the experimenter kneeled down one meter from the apparatus with her back turned to the subject, and remained there for 45 s (anticipation phase). Next, she approached the apparatus. Her behavior now differed between conditions:
-
(a)
Competitive She removed all rewards she could see and put them in a bucket. She kept observing the apparatus for 45 more seconds (reaction phase) and then opened the trap door above the apparatus and left with the bucket. The keeper came back, opened all covers, let the subject get the remaining banana through the open trap door, and closed it.
-
(b)
Cooperative The experimenter fed all rewards to the subject she could see, kept observing the apparatus for 45 more seconds (reaction phase), and then left. The keeper came back in with a bucket, opened all covers, and put the remaining food in the bucket.
Then a new trial started in both conditions: The keeper re-baited the apparatus, rearranged the covers and left (with the bucket in the competitive condition).
Test
The procedure was the same as in the training, but now the apparatus was within reach of the subject and she could manipulate whether the food was visible or hidden. When the trap door was opened in the competitive condition, subjects could now reach into the apparatus and get the remaining hidden banana themselves. Note that both conditions thus had a competitive and a cooperative component (as the keeper always behaved the opposite way of the experimenter, e.g., the keeper was cooperative when the experimenter was competitive); this was important for the purpose that chimpanzees received the same amount of rewards in both conditions and behavior differences could not arise due to differential rewarding.
Data scoring and analysis
We counted the number of rewards hidden after the anticipation phase (t1) and the reaction phase (t2) (range 0–4). As the starting number of hidden rewards differed between trials, we calculated hiding scores for t1 and t2 by subtracting the number of rewards hidden (H) at the beginning of the phase from these numbers, i.e., score at t1 = H(t1)−H(t0) and score at t2 = H(t2)−H(t1). The scores at t1 thus ranged from −4 (all pieces hidden at start, but then revealed) to +4 (all pieces visible at start, but hidden thereafter). As all visible rewards were gone at t1 (either fed or removed), tray movements during the reaction phase could only lead to revealing. Scores at t2 hence ranged from −4 to 0. Scores for t2 were only calculated if there were rewards left at t1. We also looked at only the rewards hidden at the beginning and calculated the proportion of rewards that were revealed by the subject. We did the same for rewards visible at the beginning that were later hidden by the subjects.
A second independent observer coded a random sample of 20 % of the data. The inter-rater agreement was excellent (Cohen’s kappa = .98, p < .001).
We additionally coded the time not spent at the apparatus in the reaction phase as an indicator for inhibitory behaviors. We used independent-sample t tests when comparing between conditions, one-sample t tests to compare to chance, and a repeated-measures ANOVA (with session number as within-subject factor and condition as between-subject factor) to compare between sessions.
Results
Overall, chimpanzees were more successful in the cooperative than in the competitive condition, indicated by the percentage of bananas received, t(22) = 13.06, p < .001 (cooperative: 91.7 %; competitive: 29.2 %) (Fig. 2). The hiding scores after the anticipation and the planning phase are depicted in Fig. 3.
Average hiding score in the anticipation and the reaction phases. Positive and negative values indicate evidence of hiding visible and revealing hidden banana pieces, respectively. The score ranges are [−4; +4] for the anticipation phase and [−4; 0] for the reaction phase. Error bars refer to 95 % confidence intervals
Anticipation phase
Chimpanzees hid significantly more food in the competitive than in the cooperative condition, t(22) = 2.14, p = .044. In the competitive condition, 58 % of subjects had a positive hiding score, while in the cooperative condition, only 25 % did so. This trend was evident already in the first trial (competitive: M = 50.0 %; cooperative: M = 41.6 %; χ 2(1, 22) = .20; p = .65). However, in none of the conditions, the hiding scores differed significantly from chance (cooperative: t(11) = 1.56, p = .15; competitive: t(11) = 1.49, p = .17), i.e., they did not systematically hide or reveal food. When considering only pre-hidden rewards, chimpanzees kept significantly more of them covered in the competitive condition (73.4 %) compared to the cooperative condition (56.2 %), t(22) = 3.16, p = .005. In contrast, there was no difference between conditions in actively hiding rewards that were visible at the beginning, t(22) = .51, p = .62.
Reaction phase
As all visible rewards were removed right before the beginning of this phase (either removed or fed to the subject), all rewards were hidden at start and chimpanzees could only reveal food or refrain from doing so. In both conditions, there were significantly fewer rewards hidden than at start (cooperative: t(11) = 13.07, p < .001; competitive: t(11) = 5.51, p < .001), implying that chimpanzees successfully revealed food to the collaborator, but also lost food to the competitor. However, chimpanzees revealed significantly fewer rewards in the competitive condition (48.2 %) than in the cooperative condition (84.1 %), t(22) = 2.78, p = .011. They achieved this by engaging in various behaviors, particularly by spending more time away from the apparatus in the competitive condition (average sum over 12 trials: M = 2.1 min, SE = .5) compared to the cooperative condition (M = .6 min, SE = .2), t(15) = 2.76, p = .014.
We investigated learning effects by comparing scores between the three sessions. We found no learning effects in the anticipation phase, F(2, 44) = 2.2, p = .12, η 2 = .09 (Online Resource, Fig. S1). In contrast, there was a significant session–condition interaction for the reaction phase, F(1, 22) = 6.69, p = .017, η 2 = .23. Chimpanzees revealed less over time in the competitive condition, F(2, 22) = 5.92, p = .009, η 2 = .35, while their hiding behavior stayed the same in the cooperative condition, F(2, 22) = .45, p = .65, η 2 = .04 (Online Resource, Fig. S2). In pairwise post hoc comparisons with Bonferroni correction, we found that this was due to a significant increase in hiding in the last compared to the first session, p = .031 (the other pairwise comparisons were nonsignificant).
Discussion
Chimpanzees were highly successful in revealing food to a collaborator who gave it to them; they were less successful in hiding food from a competitor who removed the food. However, they flexibly adjusted their behavior to the different experimenters in two important ways.
First, they kept significantly more food hidden when anticipating the approach of the competitor as compared to the collaborator. This implies that chimpanzees anticipated the experimenter’s intentions based on the training and adjusted their strategy accordingly. However, while chimpanzees successfully revealed fewer hidden rewards in the competitive condition, they did not actively hide those rewards that were visible at start. Second, after the experimenter arrived at the food, the chimpanzees revealed significantly less food to the competitor than to the collaborator.
These results confirm previous findings that chimpanzees refrain from revealing hidden food in the presence of a competitor (Hirata and Matsuzawa 2001; Menzel 1974; Woodruff and Premack 1979) and are consistent with other findings showing that chimpanzees indicate their presence for a collaborator (Roberts et al. 2014; Woodruff and Premack 1979). While it was unclear in previous studies whether chimpanzees do so by trial-and-error-learning, subjects in our study kept more food hidden from the competitor already from the first trial on (although the difference was not significant for the first trial), after having observed the experimenter’s behavior in the training, but devoid of any learning experiences concerning the effect of their own hiding/revealing actions. As in previous studies (Call et al. 2004; Call and Tomasello 1998; Premack and Woodruff 1978), they thus proved an understanding of others’ intentions or goals. One could still argue that they have formed an association such as “food visible—good” in the cooperative condition and “food visible—bad” in the competitive condition. However, forming such a rule was not straightforward as the training was non-differentially rewarded—in both the competitive and the cooperative training, the subject received 50 % of the rewards (either the experimenter fed them the 50 % visible rewards in the cooperative training or they received the 50 % hidden rewards a couple of seconds later from the keeper). We thus do not think that simple association can account for the chimpanzees’ behavior—rather, they combined their knowledge about the experimenter’s vision and her goals inferred from the training and acted accordingly in the test.
Interestingly, although chimpanzees kept more covered food hidden from the competitor, they did not actively hide visible food from her, although it was clear from the apparatus training and from their active revealing in the test that they knew how to move the food. The reduced rate of revealing in the competitive condition indicates that subjects understood something about the negative consequences of the competitor seeing the food. Still, they lost a significant amount of food to the competitor by revealing it to her. One reason for this could be difficulties with inhibiting actions in the presence of food (see Boysen 1996; Boysen et al. 2001). Engaging in distracting behaviors such as leaving the apparatus might have helped them to overcome these difficulties (see Evans and Beran 2007, for a study on self-distraction to overcome impulsivity in a food accumulation task). Also, chimpanzees did not actively prevent her from seeing the food by covering it. Although great apes are proficient in object permanence (Call 2001), this could be due to a reluctance to make food “disappear” that they can already see. It might also not lay in their natural behavioral repertoire to actively hide objects, and our small number of trials might not have been enough for them to learn it. More learning opportunities, as usually experienced by human children, might significantly increase apes’ performance. However, it is also possible that active misleading is a cognitive challenge for them.
Our results support recent findings that great apes and corvids do not just react to current states, but also plan for the future situations (Mulcahy and Call 2006; Osvath and Osvath 2008; Raby et al. 2007). Chimpanzees can not only save tools (Mulcahy and Call 2006; Osvath and Osvath 2008) or stones (Osvath 2009) for future use, but also hidden food for a safer situation later. They successfully inferred others’ intentions and flexibly used this knowledge to maximize their payoffs.
References
Anderson JR, Kuroshima H, Kuwahata H, Fujita K, Vick S-J (2001) Training squirrel monkeys (Saimiri sciureus) to deceive: acquisition and analysis of behavior toward cooperative and competitive trainers. J Comp Psychol 115:282–293. doi:10.1037/0735-7036.115.3.282
Boysen ST (1996) “More is less”: the elicitation of rule-governed resource distribution in chimpanzees. In: Russon AE, Bard KA, Parker ST (eds) Reaching into thought: the minds of the great apes. Cambridge University Press, Cambridge, pp 177–189
Boysen ST, Berntson GG, Mukobi KL (2001) Size matters: impact of item size and quantity on array choice by chimpanzees (Pan troglodytes). J Comp Psychol 115:106–110. doi:10.1037/0735-7036.115.1.106
Call J (2001) Object permanence in orangutans (Pongo pygmaeus), chimpanzees (Pan troglodytes), and children (Homo sapiens). J Comp Psychol 115:159–171. doi:10.1037/0735-7036.115.2.159
Call J, Tomasello M (1994) Production and comprehension of referential pointing by orangutans (Pongo pygmaeus). J Comp Psychol 108:307–317. doi:10.1037//0735-7036.108.4.307
Call J, Tomasello M (1998) Distinguishing intentional from accidental actions in orangutans (Pongo pygmaeus), chimpanzees (Pan troglodytes) and human children (Homo sapiens). J Comp Psychol 112:192–206. doi:10.1037/0735-7036.112.2.192
Call J, Tomasello M (2008) Does the chimpanzee have a theory of mind? 30 years later. Trends Cogn Sci 12:187–192. doi:10.1016/j.tics.2008.02.010
Call J, Hare B, Carpenter M, Tomasello M (2004) ‘Unwilling’ versus ‘unable’: chimpanzees’ understanding of human intentional action. Dev Sci 7:488–498. doi:10.1111/j.1467-7687.2004.00368.x
Caro T (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Cheke LG, Clayton NS (2012) Eurasian jays (Garrulus glandarius) overcome their current desires to anticipate two distinct future needs and plan for them appropriately. Biol Lett 8:171–175. doi:10.1098/rsbl.2011.0909
Correia SPC, Dickinson A, Clayton NS (2007) Western scrub-jays anticipate future needs independently of their current motivational state. Curr Biol 17:856–861. doi:10.1016/j.cub.2007.03.063
Coussi-Korbel S (1994) Learning to outwit a competitor in mangabeys (Cercocebus torquatus torquatus). J Comp Psychol 108:164–171. doi:10.1037/0735-7036.108.2.164
de Waal FBM (1986) Deception in the natural communication of chimpanzees. In: Mitchell RW, Thompson NS (eds) Deception: perspectives on human and nonhuman deceit. State University of New York Press, Albany, pp 221–244
de Waal FBM (1998) Chimpanzee politics: power and sex among apes. Johns Hopkins University Press, Baltimore
Dufour V, Sterck EHM (2008) Chimpanzees fail to plan in an exchange task but succeed in a tool-using procedure. Behav Process 79:19–27. doi:10.1016/j.beproc.2008.04.002
Evans TA, Beran MJ (2007) Chimpanzees use self-distraction to cope with impulsivity. Biol Lett 3(6):599–602. doi:10.1098/rsbl.2007.0399
Flavell JH, Shipstead SG, Croft K (1978) Young children’s knowledge about visual perception: hiding objects from others. Child Dev 49:1208–1211. doi:10.2307/1128761
Flombaum JI, Santos LR (2005) Rhesus monkeys attribute perceptions to others. Curr Biol 15:447–452. doi:10.1016/j.cub.2004.12.076
Gómez JC (1998) Assessing theory of mind with nonverbal procedures: problems with training methods and an alternative “key” procedure. Behav Brain Sci 21:119–120. doi:10.1017/S0140525X98280708
Gygax L (2000) Hiding behaviour of long-tailed macaques (Macaca fascicularis): II. Use of hiding places during aggressive interactions. Ethology 106:441–451. doi:10.1046/j.1439-0310.2000.00549.x
Hare B, Call J, Tomasello M (2006) Chimpanzees deceive a human competitor by hiding. Cognition 101:495–514. doi:10.1016/j.cognition.2005.01.011
Heyes CM (1993) Anecdotes, training, trapping and triangulating: do animals attribute mental states? Anim Behav 46:177–188. doi:10.1006/anbe.1993.1173
Heyes CM (1998) Theory of mind in nonhuman primates. Behav Brain Sci 21:101–114. doi:10.1017/S0140525X98000703
Hirata S, Matsuzawa T (2001) Tactics to obtain a hidden food item in chimpanzee pairs (Pan troglodytes). Anim Cogn 4:285–295. doi:10.1007/s100710100096
Janmaat KR, Polansky L, Ban SD, Boesch C (2014) Wild chimpanzees plan their breakfast time, type, and location. PNAS 111(46):16343–16348. doi:10.1073/pnas.1407524111
Kummer H, Anzenberger G, Hemelrijk CK (1996) Hiding and perspective taking in long-tailed macaques (Macaca fascicularis). J Comp Psychol 110:97–102. doi:10.1037/0735-7036.110.1.97
Leavens DA, Hopkins WD, Thomas RK (2004) Referential communication by chimpanzees (Pan troglodytes). J Comp Psychol 118:48–57. doi:10.1037/0735-7036.118.1.48
McGuigan N, Doherty MJ (2002) The relation between hiding skill and judgment of eye direction in preschool children. Dev Psychol 38:418–427. doi:10.1037/0012-1649.38.3.418
Melis AP, Call J, Tomasello M (2006) Chimpanzees (Pan troglodytes) conceal visual and auditory information from others. J Comp Psychol 120:154–162. doi:10.1037/0735-7036.120.2.154
Menzel EW Jr (1974) A group of young chimpanzees in a one-acre field. In: Schrier AM, Stollnitz F (eds) Behavior of nonhuman primates, vol 5. Academic Press, New York, pp 83–153
Menzel CR (1999) Unprompted recall and reporting of hidden objects by a chimpanzee (Pan troglodytes) after extended delays. J Comp Psychol 113:426–434. doi:10.1037/0735-7036.113.4.426
Mitchell RW, Anderson JR (1997) Pointing, withholding information, and deception in capuchin monkeys (Cebus apella). J Comp Psychol 111:351–361. doi:10.1037/0735-7036.111.4.351
Mitchell RW, Thompson NS (1986) Deception: perspectives on human and nonhuman deceit. State University of New York Press, Albany
Mulcahy NJ, Call J (2006) Apes save tools for future use. Science 312:1038–1040. doi:10.1126/science.1125456
Osvath M (2009) Spontaneous planning for future stone throwing by a male chimpanzee. Curr Biol 19:R190–R191. doi:10.1016/j.cub.2009.01.010
Osvath M, Osvath H (2008) Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: self-control and pre-experience in the face of future tool use. Anim Cogn 11:661–674. doi:10.1007/s10071-008-0157-0
Premack D, Woodruff G (1978) Chimpanzee problem-solving: a test for comprehension. Science 202:532–535. doi:10.1126/science.705342
Raby CR, Alexis DM, Dickinson A, Clayton NS (2007) Planning for the future by western scrub-jays. Nature 445:919–921. doi:10.1038/nature05575
Roberts WA (2002) Are animals stuck in time? Psychol Bull 128:473–489. doi:10.1037/0033-2909.128.3.473
Roberts AI, Vick SJ, Roberts SG, Menzel CR (2014) Chimpanzees modify intentional gestures to coordinate a search for hidden food. Nat Commun 5:3088. doi:10.1038/ncomms4088
Sandel AA, MacLean EL, Hare B (2011) Evidence from four lemur species that ringtailed lemur social cognition converges with that of haplorhine primates. Anim Behav 81:925–931. doi:10.1016/j.anbehav.2011.01.020
Shettleworth SJ (2010) Clever animals and killjoy explanations in comparative psychology. Trends Cogn Sci 14:477–481. doi:10.1016/j.tics.2010.07.002
Sodian B, Taylor C, Harris PL, Perner J (1991) Early deception and the child’s theory of mind: false trails and genuine markers. Child Dev 62:468–483. doi:10.1111/j.1467-8624.1991.tb01545.x
Suddendorf T, Corballis M, Collier-Baker E (2009) How great is great ape foresight? Anim Cogn 12:751–754. doi:10.1007/s10071-009-0253-9
Tomasello M, Call J, Hare B (2003) Chimpanzees understand psychological states—the question is which ones and to what extent. Trends Cogn Sci 7:153–156. doi:10.1016/S1364-6613(03)00035-4
van Schaik CP, Damerius L, Isler K (2013) Wild orangutan males plan and communicate their travel direction one day in advance. PLoS one 8(9):e74896. doi:10.1371/journal.pone.0074896
Vander Wall SB (1990) Food hoarding in animals. The University of Chicago Press, Chicago
Whiten A (2013) Humans are not alone in computing how others see the world. Anim Behav 86:213–221. doi:10.1016/j.anbehav.2013.04.021
Whiten A, Byrne RW (1988) Tactical deception in primates. Behav Brain Sci 11(2):233–244. doi:10.1017/S0140525X00049682
Woodruff G, Premack D (1979) Intentional communication in the chimpanzee: the development of deception. Cognition 7:333–362. doi:10.1016/0010-0277(79)90021-0
Zimmermann F, Zemke F, Call J, Gómez JC (2009) Orangutans (Pongo pygmaeus) and bonobos (Pan paniscus) point to inform a human about the location of a tool. Anim Cogn 12:347–358. doi:10.1007/s10071-008-0194-8
Acknowledgments
We thank L. Ajarova, the Chimpanzee Sanctuary and Wildlife Conservation Trust, the Uganda Wildlife Authority and the Uganda National Council for Science and Technology, for allowing us to conduct research on Ngamba Island. We are grateful to the Ngamba staff for their tremendous help and support. We also thank S. Schuette for building the apparatus, S. Tuepke for creating the graphics, and M. Yucel for reliability coding. K. Karg also gratefully acknowledges financial support by the German National Academic Foundation.
Ethical standard
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Our research was approved by the Uganda Wildlife Authority and the Uganda National Council for Science and Technology and is in line with the ethical standards for animal research of the Max Planck Institute for Evolutionary Anthropology, Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karg, K., Schmelz, M., Call, J. et al. Chimpanzees strategically manipulate what others can see. Anim Cogn 18, 1069–1076 (2015). https://doi.org/10.1007/s10071-015-0875-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-015-0875-z