Abstract
The study aimed to characterize phenolic compounds of the Inonotus sanghuang’s ethyl-acetate fraction (EAF) and assess the neuroprotective effect of EAF using the H2O2-treated primary cortical neuronal cells (PCNC) model. Using HPLC-ECD, 5 phenolics were identified and quantified from EAF. H2O2-treated PCNC experiments in vitro showed that pretreatment with EAF increased the GSH-PX and SOD activities and reduced the NO, MDA, and Aβ contents. Furthermore, EAF suppressed the production of IL-1β, IFN-γ, IL-6, and TNF-α in H2O2-treated PCNC. Other mechanisms found that EAF reduced Bax, caspase 9, and caspase 3 expressions at the mRNA and protein levels while increasing Bcl-2 expression at the mRNA and protein levels. These results showed that EAF could serve as potential agents for anti-NDD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodegenerative diseases (NDD), such as Parkinson’s disease (PD) and Alzheimer’s disease (AD), have gradually become the major diseases endangering the health of the elderly(Mattson and Arumugam, 2018; Wu et al., 2014). Free radicals are vulnerable to inducing damage to nerve cells, while oxidative stress plays a substantial effect in the pathogenesis of AD and PD (Jayasena et al., 2013). Currently, synthetic antioxidants have been implied to have the potential to promote the development of cancer that further limiting their use. Thus, it is crucial to develop natural antioxidants from natural resources to replace synthetic antioxidants. Mushroom-derived natural antioxidants may also protect against NDD (Smith et al., 1996).
Mushroom Sanghuang (Sanghuang) is well recognized for its neuroprotective effects and pharmacological application. Except for mushrooms for food, they have been found to have potential therapeutic effects against NDD (Phan et al., 2015). For example, an ethyl acetate extract from Phellinus linteus bodies had a neuroprotective effect against neuronal cell death induced by H2O2 through its antioxidant and anti-apoptotic properties (Choi et al., 2016), and Inonotus obliquus polysaccharide has neuroprotective effects on L-Glu-damaged HT22 cells and APP/PS1 mice with AD (Han et al., 2019). Inonotus sanghuang, not the same species as P. linteus, is one mushroom Sanghuang and has been known as a legendary medicinal herb in China for more than 2000 years (Wu et al., 2012; Wu and Dai, 2020). The bionically cultured mushrooms Sanghuang (I. sanghuang) showed an immune-regenerative effect on the immunodeficient mice, and both the wild and cultured mushroom extracts had comparable immune-regenerative properties (Wen et al., 2019). Meanwhile, our previous studies have proved the antioxidant, antiproliferative, antimicrobial (Liu et al., 2017), and anti-inflammatory (Zhang et al., 2019a) activities of the Ethyl-acetate fraction of I. sanghuang (EAF). However, no studies are exploring the neuroprotection of EAF.
The EAF showed high antioxidant activity and neuroprotective constituents such as chlorogenic acid and quercetin (Liu et al., 2017). Therefore, we hypothesized that EAF might have neuroprotective roles via modulating oxidative stress. We first determined the phenolic composition in the EAF using HPLC-ECD and then determined the EAF’s potential neuroprotective activity using H2O2-induced oxidative damage of the primary cortical neuronal cells.
Materials and methods
Materials
We collected I. sanghuang from the Aershan Region of Inner Mongolia (Inner Mongolia, China) between July and September 2018. Professor Xiangmei Zhang authenticated this mushroom and a voucher specimen (NoF121725) deposited in the College of Biology Science and Engineering of Hebei University of Economics and Business, Shijiazhuang, China. Neonatal SD rats were obtained from Tianjin Medical University. Neurobasal-A Medium, L-DMEM, B27, trypsase, and fetal bovine serum (FBS) were from Gibco-BRL (Grand Island, NY, USA). Hydrogen peroxide solution (H2O2) and cytosine arabinoside were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other chemicals are reagent grade.
Preparation and chemical composition of EAF
The ethyl acetate fraction of I. sanghuang (EAF) was isolated, and then total phenolics and flavonoids were evaluated as previously described (Liu et al., 2017).
Identification and quantification of phenolics
Phenolics in EAF were determined using high-performance liquid chromatography equipped with electrochemical detection (HPLC–ECD). EAF (10 mg) was dissolved in 10 mL methanol, followed by 5 min sonication. Phenolics were then analyzed by the CoulArray 5600A system (ESA Inc., Chelmsford, MA) using a Zorbox SB-C18 column (4.6 × 250 mm, 5 µm). The injection volume was 50 µL, and the flow rate was 1 mL/min. The autosampler was maintained at 4 °C, and the LC column chamber was set at 40 °C. The separation was performed using two mobile phases (A: 1.44% citric acid, 0.19% aminoethanoec acid and 5% acetonitrile in water; B: 1.44% citric acid, 0.19% aminoethanoec acid and 50% acetonitrile in water) with the following gradient: 0 min (95% A + 5% B), 12 min (90% A + 10% B), 32 min (80% A + 20% B), 44 min (70% A + 30% B), 55 min (60% A + 40% B), 75 min (50% A + 60% B).
Primary cortical neuronal culture (PCNC)
We prepared primary cortical neurons from the brain of neonatal SD rats (within 24 h). Briefly, the cortex was digested in 0.1% trypsin and clostridiopeptidase A at 37 °C for 20 min, and the digestion was stopped using PBS. Next, we collected the cells by the centrifuge for 5 min at 800 rpm, the supernatant was removed, and the pellet was resuspended with DMEM containing 20% FBS and diluted to a cell suspension containing 1 × 1010 cells/mL. The cells were incubated in a 6- or 96-well plate and maintained at 37 °C for 24 h (humidified with 5% CO2). After that, the medium was changed to Neurobasic-A containing 1% B27, then after 72 h, Ara-C (0.3 mg/mL) was administrated into each well, and the medium was replaced with Neurobasal-A/B27 every 3 d.
Treatment of cell culture
Cells at 1 × 1010 cells/well in 6 well plates were cultured for 72 h before treatment. After neuronal cells were incubated with different doses of EAF (40, 8, and 1.6 μg/mL) or Huperzine A (2 μg/mL) for 8 h, they were exposed to H2O2 at 900 μM for another 6 h. The control cells were administrated with the same medium without EAF and H2O2.
MTS assay and cell morphology
After primary cortical neuronal cells were incubated following the treatment mentioned above, an inverted microscope (Olympus, Japan) was used to observe the cell morphology. Additionally, we used an MTS assay to assess the cell viability using an MTS assay kit (BestBio, Shanghai, China).
ELISA for Aβ
The amyloid-beta (Aβ) concentration in the supernatant from the above-cultured cells was determined utilizing an Aβ ELISA kit (Shanghai Yinggong Biotechnology CO., LTD) following the manufacturer’s instructions.
Measurement of SOD, GSH-PX activities, and NO and MDA content
We used the glutathione peroxidase (GSH-Px), superoxide dismutase (SOD), Nitric oxide (NO), and malondialdehyde (MDA) kits (all from Nanjing Jian Cheng BIO, Nanjing, China) to determine the SOD and GSH-PX activity and the content of MDA and NO, respectively.
ELISA for IFN-γ, TNF-α, IL-1β, and IL-6
Cell-free supernatant was collected from the above-cultured cells to determine IFN-γ, TNF-α, IL-1β, and IL-6 using ELISA kits (all from Shanghai Ruifan Biotechnology co., LTD, Shanghai, China) following the manufacturer’s instructions.
Flow cytometry
To determine H2O2-induced cell apoptosis, we costained cells with Annexin V-FITC and PI using an Apoptosis Detection Kit (Beyotime, Shanghai, China) following the manufacturer’s instruction. Cells were acquired, and data were analyzed using BD FACSCalibur flow cytometry (San Jose, CA, USA).
Real-time PCR
According to the manufacturer’s instruction, THE total RNA of primary cortical neuronal cells was extracted using TRIzol reagent (Invitrogen, USA), and the first-strand cDNA was synthesized using a cDNA synthesis kit (Promega Corporation, USA). The mRNA expression levels of caspase-3, caspase-9, Bax, and Bcl-2 were measured by qRT-PCR in the 96-well plates with the real-time detection PCR system (TaKaRa, Japan) with SYBR Premix ExTaq (Takara, Japan), and the gene β-actin was used as an endogenous control. All samples were performed in triplicate, and data were analyzed by the 2−ΔΔCT method. The primer sequences for Caspase-9, Caspase-3, Bcl-2, Bax, and β-actin were as follows: Caspase-9 forward primer 5′ AGCTGGCCCAGTGTGAATAC 3′, reverse primer 5′ GCTCCCACCTCAGTCAACTC 3′; Caspase-3 forward primer 5′ GGAGCTTGGAACGCGAAGAA 3′, reverse primer 5′ ACACAAGCCCATTTCAGGGT 3′; Bcl-2 forward primer 5′ GGATCCAGGATAACGGAGGC 3′, reverse primer 5′ ATGCACCCAGAGTGATGCAG 3′; Bax forward primer 5′ AAGAAGCTGAGCGAGTGTCTC 3′, reverse primer 5′ ATGGTTCTGATCAGCTCGGG 3′; β-actin forward primer 5′ CACTGCCGCATCCTCTTCCTC 3′, reverse primer 5′ GGCATAGAGGTCTTTACGGA 3′.
Western blot analysis
The expressions of caspase-3, caspase-9, Bax, and Bcl-2 were measured by Western blot. Briefly, total protein was extracted from primary cortical neurons cells. Then the specimens were loaded on 10% SDS-PAGE, and the separated proteins were transferred onto a PVDF membrane. We incubated the membranes with a blocking buffer for 2 h at 4 °C and then incubated it overnight at 4 °C with primary antibodies: caspase-3, caspase-9, Bax, Bcl-2, GADPH (1:1000; all from Tianjin Saierbio co, Tianjin, China). Subsequently, the membranes were incubated with secondary antibodies, followed by exposure to enhanced chemiluminescent reagents (Millipore, Burlington, MA). We used the Image J software to quantify the optical density of each band. Relative expression of the protein was normalized to the respective GAPDH bands.
Animals
After receiving approval from the Animal Experimentation Ethics Committee at Guangdong Medical University (ID Number: GDY1902247), mice were housed at a relative humidity of 40–70%, at 22 °C, and in 12 h dark/light cycles. They received food and water ad libitum.
Statistical analysis
We used SPSS 22.0 to perform the statistical analysis (SPSS Inc., Chicago, IL, USA), a statistical software package via one-way ANOVA followed by Tukey’s HSD multi-comparison test. p < 0.05 was set statistically significant.
Results and discussion
Chemical composition of EAF
The yield of EAF was 2.02% (w/w). EAF contains TPC (43.12 ± 1.52 mg gallic acid equivalents/g dry-extract weight; mg GAE/g DW), and TFC (57.23 ± 1.65 mg rutin equivalents/g dry-extract weight; mg RE/g DW), which is in accord with our previous results (Liu et al., 2017).
Five phenolic compounds of EAF were identified and quantified by HPLC-ECD (Table 1 and Fig. S1). Four compounds (vanilic acid, protocatechuic acid, p-coumaric acid, and caffeic acid) (Al Olayan et al., 2020; Daroi et al., 2021a, b; Salau et al., 2020; Zhang et al., 2019b) have been proved with neuroprotective activities. Thus, EAF may be an effective neuroprotective agent attributed to these compounds. Meanwhile, their neuroprotective effects manifested different aspects: for example, protocatechuic acid and p-coumaric acid showed antioxidant, anti-inflammatory, and anti-apoptotic activities (Al Olayan et al., 2020; Daroi et al., 2021a, b). We speculate that EAF may be regarded as an effective neuroprotective agent. Meanwhile, whether the mixture of 5 components in Table 1 shows similar critical effects with the extract needs further study.
Changes in primary cortical neuronal cell morphology
Corresponding to the report (Hu et al., 2017), the H2O2-treated cells could inhibit cell density and negative change in cell morphology, including irregular cell morphology, impairment, breakdown of cell processes, and decreased cell adhesion ability. The morphology of PCNC is shown in Fig. 1(A). The recovery of proliferation ability of all the experimental groups, except the EAF (1.6 μg/mL) treated group, increased remarkably, along with a decrease in cell clustering. Besides, the EAF improved the cell morphology of the PCNC. Significantly, the EAF (40 μg/mL) treated group and the Huperzine A (2 μg/mL) treated group displayed the greatest improvement for the cell morphology, which is corresponded to the cell viability changes.
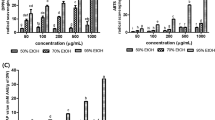
EAF impacts the cell viability and morphology in primary cortical neuronal cells treated with or without H2O2. (A) Effect of EAF on cell morphology of H2O2-damaged primary cortical neuronal cells. (B) Primary cortical neuronal cells were treated with H2O2 (300–1200 μM) for 6–24 h and (C) the EAF (1.6–200 μg/mL) for 8 h. (D) Cells were precultured with the EAF (1.6–40 μg/mL) for 8 h and then administrated with H2O2 (900 μM) for another 6 h. Results are presented as mean ± SD. These experiments were repeated four times. Values with different letters have a statistical difference (p ≤ 0.05), tested using Tukey’s HSD Test
EAF prevents H2O2-induced cell death in primary cortical neuronal cells
We first determine the cytotoxic influence of H2O2 on primary cortical neuronal cells (PCNC). PCNC was cultured at the indicated doses of H2O2 (300, 600, 900, and 1200 μM) for 6, 12, and 24 h, and then the cell viability was analyzed using an MTS assay. As shown in Fig. 1(B), the PCNC exposure to H2O2 decreased the cell viability dose-dependently, with a 54.3% reduction compared with the untreated cells. Thus, we used this dose for the subsequent study. Next, we evaluated the cytotoxic effects of EAF at the indicated doses on PCNC. The EAF at 1.6 to 40 μg/mL did not show significant cytotoxic effects for 8 h except for 200 μg/mL EAF [Fig. 1(C)]. Finally, we explored whether EAF could influence H2O2-induced cell death in PCNC. EAF dose-dependently attenuated the H2O2-induced cytotoxicity and increased cell viability to 68.74%, 76.48%, and 90.85%, respectively [Fig. 1(D)]. Huperzine A has exhibited a variety of biological actions, in particular, neuroprotective effect, and has been used as a control drug for some extracts such as Xanthoceras sorbifolia husks (Rong et al., 2019; Zhu et al., 2018). We noted that the effect of the EAF on the cell viability of H2O2-treated PCNC (90.85% compared with control) was comparable to that of the reference Huperzine A (88.25% compared with control). The current result was in agreement with the study (Choi et al., 2016) in which they found that P. linteus extract isolated by the ethyl acetate (another kind of Sanghuang mushrooms) displayed an excellent ability to prevent H2O2-induced SK-N-MC cell death.
GSH-PX and SOD activities, MDA and NO levels, and Aβ level of primary cortical neuronal cells
The oxidative damage has been involved in the pathogenesis of NDD (Jayasena et al., 2013). GSH-Px is the primary antioxidant enzyme that protects membranes from lipid peroxidation, and SOD can defend against harmful ROS in the cells (Liu et al., 2019). Thus, we next detected the GSH-Px and SOD activities. The GSH-Px activity of EAF (40 μg/mL) groups enhanced significantly (p ≤ 0.05) [Fig. 2(A)]. Meanwhile, the SOD activities of all the treatment groups increased significantly (p ≤ 0.05)[Fig. 2(B)]. The MDA and NO levels of the H2O2 treated groups rose to 1.93 ± 0.07 mmol/mL and 12.42 ± 0.13 μmol/L [Fig. 2(C) and (D)]. MDA and NO levels in the EAF-treated groups decreased significantly compared with the untreated group. It was noted that the NO levels of the EAF (40 μg/mL) treated group (9.73 ± 0.16 μmol/L) were comparable to that of the reference Huperzine A (9.92 ± 0.19 μmol/L) [Fig. 2(D)]. Due to their potent antioxidant effects, polyphenols may have potential benefits in treating age-related disorders (Jayasena et al., 2013). For example, compound caffeic acid could increase the SOD activity and show the neuroprotective action (Liang et al., 2015). The present study suggests that EAF could reverse the decrease of GSH-PX and SOD activities and reduce MDA, and these compounds may cause NO production in PCNC and the antioxidant activity of EAF, perhaps.
Effects of EAF on GSH-PX and SOD activities, MDA, NO, Aβ, and pro-inflammatory cytokines levels in H2O2-damaged primary cortical neuronal cells. (A) GSH-PX activity; (B) SOD activity; (C) MAD level; (D) NO level; (E) Aβ level; (F) IFN-γ level; (G) IL-1β level, (H) IL-6 level; (D) TNF-α level (mean ± SD, n = 4). Values with different letters have a statistical difference (p ≤ 0.05), tested using Tukey’s HSD Test
Aggregation of the Aβ is commonly considered the basis of a large group of NDD, including AD, PD, and other diseases (Chiti and Dobson, 2006). The Aβ levels were 66.94, 75.80, 70.58, 70.58, 66.63, and 66.38 ng/mL for control, H2O2, EAF (40 μg/mL), EAF (8 μg/mL), EAF (1.6 μg/mL) and HA (2 μg/mL), respectively [Fig. 2(E)]. All groups, H2O2-induced following pretreatment with EAF, produced a remarkable inhibition of aggregation compared with the H2O2-induced PCNC. Meanwhile, the Aβ levels in EAF (1.6 μg/mL) and HA (2 μg/mL) treated groups even were similar to the control (p > 0.05) [Fig. 2(E)]. Its potent activity in counteracting amyloid aggregates of medicinal plant extracts might be due to alkaloids, terpenoids, phenolic acids (Huang et al., 2020; Li et al., 2017). Thus, the noted inhibition of aggregation property of EAF is most likely attributed to its phenolic acids. Moreover, these results also indicate that EAF may inhibit the production of Aβ in PCNC by preventing oxidative stress and have the potential action to prevent and/or treat NDD.
Effects of EAF on the pro-inflammatory cytokines secreted by H2O2-induced primary cortical neuronal cells
Pro-inflammatory cytokines are one of the key players in the neurodegenerative process of PD (McGeer and McGeer, 2008). To study the anti-inflammatory impact of the EAF in H2O2-induced primary cortical neuronal cells, we determined the pro-inflammatory cytokine level including IFN-γ, IL-1β, IL-6, and TNF-α in H2O2-treated PCNC with or without EAF. As shown in Fig. 2, H2O2 (900 µM) administration for 6 h upregulated these inflammatory mediator productions. However, the production of these pro-inflammatory cytokines was decreased by EAF in a dose-dependent manner (p ≤ 0.05). Especially, EAF at 1.6 μg/mL had the most inhibitory effect on the production of IL-1β, IFN-γ, TNF-α, and IL-6, which is concerted with our previous study that EAF at 2 μg/mL had the most effective inhibition of the NO, IL-6, and TNF-α production in the RAW264.7 macrophages (Zhang et al., 2019a). However, a high concentration of EAF (40 μg/mL) may stimulate cells and reverse its role in suppressing these inflammatory cytokine productions, which was in agreement with the study of Chu et al. (2020) in which they found that a high concentration of GSEs increased the production of IL-1β. Caffeic acid (Zaitone et al., 2019) and vanilic acid (Ullah et al., 2020) have demonstrated a neuroprotective effect in decreasing pro-inflammatory cytokines. Thus, the noted anti-inflammatory property of EAF is most likely attributed to these compounds.
Effect of EAF on primary cortical neuronal cell apoptosis
To examine whether EAF can attenuate H2O2-induced apoptotic neuronal cell death, we costained PCNC with Annexin V-FITC/PI and determined the cell apoptosis by flow cytometry described in the “Materials and Methods” section. We found that the apoptosis rate in untreated controls was 17.46% and elevated to 45.51% post-treatment with 900 μM H2O2. In contrast, EAF at 40, 8, 1.6 μg/mL attenuated this increasing rate to 24.40%, 27.39% and 33.54% respectively (p < 0.05). The results indicated that EAF dose-dependently inhibited the H2O2-induced apoptosis of PCNC, while the HA treated group resulted in the lowest apoptosis rate (19.27%) [Fig. 3(G)]. Choi et al. reported that P. linteus’s acetate extract could inhibit the H2O2-induced apoptosis. Protocatechuic acid (Choi et al., 2020) has been reported to have the ability to reduce the percentage of apoptosis induced by Aβ.
Effect of EAF on the apoptosis-related gene expression at the mRNA level in H2O2-induced primary cortical neuronal cells
Apoptosis is strictly regulated by the caspase family members (caspase 9 and caspase 3) and Bcl-2 family members (Bcl-2 and Bax) (Xu et al., 2018). The upregulated Bax/Bcl-2 ratio first activates caspase-9 and subsequently results in the caspase-3 activation (Bucchieri et al., 2015). To explore the effect of EAF on the influence of inhibiting the H2O2-induced apoptosis, the apoptosis-related genes (caspase-9 and caspase 3, Bax, and Bcl-2) were measured by real-time RT-PCR. The analysis of relative mRNA levels indicates that the cells treated with H2O2 increased the caspase 3, caspase 9, and Bax expression [Fig. 4(A), (B), and (C)]. Treated cells with EAF dose-dependently reduced the mRNA expression of the apoptotic upstream genes: Caspase 3, caspase 9, and Bax. The mRNA expression level of the caspase 9 from cells pre-treated with EAF (40 μg/mL) showed no significant alterations compared with HA (2 μg/mL). Meanwhile, treated cells with EAF dose-dependently increased the expression of Bcl-2 compared to the untreated cells at the mRNA level [Fig. 4(D)]. The cells treated with EAF dose-dependently reduced the Bax/Bcl-2 ratios markedly [Fig. 4(E), p ≤ 0.05]. It is noteworthy that the Bax/Bcl-2 ratios of the control group, EAF group (40 μg/mL), and HA group were not significant [Fig. 4(E), p > 0.05]. Our results indicated that EAFI groups, especially the EAF group at 40 μg/mL, could inhibit the H2O2-induced apoptosis.
Impact of EAF on the expression of caspases3, caspase 9, Bax, and Bcl-2 in H2O2-damaged primary cortical neuronal cells. (A, B, C, D, and E) represents an analysis of mRNA expression of caspase 3, caspase 9, Bcl-2, Bax, and Bcl-2/Bax (mean ± SD, n = 3). F Represents western blot analysis of caspase 3, caspase 9, Bcl-2, Bax, and GAPDH is shown as the loading control. (G, H, I, J, and K) represents a quantitative analysis of the expression of caspase 3, caspase 9, Bcl-2, Bax, and Bcl-2/Bax. Cells were no treated (Control) or only treated with H2O2, EAF (40 μg/mL + H2O2), EAF (8 μg/mL + H2O2), EAF (1.6 μg/mL + H2O2) and HA (2 μg/mL + H2O2) (mean ± SD, n = 3). Values with different letters have a statistical difference (p ≤ 0.05), tested using Tukey’s HSD Test
The inhibitory impact of EAF on the expression of the apoptosis-related genes at the protein level in H2O2-induced primary cortical neuronal cells
Bcl-2 family proteins have a critical role in inducing and promoting programmed cell death in the developing nervous system (Zhao et al., 2016). In addition, Bax is mainly present in the healthy cell cytosol and is involved in apoptosis (Mark et al., 2006). Generally, when the initiator caspases (such as caspase-9) become activated, they could, in turn, activate the effector procaspases-3 by a proteolytic cascade, leading to the cleavage of various cellular substrates involved in apoptosis (Jing et al., 2013).
As shown in Fig. 4(F)–(K), the H2O2 treatment increased the Bcl-2 expression and decreased caspase 3, caspase 9, and Bax to 1.69, 2.37, and 4.77, respectively. However, EAF treatment initiated the opposite impact to the H2O2 treatment and prevented the apoptosis of primary cortical neuronal cells [Fig. 4(F)–(K)]. Bax protein expressions were significantly reduced by 65.86%, 58.84%, and 52.36% when treated with EAF (40, 8, 1.6 μg/mL). Bcl-2, an anti-apoptotic protein, can restrain the effect of Bax (Hu et al., 2014). The expressions of Bcl-2 at the mRNA level were 4.94-fold, 2.92-fold, and 1.99-fold higher in EAF (40, 8, 1.6 μg/mL) treated groups than in only the H2O2-treated group, respectively [Fig. 4(D)]. Furthermore, a Western blot assay showed that EAF (40, 8, 1.6 μg/mL) also led to an upregulation of Bcl-2 by 3.02-fold, 1.73-fold, and 1.30-fold. Meanwhile, EAF decreased Bax/Bcl-2 ratio (p ≤ 0.05) [Fig. 4(k)], and the ratio between HA and EAF (40 μg/mL) group showed no significant difference (p > 0.05) [Fig. 4(k)]. Besides, EAF at the 40, 8, 1.6 μg/mL suppressed the caspase-9 and caspase-3 expression at the protein level (p ≤ 0.05). Especially, the caspase-9 and caspase-3 expression in the EAF treatment were more enhanced than in the only H2O2-treatment at the mRNA level. Zhang et al. (2019b) reported that the protective effect of Caffeic acid against A53T α-synuclein apoptosis might be, at least in part, mediated via modulating the expression of Bcl-2. These results evidenced that EAF treatment inhibited H2O2-induced primary neuronal apoptosis.
In summary, our findings have demonstrated that EAF effectively prevents primary cortical neuronal cell damage induced by oxidative. This protection may involve its antioxidant, anti-inflammatory, and anti-apoptotic properties. Our discovery reveals the molecular mechanism underlying the neuroprotective properties of EAF. 5 phenolic compounds were identified from EAF, and among them, 4 compounds have been reported with neuroprotective effects. These results suggested that EAF and its active natural products could be designed and developed as a potential agent for neurological diseases.
References
Al Olayan EM, Aloufi AS, Alamri OD, El-Habit OH, Abdel Moneim AE. Protocatechuic acid mitigates Scadmium-induced neurotoxicity in rats: role of oxidative stress, inflammation and apoptosis. The Science of the Total Environment. 723: 137969 (2020)
Bucchieri F, Marino Gammazza A, Pitruzzella A, Fucarino A, Farina F, Howarth P, Holgate ST, Zummo G, Davies DE. Cigarette smoke causes caspase-independent apoptosis of bronchial epithelial cells from asthmatic donors. PloS ONE. 10: e0120510 (2015)
Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annual Review of Biochemistry. 75: 333-366 (2006)
Choi DJ, Cho S, Seo JY, Lee HB, Park YI. Neuroprotective effects of the Phellinus linteus ethyl acetate extract against H2O2-induced apoptotic cell death of SK-N-MC cells. Nutrition Research. 36: 31-43 (2016)
Choi JR, Kim JH, Lee S, Cho EJ, Kim HY. Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer's disease mouse model. Food and Chemical Toxicology. 144: 111571 (2020)
Chu Z, Ma G, Sun X, Xu Z, Zhang J. Grape seed extracts inhibit the overexpression of inflammatory cytokines in mouse retinas and ARPE-19 cells: potentially useful dietary supplement for age-related eye dysfunction. Journal of Medicinal Food. 23: 499-507 (2020).
Daroi PA, Dhage SN, Juvekar AR. p-Coumaric acid mitigates lipopolysaccharide induced brain damage via alleviating oxidative stress, inflammation and apoptosis. Journal of Pharmacy and Pharmacology. 1–9 (2021a)
Daroi PA, Dhage SN, Juvekar AR. p-Coumaric acid mitigates lipopolysaccharide induced brain damage via alleviating oxidative stress, inflammation and apoptosis. Journal of Pharmacy and Pharmacology. 1–9 (2021b)
Han Y, Nan S, Fan J, Chen Q, Zhang Y. Inonotus obliquus polysaccharides protect against Alzheimer's disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. International Journal of Biological Macromolecules. 131: 769-778 (2019)
Hu S, Wang J, Xu H, Wang Y, Li Z, Xue C. Fucosylated chondroitin sulphate from sea cucumber inhibits high-fat-sucrose diet-induced apoptosis in mouse pancreatic islets via down-regulating mitochondrial signaling pathway. Journal of Functional Foods. 7: 517-526 (2014)
Hu Q, Wang D, Yu J, Ma G, Pei F, Yang W. Neuroprotective effects of six components from Flammulina velutipes on H2O2-induced oxidative damage in PC12 cells. Journal of Functional Foods. 37: 586-593 (2017)
Huang L, Zhong X, Qin S, Deng M. Protocatechuic acid attenuates β‑secretase activity and okadaic acid‑induced autophagy via the Akt/GSK‑3β/MEF2D pathway in PC12 cells. Molecular Medicine Reports. 21: 1328-1335 (2020)
Jayasena T, Poljak A, Smythe G, Braidy N, Münch G, Sachdev P. The role of polyphenols in the modulation of sirtuins and other pathways involved in Alzheimer's disease. Ageing Research Reviews. 12: 867-883 (2013)
Jing L, Chen Z, Zhang Y, Zhang M, Zhu X, Yun F, Shi S, Ke Z, Liu Z. Rhein protects pancreatic β-cells from dynamin-related protein-1-mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 62: 3927-3935 (2013)
Li ZY, Chung YH, Shin EJ, Dang DK, Jeong JH, Ko SK, Nah SY, Baik TG, Jhoo JH, Ong WY, Nabeshima T, Kim HC. YY-1224, a terpene trilactone-strengthened Ginkgo biloba, attenuates neurodegenerative changes induced by β-amyloid (1-42) or double transgenic overexpression of APP and PS1 via inhibition of cyclooxygenase-2. Journal of Neuroinflammation. 14: 94 (2017)
Liang G, Shi B, Luo W, Yang J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behavioral and Brain Functions: BBF. 11: 18 (2015)
Liu K, Xiao X, Wang J, Chen CYO, Hu H. Polyphenolic composition and antioxidant, antiproliferative, and antimicrobial activities of mushroom Inonotus sanghuang. LWT - Food Science and Technology. 82: 154-161 (2017)
Liu C, Guo Y, Zhao F, Qin H, Lu H, Fang L, Wang J, Min W. Potential mechanisms mediating the protective effects of a peptide from walnut (Juglans mandshurica Maxim.) against hydrogen peroxide induced neurotoxicity in PC12 cells. Food & Function. 10: 3491-3501 (2019)
Mark, Delft, David, Huang. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Research. 16: 203 (2006)
Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metabolism. 27: 1176-1199 (2018).
Mcgeer PL, Mcgeer EG. Glial reactions in Parkinson's disease. Movement Disorders: Official Journal of the Movement Disorder Society. 23: 474-483 (2008)
Phan CW, David P, Naidu M, Wong KH, Sabaratnam V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: diversity, metabolite, and mechanism. Critical Reviews in Biotechnology. 35: 355-368 (2015)
Rong W, Ding K, Guo S, Xie F, Li Q, Bi K. Metabolomics analysis of Xanthoceras sorbifolia husks protection of rats against Alzheimer’s disease using liquid chromatography mass spectrometry. Journal of Chromatography B. 1126-1127: 121739 (2019)
Salau VF, Erukainure OL, Ibeji CU, Olasehinde TA, Koorbanally NA, Islam MS. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metabolic Brain Disease. 35: 727-738 (2020)
Smith MA, Perry G, Richey PL, Sayrec LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 382: 120-121 (1996)
Ullah R, Ikram M, Park TJ, Ahmad R, Saeed K, Alam SI, Rehman IU, Khan A, Khan I, Jo MG, Kim MO. Vanillic acid, a bioactive phenolic compound, counteracts LPS-induced neurotoxicity by regulating c-Jun N-Terminal kinase in mouse brain. International Journal of Molecular Sciences. 22: 361 (2020)
Wen Y, Wan YZ, Qiao CX, Xu XF, Wang J, Shen Y. Immunoregenerative effects of the bionically cultured Sanghuang mushrooms (Inonotus sanghuagn) on the immunodeficient mice. Journal of Ethnopharmacology. 245: 112047 (2019)
Wu SH, Dai YC. Species clarification of the medicinal fungus Sanghuang. Mycosystema. 39: 781-794 (2020)
Wu SH, Dai YC, Hattori T, Yu TW, Wang DM, Parmasto E, Chang HY, Shih SY. Species clarification for the medicinally valuable ‘sanghuang’ mushroom. Botanical Studies. 53: 135-149 (2012)
Wu C, Zhou W, Suthisisang C, Yang J. Traditional medicine for treatment of neurodegenerative diseases. Evidence-based complementary and alternative medicine : eCAM. 2014: 169821 (2014)
Xu F, Ren L, Song M, Shao B, Han Y, Cao Z, Li Y. Fas- and mitochondria-mediated signaling pathway involved in osteoblast apoptosis induced by AlCl 3. Biological Trace Element Research. 184: 173-185 (2018)
Zaitone SA, Ahmed E, Elsherbiny NM, Mehanna ET, El-Kherbetawy MK, Elsayed MH, Alshareef DM, Moustafa YM. Caffeic acid improves locomotor activity and lessens inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration: relevance to Parkinson's disease therapy. Pharmacological Reports: PR. 71: 32-41 (2019)
Zhang M, Xie Y, Su X, Liu K, Zhang Y, Pang W, Wang J. Inonotus sanghuang polyphenols attenuate inflammatory response via modulating the crosstalk between macrophages and adipocytes. Frontiers in Immunology. 10: 1-9 (2019a)
Zhang Y, Wu Q, Zhang L, Wang Q, Yang Z, Liu J, Feng L. Caffeic acid reduces A53T α-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacological Research. 150: 104538 (2019b)
Zhao Q, Cheng X, Wang X, Wang J, Zhu Y, Ma X. Neuroprotective effect and mechanism of Mu-Xiang-You-Fang on cerebral ischemia-reperfusion injury in rats. Journal of Ethnopharmacology. 192: 140-147 (2016)
Zhu HF, Yan PW, Wang LJ, Liu YT, Wen J, Zhang Q, Fan YX, Luo YH. Protective properties of Huperzine A through activation Nrf2/ARE-mediated transcriptional response in X-rays radiation-induced NIH3T3 cells. Journal of Cellular Biochemistry. 119: 8359-8367 (2018)
Acknowledgements
This study was supported by the Science Research Program for Colleges and Universities of Hebei Province (ZD2018067), the Key research and development project from Hebei Province (21327109D), Foundation of Hebei University of Economics and Business (2020ZD10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, K., Qi, C., Liu, Y. et al. Phenolic composition and neuroprotective effects of the ethyl-acetate fraction from Inonotus sanghuang against H2O2-induced apoptotic cell death of primary cortical neuronal cells. Food Sci Biotechnol 31, 1213–1223 (2022). https://doi.org/10.1007/s10068-022-01107-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-022-01107-x