Abstract
The purposes of this study were to evaluate the inactivation effects of intense pulsed light (IPL) on indigenous and inoculated microorganisms in fresh and minimally processed foods and the industrial applicability of this nonthermal sterilization method. The samples were treated with IPL by varying the treatment time and voltage. The inactivation effect tended to increase as the treatment conditions increased. Further, indigenous microorganisms showed a lower inactivation level than inoculated microorganisms, E. coli ATCC 25922, due to the variability of indigenous microorganisms and their properties. Chopped garlic showed a higher E. coli inactivation effect (2.65 log reduction after 0.185 J/cm2 of IPL) than peeled garlic (1.21 log reduction) due to its larger surface area. The manila clam showed a lower E. coli inactivation (0.93 log reduction) effect than squid (1.84 log reduction) due to its rougher surface. After the IPL treatment, there was no significant difference in temperature, moisture content, and color.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fresh and minimally processed foods have undergone minimal processing such as peeling, slicing, shredding, or chopping before being packaged for consumer use (Alarcón-Flores et al., 2014; Laurila and Ahvenainen, 2002). Since the destruction of the natural exterior barrier during processing fresh and minimally processed foods, the risk of bacterial growth and contamination increases (Alegbeleye et al., 2018; Li et al., 2017). So the processors are faced with the challenge to ensure the safety of fresh and minimally processed foods. Generally, fresh and minimally processed foods cannot be sterilized by heating because they must be kept fresh. Recently, non-thermal sterilization can be applied to ensure food safety and extend shelf life (Pareek, 2016).
Intense pulsed light (IPL) is a non-thermal sterilization technology using strong light of broad-spectrum in the 200–1100 nm range from ultraviolet ray to near-infrared ray in a short time (Oms-Oliu et al., 2010). IPL amplifies the power by accumulating energy in the capacitor for fractions of a second and then emitting it in the form of light in a short time between nanoseconds and milliseconds. This light inactivates microorganisms by damaging the microbial DNA or cell membrane (Ramos-Villarroel et al., 2012). In comparison with continuous UV light, IPL is a more efficient and fast method because it emits the same energy as UV in a very short time (Bohrerova et al., 2008; Gómez-López et al., 2012). Takeshita et al. (2003) observed vacuole swelling and membrane distortion in yeast cells after IPL treatment, while nearly identical to the control cell structure was shown after UV. Cheigh et al. (2012) found similar results in Listeria monocytogenes and E. coli O157:H7. The UV-C treated cells were similar in shape to the control except for blurry and unclear walls. On the other hand, the IPL treated cells showed destruction of cell structures such as cell walls and cytoplasmic membranes, resulting in cell death by leaking contents from the cytoplasm.
Several studies have been conducted on the microbial reduction of fresh foods using IPL. Huang and Chen (2018) achieved about 2.8 log reduction of Salmonella enterica on lettuce shreds after maximally 0.14 J/cm2 of IPL. In the study of Ramos-Villarroel et al. (2011), 2.97 log reduction of Listeria innocua and 3.33 log reduction of Escherichia coli were observed when the 0.4 J/cm2 of IPL was applied on fresh-cut avocado. Besides, many studies identified the efficacy of IPL on fresh foods like salmon fillets, various fruit of vegetables, etc., and they mentioned that IPL is a microbial inactivation technology which does not change the sensory and physical properties of the original state of foods (Bialka and Demirci, 2008; Holck et al., 2018; Lasagabaster et al., 2011; Ozer and Demirci, 2006; Pedrós-Garrido et al., 2018). As aforementioned, fresh and minimally processed foods are processed and sold in an easy-to-eat form, so their demand increases. However, unlike normal processed foods, they quickly deteriorate in quality. Therefore, this study tried to figure out the potential of IPL to the fresh food industry by applying it to sterilize peeled garlic, chopped garlic, squid, and manila clam. And whether significant quality changes occurred after IPL treatment was identified. Above all, in this study, the sterilization properties of IPL were investigated by comparing the level of inactivation of indigenous microorganisms in the food and artificially inoculated microorganisms on the food surface. This study is considered to be innovative because such research has not been carried out much yet.
Materials and methods
Sample preparation
Two agricultural products (peeled garlic and chopped garlic) and two kinds of seafood (manila clam and squid) were used to compare the IPL effect in this study. Samples were standardized to similar sizes. Peeled garlic of the same shape and size (2 × 3 cm) was selected and cut into a height of 0.5 cm. The average weight of peeled garlic was 3.24 g. Chopped garlic was weighed in 3 g and spread in a circular shape with a height of 0.5 cm. Manila clam of similar shape and size was selected, and the average weight was 3.3 g. The squid was cut into the same size (1 × 6 cm), and the average weight was 3.1 g. The initial number of indigenous microorganisms of peeled garlic, chopped garlic, manila clam, and squid were 107, 105, 105, and 104 CFU/g, respectively.
Microbial community analysis by NGS (next generation sequencing)
The indigenous microbial community of four samples, peeled garlic, chopped garlic, squid, and manila clam, were analyzed using NGS (next generation sequencing). The analysis was commissioned by the Korea Research Institute of Biomedical Science (KRIBS).
DNA extraction
QIAamp DNA Stool Mini Kit (Catalog no. 51504, Qiagen, Hilden, Germany) was used to isolate and purify genomic DNA in the sample. The 200 mg of the sample was placed in a 2 mL microcentrifuge tube and vortexed for 1 min by adding 1.4 mL of ASL buffer (Qiagen), a stool lysis buffer, followed by a reaction of 70 °C for 5 min. After the reaction, the sample was vortexed for 15 s and centrifuged for 1 min. The supernatant was transferred to a 2 mL microcentrifuge tube, mixed with InhibitEX Tablet (Qiagen) to remove PCR (polymerase chain reaction) inhibitors from DNA extraction preparation, and reacted for 1 min. The supernatant obtained by centrifugation for 3 min was transferred to a 1.5 mL microcentrifuge tube and centrifuged again for 3 min. 15 μL of proteinase K was added to the sample, 200 μL of AL buffer (Qiagen), which promotes the lysis of the cell membrane, denaturation of proteins, DNA, and other macromolecules, was added and vortexed for 15 s, followed by reaction at 70 °C for 5 min. For elution of DNA, the sample is transferred to a QIAmp spin column (Qiagen) and centrifuged for 1 min. 500 μL of AW1 buffer (Qiagen), used as a wash buffer, was added and centrifuged for 1 min. The lower solution was discarded, and 500 µl of AW2 buffer (Qiagen), another wash buffer, was added and centrifuged for 3 min. After drying the membrane by centrifugation for 1 to 2 min at 12,000 rpm, the collection tube was discarded. The sample was transferred to a recovery tube and 100 µL of AE buffer (Qiagen), which elutes the DNA from the spin column membrane into the microcentrifuge collection tube, was centrally dispensed. After incubating for 1 min at room temperature, DNA was extracted by centrifugation at 12,000 rpm for 1 min, and the total DNA concentration was quantified. The extracted DNA was used as template DNA for NGS analysis.
NGS analysis process
The extracted DNA went through a library preparation process, which is the first step of NGS. PCR is the first step of the library preparation process. When the libraries were prepared after PCR, they were checked for quality and quantified using Bioanalyzer (Agilent Technologies, Waldbronn, Germany). The samples were then loaded onto the sequencer iSeq 100 (Illumina, San Diego, CA, USA). After that, data analysis and visualization were performed using the BaseSpace Sequence Hub (Illumina).
Microorganism inoculation
Microorganism
Escherichia coli O157: H7 is one of the representative microorganisms that need to be taken care of concerning overall food contamination. In this study, E. coli ATCC 25922 (KCCM 11234) was used as a surrogate for E. coli O157: H7. The strains were grown in tryptic soy broth (Difco, Sparks, MD, USA) by shaking incubator (HB-201SF, Hanbaek scientific co., Gyeonggi, Korea) for 24 h at 37 °C, 250 rpm. Then they were transmitted to 10 mL tryptic soy broth and incubated at 37 °C for 5 h. Thereafter, centrifuging them at 4000 rpm for 10 min and washing the pellet in 1 mL sterile 0.85% NaCl solution were conducted. The supernatant was discarded and the cell pellet was stored in a 4 °C refrigerator after washing in sterile 0.85% NaCl solution.
Inoculation of microorganism
Escherichia coli ATCC 25922 (KCCM 11234) was uniformly inoculated on the samples’ surface to adjust the initial bacterial count to 107 CFU/g to evaluate the microbial inactivation. Before IPL treatment, the inoculated samples were stored at room temperature for 30 min to allow adequate penetration for the E. coli ATCC 25922 (KCCM 11234). Before the IPL treatment, it was confirmed that the initial number of microorganisms in the control samples was 107 CFU/g.
IPL treatment
This study's IPL device consists of three parts: Pulse lamp controller, temperature controller, and treatment chamber (Hwang et al., 2019). The pulse lamp controller is a part that can control the treatment conditions such as voltage, treatment time, frequency, and pulse duty. The voltage can be output up to 2400 V. The pulse duty can be adjusted to 0.5, 1.3, 2.1, and 3.0 ms. The pulse lamp controller is designed to operate only when the temperature controller is turned on. The temperature controller prevents the overheating of the lamp. The lamp is in the quartz tube filled in distilled water, and this water, allowing the lamp to maintain a temperature of 20–23 °C due to the temperature controller. The treatment chamber contains the lamp, a fan, and a plate on which the sample is placed. In this study, the NL9553 lamp (XAP series, Heraeus Noblelight, Cambridge, UK) was used. The length of the lamp was 27.1 cm, and the diameter was 1.1 cm. The inside of the lamp is filled with xenon gas, and the outside is surrounded by the quartz tube. The lamp is located at the uppermost part of the chamber. The fan is located on either side of the lamp. It helps to blow out the gas inside the chamber. Both chamber walls have grooves for inserting a rectangular plate to adjust the distance between the sample and the lamp.
IPL treatment was performed at different times (1, 3, 5, and 7 min) and voltages (1200, 1600, and 2000 V). The distance between the sample and the lamp was 13 cm, and the energy fluence of IPL applied to the sample was measured by a spectroradiometer (ILT950, International Light Technologies, Peabody, MA, USA) according to the voltage (1200, 1600, and 2000 V), and their fluence value were 0.134, 0.162, and 0.185 J/cm2·pulse, respectively.
After the treatment, the samples' pieces were transmitted to a stomacher bag (B01196, Nasco Whirl–Pak, Wisconsin, USA) with 27 mL of sterile 0.85% NaCl solution and then homogenized by stomacher (Tianjin Hengao, Technology Development Co. Ltd, Tianjin, China) for 2 min at 9 h/s. The homogenized solution was diluted in sterile 0.85% NaCl up to 106. Further, 0.1 mL of the diluted solution was spread on the plate count agar (Difco™, Sparks, MD, USA) and incubated at 37 °C for 48 h. The log reduction value was calculated using the ratio of the surviving number of microorganisms (N) to the initial number of microorganisms (N0).
Physical properties of the samples
Temperature, moisture content, and color change were measured to determine whether significant differences occurred in the sample's physical properties after the IPL treatment. The temperature change of samples before and after the IPL treatment was measured by the TES-1312A digital thermometer (TES Electrical Electronic Corp., Taipei, Taiwan). Moisture content change of samples before and after the IPL treatment was measured by an IR water content analyzer (FD-660, Kett Electric Laboratory, Tokyo, Japan). The measurement was replicated three times. The color change of samples before and after the IPL treatment was measured by colorimeter (Color Quest XE, Hunter Lab, Reston, VA, USA). The measurement was replicated three times. The measured color was expressed in L*a*b* values. These values are an international standards for color measurement developed by the CIE (Commission Internationale de L’Eclairage) in 1976. L* value means the lightness, ranging from 0 to 100. a* and b* values are chromatic components and a range from − 120 to + 120: a* represents redness from green to red, and b* represents yellowness from blue to yellow (Yam and Papadakis, 2004). The total color difference (ΔE) was calculated by the equation below.
Statistical analysis
All experiments were conducted in triplicate and data were analyzed using Excel 2016 (Microsoft, Redmond, WA, USA).
Results and discussion
Inactivation of inoculated E. coli ATCC 25922 after IPL treatment
Figure 1 showed the inactivation of E. coli ATCC 25922 on four samples with IPL treatment. The log reduction values of samples according to the treatment time and voltage were as follows. At the minimum treatment time (1 min), peeled garlic showed 0.44, 0.61, and 0.76 log reduction as the voltage increased from 1200 to 1600 and 2000 V, respectively. At the maximum treatment time (7 min), it showed 0.94, 1.09, and 1.21 log reduction as the voltage increased. When the voltage increased, the chopped garlic showed 0.50, 1.48, and 1.45 log reduction at the minimum treatment time (1 min), and 2.05, 1.99, and 2.65 reductions at the maximum time (7 min). Squid showed 0.69, 0.66, and 0.54 log reduction when treated for 1 min, 1.43, 1.99, and 1.84 log reduction when treated for 7 min. Manila clam treated for 1 min showed 0.18, 0.34, and 0.44 log reduction, and treated for 7 min showed 0.30, 0.43, and 0.93 log reduction. Though the log reduction values were different, the inactivation effect of the E. coli ATCC 25922 showed a tendency to increase as the treatment time and voltage increased in all four samples. The results were divided into two categories and interpreted: agricultural products and seafoods.
Peeled garlic only exceeded 1 log reduction at the maximum treatment condition (2000 V, 7 min), while chopped garlic showed almost 3 log reduction. This difference can be explained by the difference in surface area of the two samples. The larger the sample's surface area, the larger the area that light reached (Kim et al., 2008). Several studies identify the relationship between microbial reduction and surface property (they were mentioned below), but there is no study about the effect of degree of surface area on microbial reduction. In this study, the weight of chopped garlic and peeled garlic were the same as each other as 3 and 3.24 g, respectively. So, it is assumed that chopped garlic's microbial reduction effect was higher than peeled garlic because of the wider surface area. The wider surface area increases the exposure possibility to the IPL.
In squid and manila clam, squid showed almost 2 log reduction at the maximum treatment condition (2000 V, 7 min), while manila clam did not exceed 1 log reduction. The shadow effect can explain this. Since the IPL has low penetration power, the effect of IPL treatment depends on the sample's surface properties (Kim et al., 2019). Some studies have shown that if the surface of the sample is rough or uneven, the microorganisms have an increased chance of hiding in the crevices, which reduces the efficiency of IPL (Oms-Oliu et al., 2010; Cheigh et al., 2013; Elmnasser et al., 2007). The shadow effect refers to this phenomenon. That is why IPL is more suitable for surface microbial contamination of food with a smooth surface. Manila clam had a rough surface while the squid had a smooth surface, comparing the surface roughness of squid and manila clam. Therefore, E. coli on the manila clam tended to hide from the light, which resulted in a lower IPL sterilization effect.
NGS analysis
Figure 2 showed the NGS analysis results at the genus-level to identify the microbial community for the four samples. In the case of peeled garlic, Calothrix dominated 54.84%, followed by Thermogemmatispora (33.87%), Weissella (6.45%), and Leuconostoc (3.23%). In the chopped garlic, its microbial community was similar with peeled garlic, but the ratio was different. Leuconostoc dominated 26.35%, followed by Calothrix (25.19%), Thermogemmatispora (19.81%), Weissella (16.54%) and Lactobacillus (8.85%). For manila clam, Psychrilyobacter dominated 18.82%, followed by Lactobacillus (18.47%) and Acidaminococcus (10.45%). The squid had no similarity with the manila clam. Unlike the microbial community of manila clam mixed with various species, Photobacterium accounted for 89.75% of squid's total microbial community. Shewanella (4.51%) and Vibrio (2.46%) followed it. The NGS analysis at species-level was shown in Fig. 3. In peeled garlic, Calothrix parietina dominated 87.18%, followed by Weissella salipiscis (6.41%) and Leuconostoc pseudomesenteroides (2.56%). Like peeled garlic, chopped garlic was dominated by Calothrix parietina (87.18%), followed by Weissella salipiscis (24.75%), Leuconostoc garlicum (10.85%) and Leuconostoc pseudomesenteroides (3.05%). For manila clam, Lactobacillus japonicus dominated 21.62%, followed by Peptostreptococcus stomatis (15.14%), Phascolarctobacterium succinatutens (14.59%), Acidaminococcus intestine (12.97%), and Psychromonas marina (8.65%). For squid, Photobacterium aquimaris dominated 88.56%, followed by Photobacterium kishitanii (8.46%) and Yersinia massiliensis (1.00%).
As mentioned above, there is no research comparing inactivation levels between inoculated and indigenous microorganisms in the foods after IPL. Therefore, in this study, the four samples' NGS analysis was conducted, and each indigenous microorganisms were identified. Thereafter, their inactivation level after IPL was comprehensively observed to compare the inactivation level of inoculated E. coli.
Inactivation of indigenous microorganisms after IPL treatment
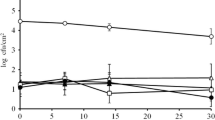
The results of the inactivation effect of indigenous microorganisms on four samples after IPL treatment were shown in Fig. 4. As the treatment time was 1 min and the voltage increased from 1200 to 1600 and 2000 V, peeled garlic showed 0.45, 0.78, and 0.59 log reduction. Under the same conditions, chopped garlic showed 0.28, 0.31, and 0.24 log reduction, squid showed 0.14, 0.47, and 0.38 log reduction, and manila clam showed 0.31, 0.53, and 0.74 log reduction. When the treatment time was 7 min, and the voltage increased from 1200 to 1600 and 2000, the log reduction was as follows. Peeled garlic showed 0.44, 0.76, and 1.33 log reduction, chopped garlic showed 0.36, 0.39, and 0.65 log reduction, squid showed 0.40, 0.33, and 0.59 log reduction, and manila clam showed 0.59, 0.97, and 1.13 log reduction. As in the samples inoculated with E. coli ATCC 25922, the inactivation effect of the indigenous microorganisms tended to increase with increasing IPL treatment time and voltage. However, the degree of increase was not visible, and this difference is considered to be the following reasons.
First, the microbial community present in each sample was different. The previous result was inoculating with E. coli ATCC 25922 in all four samples to investigate only one microbial inactivation. But in fact, each sample had different types of microorganisms, as mentioned above. The IPL effect was also affected by the microorganism type. Nicorescu et al. (2013) found that Bacillus subtilis was more resistant to IPL than yeasts like Saccharomyces cerevisiae because B.subtilis was protected by a resistant wall, while the yeast was protected only by a membrane. Anderson et al. (2000) observed that Gram-positive bacteria, such as Bacillus cereus, are less susceptible to UV-induced damage due to the recovery mechanisms against UV. On the other hand, gram-negative bacteria, such as E. coli, are more sensitive to IPL treatment damage than gram-positive bacteria. Furthermore, fungal spores with protective pigments, such as Aspergillus niger, are more resistant to IPL treatment than bacteria. Even though the sensitivity of all microorganisms present in the four samples to IPL has not been confirmed in this study, it could be considered that the microbial inactivation effect was different because of the various microbial types of the sample.
Second, the samples inoculated on the surface with E. coli ATCC 25922 uniformly adjusted the initial bacterial counts to 107 CFU/g, while the initial bacterial counts of the non-inoculated samples were all different; peeled garlic was 107 CFU/g, chopped garlic was 105 CFU/g, the squid was 103–104 CFU/g, and manila clam was 104–105. Since IPL has low penetration, it is difficult to sterilize the bacteria existing inside the sample completely. Cheigh et al. (2013) treated the shrimp, salmon, and flatfish with IPL. As a result, samples with smooth surfaces showed a higher microbial inactivation effect than rough surfaces. Similar results were found in the study comparing the IPL treated radish and pak choi seeds. The fR values, indicating resistance to IPL treatment, were as follows: radish seeds were 24.50 J/cm2, and pak choi seeds were 20.81 J/cm2. To compare the surface roughness of radish seeds and pak choi seeds, three values of the root-mean-square roughness (Rq), average surface roughness (Ra), and 10-point height roughness (Rz) were compared. As a result, all three values were higher in radish seeds. This means that there is a positive correlation between the fR value and the sample's roughness (Kim et al., 2019). The thickness of the sample also affects the IPL effect. Hillegas and Demirci (2003) found that inactivation of Clostridium sporogenes decreased as honey depth increased from 2 to 8 mm. Uesugi and Moraru (2009) examined the IPL sterilization effect by varying the thicknesses of the sausages to 0.58, 1.19, 2.46, and 3.76 mm. Sharma and Demirci (2003) treated the different thicknesses (1.02, 1.92, 3.61, and 6.25 mm) of alfalfa seeds inoculated with E. coli O157: H7 using IPL. The results showed that the thicker the seed, the lower the inactivation of E. coli. Furthermore, the thicker the seed, the longer it took to inactivate the E. coli. This experiment also revealed that IPL treatment was effective for surface sterilization but could not completely kill bacteria that existed below the surface. These studies will account for the difference between the microbial reduction effect of peeled garlic and chopped garlic. When the samples were inoculated with E. coli, the microbial reduction effect of chopped garlic was significantly higher, and it was deduced to be related to the surface area as mentioned above. When the inoculation was performed, each sample was first placed in a petri dish and inoculated with E. coli on its surface. Therefore, the microbial reduction of chopped garlic, which exposed more microorganisms to the IPL due to its large surface area, was higher. On the other hand, in indigenous microorganisms cases, the microorganisms are present on the surface and inside the sample. In chopped garlic, the small particles cover the indigenous microorganisms, so the reduction effect was lower than that of peeled garlic.
Physical properties of the samples
The temperature rise and increase of moisture content of four samples with/without IPL treatment at the maximum IPL treatment condition (2000 V, 7 min) was shown in Table 1. In peeled garlic, the temperature increased by 1.9 °C at the maximum IPL treatment condition (2000 V, 7 min). The chopped garlic showed a temperature rise of 3.4 °C at the maximum treatment condition, and the squid showed a temperature rise of 3.6 °C. The manila clam showed a temperature rise of 5.8 °C when treated with IPL under maximum conditions. The result showed that there was no significant difference between the temperature of the control and treated samples. The result also showed no significant difference between the moisture content of the control and treated samples. The color difference of four samples with/without IPL treatment at maximum IPL treatment condition (2000 V, 7 min) was also identified. ΔE value refers to the total color difference of the sample and is classified as follows: not noticeable (0–0.5), slightly noticeable (0.5–1.5), noticeable (1.5–3.0), well visible (3.0–6.0), and great (6.0–12.0). Since all four samples have ΔE values between 0.5 and 1.5, the color difference after IPL treatment is ‘slightly noticeable,’ but no significant difference with control (Cserhalmi et al., 2006).
In conclusion, it is revealed that IPL does not affect the physical properties of these four samples. Many studies have indicated that proper IPL treatment does not significantly affect the physical properties of the food. Tomašević (2015) observed no change of the appearance, total taste, and odor scores of beef after the IPL treatment of 3.4 J/cm2. Hierro et al. (2011) also mentioned that IPL treatment up to 3.4 J/cm2 did not change in color, flavor, appearance, and odor in ham.These results showed the potential of the IPL to be applied to the actual food industry and provide a stepping stone to develop the IPL systems that can handle fresh and minimally processed foods.
In conclusion, this study aimed to investigate the microbial inactivation effect of IPL on peeled garlic, chopped garlic, squid, and manila clam. Microbial inactivation increased with increasing treatment time and voltage in both non-inoculated and E. coli inoculated samples. Comparing the inactivation of E. coli inoculated peeled garlic and chopped garlic, chopped garlic's IPL effect was higher. This is due to the larger surface area of the chopped garlic and the larger area of light reaching. Comparing the inactivation of E. coli inoculated squid and manila clam, the IPL effect of squid was higher. This is due to the shadow effect caused by the rough surface. Furthermore, inoculated microorganisms showed higher inactivation levels than that of indigenous microorganisms. Indigenous microorganisms exist not only on the surface but also inside the samples, so the probability that microorganisms can expose to IPL was low. And the types of indigenous microorganisms are various, their sensitivities to IPL are also various, so it is hard to estimate the inactivation level. As a result of measuring the temperature, moisture content, and color before and after the IPL treatment, there was no significant difference. Therefore, it could be seen that the IPL can inactivate microorganisms while maintaining the quality of food.
References
Alarcón-Flores MI, Romero-González R, Vidal JLM, González FJE, Frenich AG. Monitoring of phytochemicals in fresh and fresh-cut vegetables: A comparison. Food Chemistry. 142: 392-399 (2014)
Alegbeleye OO, Singleton I, Sant’Ana AS. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: a review. Food Microbiology. 73: 177-208 (2018)
Anderson JG, Rowan NJ, MacGregor SJ, Fouracre RA, Farish O. Inactivation of food-borne enteropathogenic bacteria and spoilage fungi using pulsed-light. IEEE Transactions on Plasma Science. 28: 83-88 (2000)
Bialka KL and Demirci A. Efficacy of pulsed UV‐light for the decontamination of Escherichia coli O157: H7 and Salmonella spp. on raspberries and strawberries. . Journal of Food Science. 73: M201-M207 (2008)
Bohrerova Z, Shemer H, Lantis R, Impellitteri CA, Linden KG. Comparative disinfection efficiency of pulsed and continuous-wave UV irradiation technologies. Water Research. 42: 2975-2982. (2008)
Cserhalmi Z, Sass-Kiss A, Tóth-Markus M, Lechner N. Study of pulsed electric field treated citrus juices. Innovative Food Science & Emerging Technologies. 7: 49–54 (2006)
Cheigh CI, Hwang HJ, Chung MS. Intense pulsed light (IPL) and UV-C treatments for inactivating Listeria monocytogenes on solid medium and seafoods. Food Research International. 54: 745-752 (2013)
Cheigh CI, Park MH, Chung MS, Shin JK, Park YS. Comparison of intense pulsed light-and ultraviolet (UVC)-induced cell damage in Listeria monocytogenes and Escherichia coli O157: H7. Food control. 25: 654-659 (2012)
Elmnasser N, Guillou S, Leroi F, Orange N, Bakhrouf A, Federighi M. Pulsed-light system as a novel food decontamination technology: a review. Canadian Journal of Microbiology. 53: 813-821 (2007)
Gómez-López VM, Koutchma T, Linden K. Ultraviolet and pulsed light processing of fluid foods, In Novel thermal and non-thermal technologies for fluid foods. Academic Press, pp. 185-223 (2012)
Hierro E, Barroso E, De la Hoz L, Ordóñez, JA, Manzano S, Fernández M. Efficacy of pulsed light for shelf-life ex-tension and inactivation of Listeria monocytogenes on ready-to-eat cooked meat products. Innovative food science and emerging technologies. 12: 275-281 (2011)
Hillegas SL, Demirci A. Inactivation of Clostridium sporogenes in. clover honey by pulsed UV-light treatment, In 2003 ASAE annual meeting. American society of agricultural and biological engineers (p. 1) (2003)
Holck A, Liland KH, Carlehög M, Heir E. Reductions of Listeria monocytogenes on cold-smoked and raw salmon fillets by UV-C and pulsed UV lightInnovative . Innovative Food Science & Emerging Technologies. 50: 1-10 (2018)
Huang R, Chen H. Evaluation of inactivating Salmonella on iceberg lettuce shreds with washing process in combination with pulsed light, ultrasound and chlorine. International journal of food microbiology. 285: 144-151 (2018)
Hwang HJ, Seo JH, Jeong C, Cheigh CI and Chung MS. Analysis of bacterial inactivation by intense pulsed light using a double-Weibull survival model. Innovative Food Science & Emerging Technologies. 56: 102185 (2019)
Kim DH, Lim JJ, Lee JJ, Jang HH, Jang DI, Lee SJ, Lee HJ, Min WG, Kwon SH, Kim, S. H. Bacteriocidal effects of ultraviolet irradiation for reducing bovine mastitis derived from environmental contamination. Korean Journal of Environmental Agriculture. 27: 435-440 (2008)
Kim SM, Hwang HJ, Cheigh CI, Chung MS. Bactericidal effect of intense pulsed light on seeds without loss of viability. Food Science and Biotechnology. 28: 281-287 (2019)
Lasagabaster A, Arboleya JC, De Maranon IM. Pulsed light technology for surface decontamination of eggs: Impact on Salmonella inactivation and egg quality. Innovative Food Science & Emerging Technologies. 12: 124-128 (2011)
Laurila E, Ahvenainen R. Minimal processing of fresh fruits and vegetables. Jongen, W. Fruit and Vegetable Processing. Improving quality. Boca Raton, USA & Cambridge, England. CRC Press & Woodhead Publishing Limited (pp. 288-309) (2002)
Li K, Weidhaas J, Lemonakis L, Khouryieh H, Stone M, Jones L, Shen C. Microbiological quality and safety of fresh produce in West Virginia and Kentucky farmers’ markets and validation of a post-harvest washing practice with antimicrobials to inactivate Salmonella and Listeria monocytogenes. Food Control. 79: 101-108 (2017)
Nicorescu I, Nguyen B, Moreau-Ferret M, Agoulon A, Chevalier S, Orange N. Pulsed light inactivation of Bacillus subtilis vegetative cells in suspensions and spices. Food control. 31: 151-157 (2013)
Oms-Oliu G, Martín-Belloso O, Soliva-Fortuny R. Pulsed light treatments for food preservation. A review. Food and Bioprocess Technology. 3: 13-23 (2010)
Ozer NP, Demirci A, Inactivation of Escherichia coli O157: H7 and Listeria monocytogenes inoculated on raw salmon fillets by pulsed UV‐light treatment. International Journal of Food Science and Technology. 41: 354-360 (2006)
Pareek, S. (Ed.). Fresh-cut fruits and vegetables: technology, physiology, and safety. CRC Press (2016)
Pedrós-Garrido S, Condón-Abanto S, Clemente I, Beltrán J, Lyng J, Bolton D, Brunton N, Whyte P. Efficacy of ultraviolet light (UV-C) and pulsed light (PL) for the microbiological decontamination of raw salmon (Salmo salar) and food contact surface materials. International Journal of Food Science and Technology. 50: 124-131 (2018)
Ramos-Villarroel AY, Martín-Belloso O, Soliva-Fortuny R. Bacterial inactivation and quality changes in fresh-cut avocado treated with intense light pulses. European Food Research and Technology. 233: 395-402 (2011)
Ramos-Villarroel AY, Aron-Maftei N, Martín-Belloso O, Soliva-Fortuny R. The role of pulsed light spectral distribution in the inactivation of Escherichia coli and Listeria innocua on fresh-cut mushrooms. Food Control. 24: 206-213 (2012)
Sharma R, Demirci A. Inactivation of Escherichia coli O157: H7 on inoculated alfalfa seeds with pulsed ultraviolet light and response surface modeling. Journal of Food Science. 68: 1448-1453 (2003)
Takeshita K, Shibato J, Sameshima T, Fukunaga S, Isobe S, Arihara K. Itoh M. Damage of yeast cells induced by pulsed light irradiation. International Journal of Food Microbiology. 85: 151-158 (2003)
Tomašević I. The effect of intense light pulses on the sensory quality and instrumental color of meat from different animal breeds. Biotechnology in Animal Husbandry. 31: 273-281 (2015)
Uesugi AR, Moraru CI, Reduction of Listeria on ready-to-eat sausages after exposure to a combination of pulsed light and nisin. Journal of Food Protection. 72: 347-353 (2009)
Yam KL, Papadakis SE. A simple digital imaging method for measuring and analyzing color of food surfaces. Journal of Food Engineering. 61: 137-142 (2004)
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A3B03931649) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (317030-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, HJ., Park, JY., Chung, MS. et al. Microbial inactivation in fresh and minimally processed foods by intense pulsed light (IPL) treatment. Food Sci Biotechnol 30, 939–948 (2021). https://doi.org/10.1007/s10068-021-00937-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-00937-5