Abstract
This study aimed to assess apple blossom extracts as potential natural whitening agents due to their ability to inhibit melanogenesis. Ethanol extracts of apple blossom (ABE) were assessed for biological activity in the B16F10 mouse melanoma cell line. ABE toxicity was assessed by thiazolyl blue tetrazolium bromide (MTT) assay. Levels of melanogenic enzyme expression in response to ABE supplementation were assessed by western blotting. Also assessed purified kaempferol, one of the phenolic compounds extracted from apple blossom, was evaluated using western blot analysis. The expression levels of cellular tyrosinase, microphthalmia-associated transcription factor (MITF), tyrosinase-related protein (TRP)-1, and TRP-2 proteins related to melanogenesis decreased in a dose-dependent manner with ABE treatment of cells. Using nuclear magnetic resonance, we identified kaempferol in the ABE. Treatment of cells with purified kaempferol decreased the expression levels of tyrosinase and the MITF protein to a similar degree as that observed with ABE treatment. This suggests that the efficacy of melanogenesis-related inhibition demonstrated by ABE was due to kaempferol. ABE has an inhibitive effect on melanogenic enzymes and potentially can be applied to functional foods and cosmetics having a whitening effect as a natural material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In today, white and clear skin beacme a measure of health and beauty. As the standard of living have improved and the life expectancy of human being increases, interest in skin protection and whitening using natural food sources has increased as an approach to protect the skin from ultraviolet (UV) exposure and environmental pollution. Therefore, research toward the discovery of a whitening material in edible plant sources that is hypoallergenic on the skin is actively being conducted (Ha, 2008; lee et al., 2003).
Melanin protects the skin from external irritants such as UV rays. However, excessive pigmentation within the skin surface can result in the skin aging because it creates formation of spots and freckles (Cao et al., 2014). The synthesis of melanin begins with tyrosine, an amino acid substrate. Tyrosine is converted to 3,4-dihydroxyphenylalanine (DOPA) quinone through DOPA by tyrosinase (Seo et al., 2010). The oxidation of DOPA to DOPA quinone is then converted to DOPA chrome, and DOPA chrome is oxidized to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) by tyrosinase-related protein (TRP)-2. DHICA is oxidized by TRP-1 to promote the polymerization of DHICA melanin, which is dark brown (Parvez et al. 2006; Seiberg et al., 2000). Furthermore, microphthalmia-associated transcription factor (MITF) regulates the expression of enzymes involved in melanin formation by binding to the M-box sequences of tyrosinase and TRPs (Bentley et al., 1994). Therefore, inhibitory activities of tyrosinase, TRP-1, TRP-2, and MITF may play a significant role in inhibiting the melanogenesis, which could promote a whitening effect (Curto et al., 1999). Various sulfhydryl compounds, such as reduced glutathione, L-cysteine, N-acetyl-L-cysteine, and thiols, etc., that are contained in natural products have an antityrosinase effect (Venditti et al., 2013).
The apple, which belongs to the Rosaceae family, blossoms with white flowers in April–May, which hang with leaves from the leaf axil at the end of branches. Rosaceae plants reportedly enhance tyrosinase inhibitors (Gao et al., 2003) and antioxidants (Cho et al. 2003). Apple blossoms have also been found to contain phenolic compounds that are similar to those found in apple fruit (Choi et al., 2011); however, apple blossoms are discarded during the fruiting process and there is limited research on the active use of apple blossom, including their whitening properties. Therefore, eco-friendly research to develop functional materials from waste by-products such as apple blossoms is very necessary.
Existing natural and synthetic whitening agents can be classified as tyrosinase inhibitors that block UV ray, such as hydroquinone, ascorbic acid, arbutin, kojic acid, tropolone, and polyphenols (Choi and Shin, 2016). However, some synthetic whitening agents are not suitable as cosmetic whitening materials because they can cause allergic skin reactions as well as toxicity at certain concentrations (Peng et al., 2021). Therefore, there is an urgent need to develop a stable and effective natural materials to overcome limitations such as skin erythema, sensitivity reactions, and cytotoxicity.
This study, apple blossom extracts (ABE) and purified compounds from ABE were used to treat B16F10 mouse melanoma cells, followed by an assessment of their tyrosinase inhibitory activity and melanogenesis. Western blotting was used to confirm the expression inhibition of proteins involved in melanogenesis, such as tyrosinase, MITF, TRP-1, and TRP-2. These results indicate the potential of apple blossom as a natural whitening agent.
Materials and methods
Preparation of apple blossom extracts
Apple blossom used in this experiment was collected in April 2019 from the “Hongro” cultivars of Korean apple trees (Malus pumila Borkh) at the Apple Research Institute (Gunwi-Gun, Republic of Korea). The Hongro cultivars were developed by the Horticultural Experiment Station (Rural Development Administration, Suwon, Republic of Korea) in 1981 (Chun et al., 2012; Shin et al., 1989) and registered for plant variety protection in 1998 by the Korea Seed & Variety Service (application number: 1998-28). Botanical identification of the plant was carried out by Prof. Dr. In-Kyu Kang (Department of Horticultural Science, Kyungpook National University, Republic of Korea). After drying in a dry oven (Jeiotech, Daejeon, Republic of Korea) at 45 °C, the apple blossoms were ground using a high-speed grinder at 25,000 rpm (RT-08, Rong Tsong Precision Technology, Taichung, Taiwan). The material was pulverized using a 40-mesh sieve and stored at 4 °C. For hot water extract, 1 g of powdered apple blossom was added to 200 mL of distilled water (DW) and heated until reached 100 mL. The concentrate was cooled to room temperature and stirred in a shaking incubator at 120 rpm for 24 h. In the case of ethanol extract, 100 mL of 40% ethanol was added to 1 g of the sample, followed by stirring in a shaking incubator at 120 rpm for 24 h. The apple blossom extracts (ABE) were filtered using a filter paper (No. 1, Whatman, Maidstone, UK) and vacuum-dried in a rotary evaporator (Eyela NE, Tokyo, Japan) at 45 °C to the target concentration of phenolic compounds (50, 100, 150, and 200 μg/mL) for the in vitro experiment. And solid powder was prepared by freeze drying at − 80 °C for 96 h using a freeze dryer (FDS8518, Ilshin Bio Base Co. Ltd, Dongducheon, Republic of Korea) for the cell-line experiment.
Measurement of total phenolic content (TPC)
Total phenolic content (TPC) was measured as described by Folin and Denis (1912). 1 mL of sample extract was added 1 mL of ethanol, 5 mL of DW, and 0.5 mL of 1 N Folin–Ciocalteu reagent then incubated for 5 min at room temperature. After, adding 1 mL of Na2CO3 to the mixture, react within 1 h in the dark. The consequent absorbance was measured using by UV–Vis spectrophotometer (Optizen 3220 UV, Merasys Co. Ltd., Seoul, Korea) at 725 nm. The TPC was calculated by conversion from a standard curve using gallic acid.
Purification and chemical structure assay of purified active compounds isolated from apple blossom
The level of purified active compounds isolated from apple blossom was quantified using high-performance liquid chromatography (HPLC) with an HPLC–diode-array detection (DAD) system (HP 1100, Hewlett Packard, Agilent Technologies, Santa Clara, CA, USA) equipped with a Zorbax Eclipse Plus C18 column (4.6 × 150 mm, 5 μm, Agilent Technologies). Then, 0.5-µL samples were injected to the HPLC–DAD system. The mobile phase consisted of deuterium depleted water (DDW) as solvent A (30%) and methanol as solvent B (70%), with the flow rate kept constant at 0.4 mL/min. The UV detector was set to monitor at 254 nm (Xu et al., 2006). Chemical structural analysis of the purified active compounds isolated from apple blossom was performed using a 600 MHz nuclear magnetic resonance (NMR) spectrometer (ADVANCE III HD 600; Bruker, Rheinstetten, Germany) at Gyeongbuk Technopark to measure the 1H- and 13C-NMR spectra. The filling agent used for column chromatography in the NMR was Sephadex LH-20 (10–25 μm, GE Healthcare Bio-Science AB, Sweden) and ODS-SM-50B (50 μm, YAMAZEN, Osaka, Japan). Thin layer chromatography (TLC) silica gel 60 (Merck, Darmstadt, Germany) was used for the TLC plate. Medium pressure liquid chromatography was performed using Buchi Sepacore Flash System (Sepacore 50, Swiss) equipped with a glass column (26 × 300, 50 × 600 mm).
High-performance liquid chromatography and nuclear magnetic resonance data
From the HPLC data for Compound 1, only a single peak can be observed on the chromatogram (Fig. 1B). In the 1H-NMR spectrum of Compound 1, it was confirmed that 1,4-substituted aromatic rings of the A2B2 type existed in δ8.05 (1H, d, J = 8.4 Hz, H-3′, H-5′) and δ6.93 (1H, d, J = 8.4 Hz, H-2′, H-6′). In addition, each broad singlet shown in δ6.44 and δ6.19 ppm is presumed to be a flavanol moiety with H-8 and H-6 of the flavonoid A ring composed of meta coupling. The results for the above experimental 1H- and 13C-NMR data were compatible with the data reported by Zhang et al. (2012), and Compound 1 was identified as kaempferol (Fig. 1A).
Compound 1—1H-NMR (600 MHz, dimethyl sulfoxide-d6 [DMSO-d6]) δ: 8.05 (2H, d, J = 8.4 Hz, H-2′, H-6′), 6.93 (2H, d, J = 8.4 Hz, H-3′, H-5′), 6.44 (1H, d, J = 1.8 Hz, H-6), 6.19 (1H, d, J = 1.8 Hz, H-8). 13C-NMR (150 MHz, DMSO-d6) δ: 176.3 (C-4), 164.3 (C-7), 161.1 (C-5), 159.6 (C-4′), 156.6 (C-9), 147.2 (C-2), 136.1 (C-3), 129.9 (C-2′, C-6′), 122.1 (C-1′), 115.9 (C-3′, C-5′), 103.5 (C-10), 98.6 (C-6), and 93.9 (C-8).
Measurement of extracellular tyrosinase inhibitory activity
Extracellular tyrosinase inhibitory activity was performed as described by Hearing (1987). The reaction mixture consisted of 2.3 mL of 0.1 M sodium phosphate buffer (pH 6.8), 0.4 mL of 1.5 mM substrate tyrosine solution, and 0.1 mL of mushroom tyrosinase (250 U/mL, Sigma-Aldrich Co, St. Louis, MO, USA). Then 0.2 mL of Sample extracts (50–200 μg/mL phenolic concentration) was added to the mixture. And 0.2 mL of kojic acid (50–200 μg/mL) was added to the positive control. After mixture was reacted for 20 min at 37 °C, the absorbance was measured at 475 nm. The inhibition rate of extracellular tyrosinase was calculated as follows:
B16F10 melanoma cell culture and stimulation
The B16F10 mouse melanoma cell line was purchased from Korea Cell Line Bank (Seoul, Republic of Korea). For cells, in Dulbecco’s Modified Eagle Medium (DMEM, HyClone Laboratories, Inc., Logan, Utah, USA) supplemented with 10% fetal bovine serum (HyClone Laboratories, Inc.) and 1% penicillin/streptomycin (100 U/mL, HyClone Laboratories, Inc.) was used as a culture medium and cultured in an incubator (311, Thermo Fisher Scientific., Waltham, MA, USA) with 5% CO2 at 37 °C. Cells were observed using an inverted microscope (Nikon, Tokyo, Japan). Cells under 10 passages were used and were subcultured at a confluence of approximately 80%. B16F10 cells were stimulated with 1 μM α-melanocyte stimulating hormone (α-MSH) for 1 h. Then, a sample solutions (25–200 μg/mL) was then treated to cells and incubated for 24–48 h.
B16F10 melanoma cell viability assay
The toxicity of ABE in B16F10 cells was performed as described in a previous study (Carmichael et al., 1987). B16F10 cells (2 × 104 cells/well) were seeded in a 48 well plates in a DMEM and incubated for 24 h with 5% CO2. Subsequently, 500 μL of sample solutions (25–200 μg/mL) was added to the cells and were incubated for 48 h at 37°. The control group was cultured under the same conditions using DMEM instead of the sample solution. After incubation, 20 µL of thiazolyl blue tetrazolium bromide (5 mg/mL, MTT, Sigma-Aldrich Co.) reagent was added to wells, and the culture solution was removed after reacted for 4 h. Dissolved formazan crystals with 500 µL DMSO, then left to react for 10 min, and its absorbance at 540 nm was measured by SPECTROstar Nano Microplate Reader (BMG LABTECH., Germany). The cell viability rate was calculated as follows:
Melanin contents in α-MSH treated B16F10 melanoma cells
The melanin contents was measured following the method of Hosoi et al. (1985). B16F10 cells (3 × 105 cells/well) were seeded in a 100-mm tissue culture dish and cultured for 24 h for stabilization and stabilization. Therefter, the cultured medium was removed, new DMEM was added, and 8 mL of 1 μM α-MSH was added as a stimulant to wells except for the normal group. Sample solutions (25–100 μg/mL) were then added and incubated for 48 h with 5% CO2 at 37 °C. After incubation, the medium was removed, and cells were washed with cold phosphate-buffered saline (PBS) and then stored at − 80 °C. Thereafter, 200 μL of lysis buffer mixed with protease inhibitor cocktail (Thermo Fisher Scientific, San Joes, CA, USA) and mammalian protein extraction reagent (M-PER, Thermo Fisher Scientific, Rockford, USA) at a ratio of 1:100 was added to lyse cells and release soluble protein, after which the cell debris was collected by centrifugation at 16,600×g (Micro 17 TR, Hanil, Incheon, Korea) and 4 °C for 15 min. The pellet was dissolved in 500 μL of 1 N NaOH in 10% DMSO and heated using a heating block (MaXtable H10-set, DAIHAN scientific Co., LTD., Wonju, Republic of Korea) at 70 °C for 1 h. The rate of melanin content was calculated as follows:
Cellular tyrosinase inhibitory activity in α-MSH-treated B16F10 melanoma cells
The cellular tyrosinase inhibitory activity was measured by the method of Kim et al. (2011). B16F10 cells (3 × 105 cells/well) were seeded in a 100-mm tissue culture dish and cultured for 24 h. After culturing and stimulating cells, the sample solutions were treated and then the culture media were removed. Cells were washed with cold PBS, and then 200 μL of lysis buffer per well was added to lyse cells. The mixture was centrifuged at 16,600×g for 15 min at 4 °C to remove the protein components. Therefter, 40 μL of the supernatant was added to a 96-well plate, and 160 μL 10 mM substrate 3,4-dihydroxy-L-phenylalanine (L-DOPA) dissolved in 0.1 M sodium phosphate buffer (pH 6.8) was mixed and reacted for 1 h at 37 °C. The amount of DOPA chrome produced was measured by SPECTROstar Nano Microplate Reader at 490 nm to confirm cellular tyrosinase inhibitory activity.
Western blot analysis
Western blot analysis was performed according to Tsareva et al. (2007), B16F10 cells (3 × 105 cells/well) were seeded in a 100-mm tissue culture dish and cultured for 24 h. After culturing and stimulating by 1 μM α-MSH to B16F10 cells, the sample solutions were treated and then the culture media were removed. Cells were washed with cold PBS, and then 200 μL of lysis buffer per well was added to lyse cells. The mixture was centrifuged (16,600×g, 15 min, 4 °C) to remove the protein components. Proteins obtained by centrifugation were quantified using a BCA assay kit (Thermo Fisher Scientific), then 20 μL proteins were separated by applyiong 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for 1.5 h. Proteins were transferred to polyvinylidene fluoride membrane (Immobilon-P™, Millipore, Bedford, MA, USA) for 2.5 h at 60 V. In order to block, the membrane was immersed in 5% bovine serum albumin (BSA) for 1 h. After washing with 1× Tris-buffered saline plus Tween 20 (TBST) for 10 min, the primary antibody (1:500) was incubated overnight. The primary antibodies were tyrosinase, TRP-1, TRP-2 (Santa Cruz Biotechnology Inc., Dallas, TX, USA) and MITF (Bioworld Technology, Inc., Louis Park, MN, USA). Next, the secondary antibody (1:1000, Santa Cruz Biotechnology Inc.) was incubated for 1 h. Bands were detected with enhanced chemiluminescent (ECL) solution (Millipore, Bedford, USA), and the images of the blots were captured by C 300 image analyzer (Azure Biosystems, Dublin, CA, USA).
Statistical analysis
Statistical analysis was performed using a one-way analysis of variance (ANOVA) in SPSS 26 for windows (Statistical Package for Social Science, Chicago, IL, USA). Results are presented as the mean ± standard deviation. Furthermore differences between the mean of treatments were further analyzed using Duncan’s multiple-range tests with significance level of P < 0.05.
Results and discussion
Extracellular tyrosinase inhibition effect of apple blossom extracts on the phenolic concentration
The extraction separates the physiologically active or functional substances contained in the sample differently depending on the properties of the solvent used. Therefore, the selection of the solvent used in the extraction process has a significant impact on all stages of evaluating physiological activity. Hot water extraction is widely used in extracting water-soluble substances because water that is safe for the human as a solvent to minimal harm and obtain a high yield. On the other hand, polar solvents such as ethanol, have been widely used to extract various useful components of plants, since the extract is easy to purify after extraction (Cheon, 2015). The total phenolic content (TPC) of ABE yielded via different ethanol concentration extractions are shown in Fig. 2A. The highest extraction yield of the phenolic content from apple blossom was produced by 40% ethanol extraction. Therefore, the study continued using ABE prepared from both hot water and the 40% ethanol extraction.
The total phenolic content (TPC) in apple blossom extracts (ABE) in various concentration of ethanol (A), and tyrosinase inhibitory activity on phenolic concentration in ABE (B). The activity of tyrosinase inhibitors was measured using a concentration of phenolic compounds from ABE containing both extracts of hot water and 40% ethanol. As a positive control, kojic acid (50 to 200 μg/mL) was used. Mean ± standard deviation (n = 3). Mean with different letters (a–g) above the bars represent significant differences (P < 0.05), as assessed by Duncan’s multiple-range test
Tyrosinase, a peptide in melanocyte in the skin, produces dark brown pigment melanin. Therefore, the inhibition of melanin biosynthesis in skin is an effective measure of the tyrosinase inhibitory activity. Hot water and 40% ethanol extracts of apple blossom, the inhibitory effect of extracellular tyrosinase was measured. The results were compared with the well-known tyrosinase inhibitor kojic acid, which was used as the positive control. In the hot water extract, no inhibitory effects were noted. In contrast, there was 50% inhibitory activity at 200 μg/mL concentrations of phenolic compounds in the 40% ethanol extract, and inhibition was present in a dose-dependent manner (Fig. 2B). The results of the experiments are consistent with the previously reported results that were correlated to the total phenolic content, antioxidant effects, and inhibition of tyrosinase activity of onion skin (Ra et al., 1997). We determined that the apple blossom hot water and ethanol extracts had inhibitory effects on tyrosinase, an enzyme associated with skin whitening, thus, demonstrating their potential for use as a whitening agent in cosmetics or functional foods.
Cell viability, melanin content, and cellular tyrosinase activity of B16F10 cells
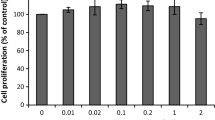
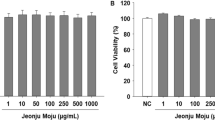
To evaluate the whitening effect of apple blossom, the effects of lyophilized powder of 40% ethanol apple blossom on B16F10 melanoma cell viability was analyzed. The result of MTT assay on cells treated with ABE at 25, 50, 100, and 200 μg/mL, showed cell viability of 97.0%, 89.2%, 84.7%, and 76.3%, respectively, compared with those of the control group (Fig. 3A). At a concentration of 200 μg/mL, cell viability was slightly reduced, which was judged to be some toxic. We found that ABE reduced the cell viability of B16F10 cells in a concentration-dependent manner, and therefore we used 25, 50, and 100 μg/mL ABE for this study.
Cell viability (A), inhibition of melanin production (B), and cellular tyrosinase activity (C) of ethanol extracts from apple blossom (ABE) on B16F10 melanoma cells. Melanin contents were measured after treatment with the ABE and α-MSH (1 µM) for 24 h. Nor: not treated with α-MSH; Con: only treated with α-MSH. Mean ± standard deviation (n = 3). Mean with different letters (a–d) above the bars represent significant differences (P < 0.05), as assessed by Duncan’s multiple-range test
Melanin determines the skin color, and melanin pigments in the skin basal layer protects the skin from harmful UV rays or free radicals. Melanin biosynthesis is regulated by various enzymes, including tyrosinase enzymes (Jimenez-Cervantes et al., 1994; Pavel, 1993). Therefore, we investigated the effect of using ABE to inhibit melanogenesis. As a result, the higher concentration treated with ABE showed a tendency to decrease melanin production in a concentration-dependent manner compared with those of the control group (Fig. 3B). At the concentration of 100 μg/mL ABE, production of melanin was inhibited to approximately 71.0% of the stimulated control value.
B16F10 melanoma cells were stimulated with α-MSH to confirm tyrosinase inhibitory activity and were then treated with ABE to assess the inhibition of this activity (Fig. 3C). The activity level of cellular tyrosinase was 74.7%, 65.6%, and 56.2% for 25, 50, and 100 μg/mL extracts, respectively, compared with that of the stimulated control. At 100 μg/mL, cellular tyrosinase activity was similar to that of cells without stimulation. These results suggest that ABE inhibits the synthesis of intracellular tyrosinase in melanocyte and inhibits melanin biosynthesis.
Effect of ABE on tyrosinase, MITF, TRP-1, and TRP-2 protein expression
In the melanosomes of melanocytes, tyrosinase acts as a tyrosine hydroxylase, to oxidize tyrosine to produce DOPA. Tyrosinase, a kye enzyme, is a type 1 membrane glycoprotein produced by the N-linked glycosylation (Park et al., 2013). Western blot analysis was performed to examine the inhibitory effects of ABE on expression of proteins related to melanogenesis. This confirmed that α-MSH stimulated tyrosinase, MITF, TRP-1, and TRP-2 protein expressions compared with that of the control group (Fig. 4A). The treated with 100 μg/mL ABE inhibited the tyrosinase expression by 64.0% compared with that of the stimulated control group (Fig. 4B).
The expression levels of proteins related to melanogenesis in B16F10 melanoma cells to evaluate potential whitening effect by apple blossom ethanol extracts (ABE). The protein expression levels of tyrosinase, MITF, TRP-1, and TRP-2 were measured after treatment with the ABE and α-MSH (1 µM) for 24 h. Western blot (A), inhibition effect of tyrosinase (B), TRP-1 (C), TRP-2 (D), and MITF (E) protein expression of ABE. Nor: not treated with α-MSH, Con: only treated with α-MSH. Mean ± standard deviation (n = 3). Mean with different letters (a–e) above the bars represent significant differences (P < 0.05), as assessed by Duncan’s multiple-range test
TRP-1 is a DHICA oxidase that converts DHICA into indole-5,6-carboxylic acid, which is an important factor in indirectly regulates melanogenesis. TRP-2 is a dopachrome tautomerase that converts dopachrome to DHICA (Hearing et al., 1992; Takechi et al., 1996). Treatment of B16F10 melanoma cells with ABE inhibited TRP-1 and TRP-2 protein expressions compared with those of the stimulated control group (Fig. 4B, C). In the treated with 100 μg/mL ABE, the protein expression levels of TRP-1 and TRP-2 were approximately 70.0% and 64.0%, respectively.
MITF, a microphthalmia transcription factor, binds to the M-box sequences of tyrosinase and TRPs, and then promotes transcription of tyrosinase, TRP-1, and TRP-2 (Branza-Nichita et al., 2000; Yoon et al., 2007) MITF regulates melanogenesis in melanocytes by stimulating the expressions of TRP-1 and TRP-2 (Debbache et al., 2012). α-MSH stimulated an increase in the MITF protein expression compared with that of the control, whereas this was strongly inhibited to 13% of the simulated control levels by the addition of 100 μg/mL ABE (Fig. 4E).
Effect of purified kaempferol extracted from apple blossom on tyrosinase and MITF protein expression
The compound 1, Kaempferol (5,7,4-trihydroxy flavonol), is a natural flavonol that flavonoid inhibitor who effect of anti-tyrosinase have been proved generally (Farasat et al., 2020). It was estimated that kaempferol was one of the representative ingredients showing the whitening effect in apple blossoms. We further used melanin induced by α-MSH stimulation to compare the inhibitory effects of ABE and purified kaempferol compared with ABE on the expression of proteins related to melanogenesis (Fig. 5A). Treatment of 15 μg/mL of purified kaempferol from apple blossom successfully inhibited the expression of tyrosinase in a dose-dependent manner to only 42.0% of the concentration found in the control (Fig. 5B).
Expression levels of proteins related to melanogenesis in B16F10 melanoma cells for whitening using purified kaempferol from apple blossom. The protein expression levels of tyrosinase and MITF were measured after treatment of the kaempferol and α-MSH (1 µM) for 24 h. Western blot (A), inhibition effect on tyrosinase (B), and MITF (C) protein expression by kaempferol. Nor: not treated with α-MSH, Con: only treated with α-MSH. Mean ± standard deviation (n = 3). Mean with different letters (a–e) above the bars represent significant differences at (P < 0.05), as assessed by Duncan’s multiple-range test
The α-MSH-stimulated MITF expression was less strongly inhibited by purified kaempferol (Fig. 5C). The inhibition of the MITF protein expression was 71.0% of the unstimulated control at 15 μg/mL of purified kaempferol extract from apple blossom, although the inhibition was still dose-dependent. The results were similar to those presented by ABE, which suggests that the efficacy of melanogenesis-related inhibition shown by ABE was due to kaempferol.
Conclusion
This study aimed to investigate the whitening effect of ABE, and examined the mechanisms of inhibiting the enzyme involved in melanogenesis. After extracting apple blossom using both hot water and 40% ethanol, the extracts adjusted to 50–200-μg/mL phenolic concentrations were used to investigate the extracellular tyrosinase inhibitory activity. In the case of hot water extracts, the effect was not significant but 40% ethanol ABE produced concentration-dependent inhibition, which indicated that melanin production was inhibited. We determined that lyophilized ABE was cytotoxic to B16F10 melanoma cells at 200 μg/mL. Therefore, the experiments were carried out at or below 100 μg/mL. The inhibitory effects on cellular tyrosinase by ABE were determined in α-MSH-stimulated B16F10 melanoma cells. The inhibitory effects on protein expression on tyrosinase, MITF, TRP-1, and TRP-2 was confirmed by western blot. As the concentration treated with ABE increased, the concentration of melanin decreased in a concentration-dependent manner. We confirmed a decrease in the expression levels of tyrosinase, MITF, TRP-1, and TRP-2. The purified active compound was separated from ABE by column chromatography and identified as kaempferol by 1H-NMR and 13C-NMR spectra. Adding purified kaempferol to cell assays produced similar dose-dependent decreases in the protein expression levels of tyrosinase and MITF. The above results suggest that the efficacy of melanogenesis-related inhibition present in ABE was due to kaempferol. Thus, ABE inhibited melanin production and the protein expression of enzymes related to melanin production, including tyrosinase, MITF, TRP-1, and TRP-2 in B16F10 melanoma cells. Therefore, ABE and kaempferol, a phenolic compound, extracted from apple blossom, have the potential to be used for whitening functional foods and materials. However, this study was only validated by the cellular level used for ABE and kaempferol extraction. Therefore, further research should be conducted in an animal model and human skin before developing industrial applications.
References
Bentley NJ, Eisen T, Goding CR. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator Molecular and Cellular Biology. 14: 7996-8006 (1994)
Branza-Nichita N, Negroiu G, Petrescu AJ, Garman EF, Platt FM, Wormald MR, Petrescu SM. Mutations at critical N-glycosylation sites reduce tyrosinase activity by altering folding and quality control. Journal of Biological Chemistry. 275: 8169-8175 (2000)
Cao HH, Tse AK, Kwan HY, Yu H, Cheng CY, Su T, Fong WF, Yu ZL. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochemical Pharmacology. 87: 424-434 (2014)
Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Research. 47: 936-942 (1987)
Cheon JH. Effects of Backhousia citriodora extracts on antioxidant activity and bone formation (Doctoral dissertation, MS Thesis), University of Silla, Busan, Republic of Korea. (2015)
Cho EJ, Yokozawa T, Rhyu DY, Kim SC, Shibahara N, Park JC. Study on the inhibitory effects of Korean medicinal plants and their main compounds on the 1, 1-diphenyl-2-picrylhydrazyl radical. Phytomedicine. 10: 544-551 (2003)
Choi MH, Shin HJ. Anti-melanogenesis effect of quercetin. Cosmetics. 3: 18 (2016)
Choi SJ, Cho EA, Cho EH, Jeong YJ, Ku CS, Ha BJ, Chae HJ. Screening of functional materials from solvent fractions of apple flower leaf extract. KSBB Journal. 26: 165-171 (2011)
Chun IJ, Zheng WW, Choi C, Song YY, Kwang IK, Hirst P. Multiple applications of lime sulfur for fruit thinning of ‘Fuji’and ‘Hongro’apple trees. Protected Horticulture and Plant Factory. 21: 445-451 (2012)
Curto EV, Kwong C, Hermersdörfer H, Glatt H, Santis C, Virador V, Hearing Jr VJ, Dooley TP. Inhibitors of mammalian melanocyte tyrosinase: in vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochemical pharmacology. 57: 663-672 (1999)
Debbache J, Raza Zaidi M, Davis S, Guo T, Bismuth K, Wang X, Arnheiter H. In vivo role of alternative splicing and serine phosphorylation of the microphthalmia-associated transcription factor. Genetics. 191: 133-144 (2012)
Farasat A, Ghorbani M, Gheibi N, Shariatifar H. In silico assessment of the inhibitory effect of four flavonoids (Chrysin, Naringin, Quercetin, Kaempferol) on tyrosinase activity using the MD simulation approach. BioTechnologia. Journal of Biotechnology Computational Biology and Bionanotechnology. 101: 193-204 (2020)
Folin O, Denis W. On phosphotungstic-phosphomolybdic compounds as color reagents. Journal of Biological Chemistry. 12: 239–243 (1912)
Gao H, Wu L, Kuroyanagi M, Harada K, Kawahara N, Nakane T, Nakamura Y. Antitumor-promoting constituents from Chaenomeles sinensis KOEHNE and their activities in JB6 mouse epidermal cells. Chemical and Pharmaceutical Bulletin. 51: 1318-1321 (2003)
Ha BJ. Instrumental analysis of the human hair damaged by bleaching treatments-focused on ATR FT-IRM. Journal of Fashion Business. 12: 23-33 (2008)
Hearing Jr VJ. Mammalian monophenol monooxygenase (tyrosinase): purification, properties, and reactions catalyzed. Methods in Enzymology. 142: 154-165 (1987).
Hearing VJ, Tsukamoto K, Urabe K, Kameyama K, Montague PM, Jackson IJ. Functional properties of cloned melanogenic proteins. Pigment Cell Research. 5: 264-270 (1992)
Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1α, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Research. 45: 1474-1478 (1985)
Jimenez-Cervantes C, Solano F, Kobayashi T, Urabe K, Hearing VJ, Lozano JA, Garcia-Borron JC. A new enzymatic function in the melanogenic pathway. The 5, 6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). Journal of Biological Chemistry. 269: 17993-18000 (1994)
Kim DH, An BJ, Lee JY. Whitening activities of the Agrimonia pilosa L. extracts. Journal of Applied Biological Chemistry. 54: 284-289 (2011)
Lee TH, Kim HJ, Kim YB. Depigmentation activity of barley, unpolished rice, Job's-tear. The Journal of Korean Medicine Ophthalmology and Otolaryngology and Dermatology. 16: 57-77 (2003)
Park SA, Park J, Park CI, Jie YJ, Hwang YC, Kim YH, Park SN. Cellular antioxidant activity and whitening effects of Dendropanax morbifera leaf extracts. Microbiology and Biotechnology Letters. 41: 407-415 (2013)
Parvez S, Kang M, Chung HS, Cho C, Hong MC, Shin MK, Bae H. Survey and mechanism of skin depigmenting and lightening agents. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 20: 921-934 (2006)
Pavel S. Dynamics of melanogenesis intermediates. Journal of Investigative Dermatology. 100: S162-S165 (1993)
Peng Z, Wang G, Zeng QH, Li Y, Liu H, Wang JJ, Zhao Y. A systematic review of synthetic tyrosinase inhibitors and their structure-activity relationship. Critical Reviews in Food Science and Nutrition. 62: 1-42 (2021)
Ra KS, Suh HJ, Chung SH, Son JY. Antioxidant activity of solvent extract from onion skin. Korean Journal of Food Science and Technology. 29: 595-600 (1997)
Seiberg M, Paine C, Sharlow E, Eisinger M, Shapiro SS, Andrade-Gordon P, Costanzo M. Inhibition of melanosome transfer results in skin lightening. Journal of Investigative Dermatology. 115: 162-167 (2000)
Seo EJ, Hong ES, Choi MH, Kim KS, Lee SJ. Antioxidant and skin whitening effects of Rhamnus yoshinoi extracts. Korean Journal of Food Science and Technology. 42: 750-754 (2010)
Shin YU, Kim WC, Kang SJ, Moon JY, Kim JH. ‘Hongro’, high sugar, attractive red color apple cultivar for ‘Chuseok’ season. The Research Reports of the Rural Development Administration-Horticulture RDA. 31: 53-61 (1989)
Takechi Y, Hara I, Naftzger C, Xu Y, Houghton AN. A melanosomal membrane protein is a cell surface target for melanoma therapy. Clinical Cancer Research. 2: 1837-1842 (1996)
Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schutz A, Kovacic B, Friedrich K. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia. 9: 279-291 (2007)
Venditti A, Mandrone M, Serrilli AM, Bianco A, Iannello C, Poli F, Antognoni F. Dihydroasparagusic acid: antioxidant and tyrosinase inhibitory activities and improved synthesis. Journal of Agricultural and Food Chemistry. 61: 6848-6855 (2013)
Xu SJ, Yang L, Zeng X, Zhang M, Wang ZT. Characterization of compounds in the Chinese herbal drug Mu‐Dan‐Pi by liquid chromatography coupled to electrospray ionization mass spectrometry. Rapid Communications in Mass Spectrometry: An International Journal Devoted to the Rapid Dissemination of Up‐to‐the‐Minute Research in Mass Spectrometry. 20: 3275-3288 (2006)
Yoon HJ, Shin JW, Kim YK, Kim JE, Cho KH. The effect of vitamin C and E on the expression of the cutaneous basement membrane components. Korean Journal of Investigative Dermatology. 14: 87-98 (2007)
Zhang HM, Wang CF, Shen SM, Wang GL, Liu P, Liu ZM, Deng ZW. Antioxidant phenolic compounds from Pu-erh tea. Molecules. 17: 14037-14045 (2012)
Acknowledgements
No funds are received for the completion of this manuscript.
Author information
Authors and Affiliations
Contributions
E-B C, E-H L and H-J P should be considered joint first author. They contributed equally to this work. They designed the study and conducted the experiment together. Also, they analyzed the experimental results and wrote a manuscript. I-K K reviewed and supervised this paper. As a correspondence author, Y-J C discussed and checked this study. And He confirmed and submitted this paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, EB., Lee, EH., Park, HJ. et al. Phenolic from apple blossom “Hongro” inhibits the expression of proteins related to melanogenesis in B16F10 melanoma cells. Food Sci Biotechnol 32, 91–100 (2023). https://doi.org/10.1007/s10068-022-01167-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-022-01167-z