Abstract
The bacteriophages (phages) in the watery kimchis (Baek-kimchi and Dongchimi) were characterized to determine the phage ecology of lactic acid bacteria (LAB). Kimchi obtained from the Seoul markets had an average of 2.1 log phage particles/mL, corresponding to 28% of the bacterial counts on a log scale. High counts of 5.5–6.5 log particles/mL of phages were noted in the early phase of fermentation (reaching pH 4), and 2.1–3.0 log phage particles/mL were found in the later phase, with some fluctuation in numbers. The LAB hosts changed from Weissella and Leuconostoc to Lactobacillus during Dongchimi fermentation. Fifteen phages, except for those of Lactobacillus, were isolated from diverse strains in the early phase. Five Weissella phages were Podoviridae, and all 10 Leuconostoc phages were Myoviridae. Phages had narrow and different host infection spectra to strains of the same species and high acidic stability. Therefore, the mortality and diversity of LAB during natural kimchi fermentation may be related to the specific phages of the hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although there have been various studies on kimchi, a few have focused on the bacteriophages (phage) present in it; thus, the population and characteristics of lactic acid bacteria (LAB) phages in kimchi require investigation. LAB exist widely in nature and, as starters for fermentation, are known to produce organic acids for flavoring and food preservation. There are various LAB involved in kimchi fermentation, comprising three primary genera (Weissella, Leuconostoc, and Lactobacillus) and 20 different species (Jung et al., 2014; Lee et al., 2017). Among the related LAB, there are some population dynamics in the community, due to the three stages of fermentation (early, middle, and late) determined by pH and titratable acidity (TA). During the fermentation process, succession occurs for the predominant genera Weissella, Leuconostoc, and Lactobacillus; Weissella and Leuconostoc are dominant in the early and middle phases, and Lactobacillus is dominant in the late stage because of its high acid tolerance (Lee et al., 2015b). Recently, kimchi microbiota has been analyzed using metagenomics, and phages have been detected during fermentation (Jung et al., 2011). Additionally, Weissella, Leuconostoc, and Lactobacillus phages have been detected during pickle fermentation (Lu et al., 2003). Therefore, phages have been suggested to affect LAB succession in kimchi (Jung et al., 2011; Lee et al., 2015b).
Phages are abundant and ubiquitous in nature; they are estimated that there are 1031 particles, which is 10 times the estimated number of bacteria (Weitz et al., 2012). More than 90% of phages belong to the dsDNA order Caudovirales, including the families Myoviridae, Siphoviridae, and Podoviridae (Raya and H’bert, 2009). Phages have lytic and lysogenic life cycles that substantially contribute to bacterial mortality and diversity in environmental ecosystems (Parsley et al., 2010). Typically, 20–50% of daily prokaryotic mortality arises from viral infections and is a major source of dissolved organic matter in nature (Weinbauer, 2004; Wommack and Colwell, 2000). Phages are now considered to be an important component and play crucial roles in bacterial ecology, even in LAB-fermented food (Marco et al., 2017). During cheese fermentation, phages attack the LAB starter so that low acid production delays cheese formation (Chopin et al., 1976). A phage infecting a Weissella cibaria starter was isolated from Nham sausage in Thailand, and LAB phages have been isolated from sauerkraut (Kleppen et al., 2012; Lu et al., 2012). Phages induce the destruction of the principal genes by phage transduction into the prophage and obtain foreign genes for superinfection immunity and resistance to environmental stresses (Fortier and Sekulovic, 2013; Modi et al., 2013). Such evolution may lead to population changes in the microecosystem during fermentation. However, there has been few research on the direct succession of LAB in complex microbes during natural fermentation.
Kimchi is a traditional fermented Korean dish that is known to be a health-promoting food (Cho et al., 2014; Jung et al., 2014). Kimchi is generally classified into two types based on water content: watery kimchi is primarily composed of kimchi cabbage and radish (but not red pepper) and contains organic acids in a carbonated water soup. The components besides cabbage and radish are salt, ginger, green onion, and garlic, according to the region and manufacturer. Watery kimchi was thought to be a good model for this study because the bacterial diversity is relatively simple, and the experimental approaches and analysis are easy. There are precise and rapid methods for phage quantification and detection, including flow cytometry, epifluorescence microscopy, and transmission electron microscopy (TEM), that do not require the phage host for a plaque assay (Brussarrd et al., 2000; Duhamel and Jacquet, 2006; Park et al., 2018). Thus, it would be possible to investigate the phage microbiota in kimchi using these methods to quantify direct total counts, and the acquired data would elucidate the role of phages in kimchi fermentation. Therefore, LAB and phages in watery kimchi were quantitatively analyzed, and their characteristics and roles were examined to gain insight into the kimchi fermentation process.
Materials and methods
Kimchi samples Thirty kimchi samples, including 15 cabbage kimchi (kimchi cabbage with red pepper), 12 Dongchimi (radish kimchi in water without red pepper), and three Baek-kimchi (cabbage kimchi in water without red pepper), were purchased from a market in the capital area of Seoul. Each kimchi contained cabbage or radish as its major component, and the samples were in different fermentation stages. For Baek-kimchi and Dongchimi fermentation experiments, samples were collected immediately after preparation by the kimchi producers. The major components in watery kimchi were cabbage (Baek-kimchi), radish (Dongchimi), garlic, carrot, green onion, ginger, pear, sugar, apple, and salt.
Enumeration of microbes and determination of TA The TA was determined by the lactic acid standard, according to Park’s method (Park and Lee, 2005). A 25 g sample was mixed with 225 mL of tris-ethylenediaminetetraacetic acid buffer, homogenized using a stomacher (B&F Korea, Seoul, Korea), diluted, and applied to the media. The total bacterial counts (TBCs) on the plate count agar (PCA; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) were determined after incubation at 37 °C for 24 h. For LAB determination, samples were applied to PCA with bromocresol purple (BCP) and grown overnight at 30 °C in an anaerobic jar (Becton, Dickinson and Company). Yellow colonies were selected and then confirmed as LAB on de Man, Rogosa, and Sharpe (MRS) agar (Oxoid, Hampshire, UK) (Lin et al., 2006).
Phage counting in commercial kimchi and Baek-kimchi was performed via epifluorescence microscopy after removing the bacteria with a 0.22-μm syringe filter (Millipore, MA, USA) (Ortman and Suttle, 2009). The sample was applied to a 0.02 µm Anodisc™ 25 (Whatman, Darmstadt, Germany) on a 0.45 µm cellulose nitrate membrane filter (Whatman) and filtered using negative pressure. Anodisc was stained with SYBR Green (Invitrogen, Carlsbad, CA, USA) in the dark and dried. ProLong™ Gold Antifade Reagent (Invitrogen) was applied to the surface. Phages were counted three times at each of the three spots using an epifluorescence microscope (Optika SRL, Ponderanika, Italy) at 1000 × magnification and then quantified according to the following equation:
where Nv = phage mL–1, Pt = total number of phage counted, Ft = total number of fields, At = total area filtered (μm2), Af = area of each field (μm2), Vt = volume of sample filtered (mL).
The phage count for Dongchimi fermentation was determined using a flow cytometer (Accuri C6; Becton, Dickinson, and Company), according to the manufacturer’s protocol. The fluorescence height (FL-H) and side scatter height (SSC-H) were used to determine the bacterial and viral spots (Park et al., 2018).
Isolation and identification of LAB phage hosts Among the acid-producing bacteria on BCP agar, 96 strains from Baek-kimchi and Dongchimi were employed as putative phage hosts. Before use, LAB were cultured anaerobically in MRS broth (Oxoid) at 30 °C overnight, and the culture was stored in glycerol stock at − 80 °C. For genus identification of the host, polymerase chain reaction (PCR) was performed using specific primers (Schillinger et al., 2008; Zhao et al., 2013). The selected LAB strains were identified further using 16S rRNA gene sequencing. The 16S ribosomal DNA was extracted using the AccuPrep Genomic DNA Extraction Kit (Bioneer, Daejeon, Korea) and amplified using preMix (Bioneer) and the universal primers 27F and 1492R (Pringsulaka et al., 2011). PCR products were sequenced by Bioneer and Macrogen (Seoul, Korea) and analyzed by comparing consensus sequences in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank) using the Basic Local Alignment Search Tool (Benson et al., 2002). A phylogenetic tree for evolutionary analysis was constructed using Mega 7 software (www.megasoftware.net) (Kumar et al., 2016).
For the microbial community analysis of LAB during Dongchimi fermentation, pyrosequencing (454 GS FLX system; Roche/454, Pleasanton, CA, USA) was performed, and the V1/V2 regions of 16S rRNA were amplified and analyzed (Nam et al., 2012).
Phage isolation and identification To isolate phages from kimchi samples via spot assay, 96 strains were employed as putative hosts. The samples were collected and the bacteria were removed using a 0.22-µm syringe filter (Millipore). Thereafter, 10 µL of solution was applied to each host in MRS broth and cultivated. The 50 µL culture solution was applied to an MRS agar plate overlaid with 0.6% soft agar and the host, and again cultivated at 28 °C in an anaerobic jar. Phages from the different plaques on MRS agar were isolated and stored in 10% glycerol stock at − 80 °C.
To differentiate the isolated phages, restriction enzyme mapping and protein pattern analyses were performed. To extract phage DNA, 8–9 log plaque forming unit (PFU) /mL phages were prepared and concentrated with polyethylene glycol (PEG; MW 8000; Sigma-Aldrich, St Louis, MO, USA). The phage concentrate was treated with DNase, RNase, proteinase K, and then chloroform:isoamyl alcohol:phenol (24:1:24) (Sigma-Aldrich). The purified phage DNA was concentrated again with isopropanol and ethanol and suspended in diethyl pyrocarbonate water (Bioneer). Next, the DNA was digested with restriction enzymes (Sigma-Aldrich) and separated via electrophoresis on a 1% agarose gel. The fragments were visualized using ultraviolet light after staining with ethidium bromide (1 μg/mL) (Lee et al., 2013). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed to analyze the structural proteins of the isolated phages. The phage lysate (concentrated to a titer of 1010 PFU/mL) was mixed with 5× sample buffer and incubated at 100 °C for 10 min. Proteins were separated using SDS-PAGE at 200 V for 60 min and 130 V for 30 min. The gel was stained with Coomassie Brilliant Blue G-250 (Bio-Rad, Hercules, CA, USA) for 1 h and decolorized with de-staining solution for 2 h (Ortman and Suttle, 2009).
Characterization of the isolated phage To analyze the host range, each isolated phage (titer of ≥ 109 PFU/mL) was spotted on a bacterial lawn and incubated at 30 °C overnight. Plaque formation indicated phage infection and lysis of the strain (Ortman and Suttle, 2009). The morphological characteristics of the isolated phages were analyzed via TEM. The phage solution was concentrated to 109–10 PFU/mL using 20% PEG (MW 8000; Sigma-Aldrich). To remove damaged phages from the concentrated phage solution, CsCl ultracentrifugation at 28,000×g was performed for 1 h. The purified phage was applied to a copper grid (200 mesh; Ted Pella, Inc., Redding, CA, USA) and dyed with 1% uranyl acetate. Purified phages were stained with 2% (w/v) uranyl acetate on a carbon-coated grid and then observed via TEM (H-7600; HITACHI, Tokyo, Japan) at 80 kV (Ackermann, 2009).
The one-step growth curve for latent and burst size was analyzed after cultivating infected hosts that were prepared after infection with phages for 10 min and then centrifuged to remove the uninfected phage. To determine the pH stability of the phages, they were exposed to the low pH of SM buffer for 30 and 60 min, followed by a spot assay. The number of plaques was compared to that in the control group.
Results and discussion
Microbial and phage populations in kimchi Thirty kimchi samples were collected from around the capital area of Seoul and analyzed for bacterial populations using conventional culture methods and epifluorescence microscopy for phage quantification (Table 1). The pH of kimchi ranged from 3.9 to 5.8 (mean pH 4.3) and the TA was 0.51–2.3% (mean 1.25%), which is mostly in the ripened status. Mheen and Kwon (1984) report that the best kimchi flavor is achieved at pH 4.2 and TA 0.7%. The TBC ranged from 5.1 to 8.3 log CFU/mL and the total LAB ranged from 5.2 to 8.9 log CFU/mL.
Phage counts using epifluorescence microscopy ranged from 0.2 to 3.6 log phage particles/mL and varied broadly according to the samples. A mean count of 2.1 log phage particles/mL was detected at each 7.5 CFU/mL of TBC and LAB counts regardless of kimchi type. Jung et al. (2011) report a surprisingly large amount of phage DNA in kimchi (7% of the genome). The high number of phage sequences indicates that many phages may exist as template phages or prophages in kimchi. However, fewer phages exist in kimchi than in other fermented food and environmental habitats (Kleppen et al., 2012; Lu et al., 2003; Park, 2017; Pringsulaka et al., 2011). Park et al. (2011) report that less complex viral communities appear in fermented food than in other environments. Therefore, the phage count found in kimchi was low compared to the bacterial population; it was approximately 28% of the TBC on a log scale. To the best of our knowledge, this is the first report of direct viral counts in kimchi.
Microbes and bacteriophages during Baek-kimchi and Dongchimi fermentation Baek-kimchi and Dongchimi were collected immediately after preparation by each producer and fermented at 7 °C and 20 °C for population analyses (Fig. S1). The pH during fermentation ranged from 3.8 to 6.4 for Baek-kimchi and from 3.8 to 6.0 for Dongchimi, which were within the ranges previously reported (Jeong et al., 2013; Kyung et al., 2015; Park et al., 2008). The TBC was 4.9–8.5 log CFU/mL for Baek-kimchi and 7–8 log CFU/mL for Dongchimi during fermentation. The viable counts of LAB on BCP agar were 4.8–8.1 log CFU/mL for Baek-kimchi and 5.3–7.6 log CFU/mL for Dongchimi. Therefore, the bacteria in Baek-kimchi and Dongchimi were nearly 100% LAB, which was in accordance with previous reports (Kyung et al., 2015; Park et al., 2008).
The phage count obtained via epifluorescence microscopy for Baek-kimchi was 4.0–5.5 log particles/mL at the beginning of fermentation and decreased to 0.3–2.1 log particles/mL at the later stage on day 15. Phage counts obtained using flow cytometry for Dongchimi were 6.5 log events/mL at the beginning and decreased to 2.8 log events/mL at the end, with some fluctuations, which might be due to host changes that are related to the succession of LAB during fermentation. Park et al. (2008) report the analyses of the viral metagenome in this environment, and suggest that the richness and quantity of viruses may be largely determined by the corresponding microbial community. Lu et al. (2012) also suggest that LAB mortality and bacterial ecology may be influenced by abundant and diverse phages in commercial cucumbers, affecting the dynamics of fermentation.
Therefore, the phage count decreased from a high number at the beginning of fermentation to a low number when an acidic pH was reached, and fluctuations were confirmed.
LAB population changes during Dongchimi fermentation To analyze microbial population changes during fermentation, pyrosequencing was performed to analyze the microbial community (Fig. 1). During fermentation at 7 °C, Leuconostocaceae and Lactobacillaceae were initially present at low numbers and then rapidly increased to become the principal LAB for further fermentation. At the end of fermentation, the dominant family was Lactobacillaceae, which rapidly increased in the same pattern as that under fermentation at 20 °C (data not shown).
For the genus analysis, Streptophyta from plants comprised more than 70% of the total at the beginning, and the proportions of Leuconostoc, Weissella, and Lactobacillus were very low, as shown in Fig. S2. However, Leuconostoc and Lactobacillus increased remarkably with the reduction of Streptophyta on day 1. In particular, Leuconostoc rapidly increased and comprised more than 45% of the total population. On day 3, Leuconostoc decreased, and Lactobacillus increased. Finally, Lactobacillus was the dominant genus at the end of fermentation. However, Lactobacillus was dominant from day 2 to the late stage of fermentation at 20 °C. To identify LAB species during fermentation, sequencing reads were further classified at the species level. The randomly isolated species in the early phase (day 1) were W. cibaria and Leu. lactis, whereas Leu. inhae, Leu. lactis, and Leu. kimchi were confirmed in the middle phase (days 3–7). Lac. brevis and Lac. plantarum were the dominant species at the later phase (data not shown).
Jeong et al. (2013) report that the genera Leuconostoc, Lactobacillus, Pseudomonas, Pantoea, and Weissella exist at the beginning of fermentation, and Leuconostoc is the dominant population during fermentation. LAB show succession among the three predominant genera of Leuconostoc, Lactobacillus, and Weissella in cabbage kimchi, with Weissella and Lactobacillus predominating at the late fermentation stage. Generally, the predominant genera Weissella, Leuconostoc, and Lactobacillus show succession during fermentation depending on the materials and fermentation temperature (Cho et al., 2006; Lee et al., 2015b) (Fig. 1).
Therefore, there were major population changes in Weissella, Leuconostoc, and Lactobacillus during Dongchimi fermentation, and sequential succession was confirmed.
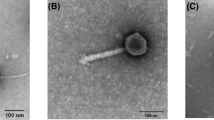
Characteristics of phages isolated during Baek-kimchi fermentation A total of 29 phages were isolated from 19 host strains at 7 °C by day 3. The hosts for the phages were identified as four Weissella spp. and 15 Leuconostoc spp. using specific PCR primers for the genera. Among the 29 phages, seven showing a high titer of 9 log PFU/mL were selected. The phages were identified by their morphologies as three Weissella (all Podoviridae) and four Leuconostoc (all Myoviridae) using TEM (Fig. 2). The phage hosts were identified using 16S rRNA sequencing, and three W. cibaria and four Leu. citreum strains were confirmed using phylogenetic trees (data not shown).
The head diameter sizes for Leuconostoc phages ranged from 41 to 49 nm and the tails were 110–126 nm long with 78–100 PFU/infected cells from burst size analysis. Regarding the host range analysis, these phages infected only four Leu. citreum strains among seven Leu. citreum, eight Weissella spp., five Lactobacillus spp., two Lactococcus spp., and one Leuconostoc sp. The narrow host spectrum seemed to be different from that reported in other studies (Lu et al., 2012; Pujato et al., 2017). ФLC093 and ФLC219 among the Leuconostoc phages had high pH stabilities even after exposure to pH 2.0 for 30 min, with 15% and 52% survival, respectively (Fig. 2).
The characteristics of Weissella phages have been reported in a previous paper (Kong and Park, 2020).
Profile and characteristics of phages during Dongchimi fermentation A total of 10 Dongchimi phages were isolated from 17 host strains by day 3 using the same procedures as those for Baek-kimchi. The hosts were identified using 16S rRNA sequencing, and comprised two W. cibaria, 12 Leu. lactis, and three Leu. citreum (Fig. S3). Lactobacillus phages were not isolated on any day during Dongchimi fermentation. Interestingly, the host strains, even in the same species, were slightly different from the Baek-kimchi phage hosts in terms of their 16S rRNA sequences. The strains involved in natural fermentation have been reported to be very diverse and different, even in the same species (Ledormand et al., 2020; Lee et al., 2015a). Therefore, the strain diversity of starters might be necessary for successful fermentation in the presence of phages in kimchi microecosystems.
Initially, two phages were isolated from two W. cibaria hosts. Eight phages were isolated from Leu. lactis and Leu. citreum by day 3 at pH 4.0. No Lactobacillus phages were isolated at further stages of fermentation (Table 2). Two Weissella phages, Ф0D4.04 and Ф0D4.58, infected two different hosts, W. cibaria 0D.4.4 and W. cibaria 0D4.58, respectively, and did not infect the 16 other Leuconostoc spp. The structural and genetic differences in the Weissella phages were confirmed using SDS-PAGE and EcoR1/HindIII digestion. These phages were Podoviridae with a non-isometric oval head and a head diameter of 67–86 nm (Fig. 3: 1, 2). The phages showed typical Weissella morphologies described in previous reports (Kleppen et al., 2012; Kong and Park, 2020; Pringsulaka et al., 2011).
Eight phages were confirmed by restriction enzyme mapping, protein structure, and host range (data not shown). All the phages for Leuconostoc were Myoviridae with an isometric hexagonal head 41–57 nm in diameter and a contractile tail 105–165 nm long (Fig. 3: 3–10).
The pH stabilities in Weissella (Ф0D4.04 and Ф0D4.58) and four Leuconostoc (Ф1D4.01, Ф1D4.63, Ф2D4.26, and Ф3D4.40) phages were determined. These phages were stable at pH 3.0 for 30 min, and had similar stabilities to Leuconostoc phages from Baek-kimchi, except for Ф2D4.26 and Ф3D4.40 (Fig. S4). Jończyk et al. (2011) report that phages may generally be stable in acidic environments. In conclusion, the Dongchimi microbial community analysis suggested that Leuconostoc was dominant during fermentation, followed by Lactobacillus, as reported in other studies (Jeong et al., 2013; Kyung et al., 2015; Park et al., 2008). During fermentation, various Leuconostoc phages existed and might contribute to the mortality of the Leuconostoc population. In addition, the phages showed very limited host infections and did not infect other strains of the same species, which might lead to diverse starters during natural fermentation.
Therefore, Weissella and Leuconostoc phages might contribute to LAB succession by killing their hosts, and no Lactobacillus phages would be helpful for the predominance of Lactobacillus spp. at the end of fermentation.
References
Ackermann HW. Basic phage electron microscopy. Vol. 1, pp. 113–126. In: Bacteriophages Methods and Protocols. Clokie MRJ, Kropinski AM (eds). Humana Press, New York, USA (2009)
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL. GenBank. Nucleic Acids Research 30: 17-20 (2002)
Brussarrd CPD, Marie D, Bratbak G. Flow cytometric detection of viruses. Journal of Virological Methods 85: 175-182 (2000)
Cho J, Lee D, Yang C. Jeon J, Kim J, Han H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiology Letters 257: 262-267 (2006)
Cho SK, Eom HJ, Moon JS, Lim SB, Kim YK, Lee KW, Han NS. An improved process of isomaltooligosaccharide production in kimchi involving the addition of a Leuconostoc starter and sugars. International Journal of Food Microbiology 170: 61-64 (2014)
Chopin MC, Chopin A, Roux C. Definition of bacteriophage groups according to their lytic action on mesophilic lactic streptococci. Applied and Environmental Microbiology 32: 741-746 (1976)
Duhamel S, Jacquet S. Flow cytometric analysis of bacteria- and virus-like particles in lake sediments. Journal of Microbiological Methods 64: 316-32 (2006)
Fortier LC, Sekulovic O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence 4: 354-365 (2013)
Jeong SH, Jung JY, Lee SH, Jin HM, Jeon CO. Microbial succession and metabolite changes during fermentation of Dongchimi, traditional Korean watery kimchi. International Journal of Food Microbiology 164:46-53 (2013)
Jończyk E, Kłak M, Międzybrodzki R, Górski A. The influence of external factors on bacteriophages-Review. Folia Microbiologica 56: 191-200 (2011)
Jung JY, Lee SH, Jeon CO. Kimchi microflora: History, current status, and perspectives for industrial kimchi production. Applied Microbiology and Biotechnology 98: 2385-2393 (2014)
Jung JY, Lee SH, Kim JM, Park MS, Bae J, Hahn Y, Madsen EL, Jeon CO. Metagenomic analysis of kimchi, a traditional Korean fermented food. Applied and Environmental Microbiology 77:2264–2274 (2011)
Kleppen HP, Holo H, Jeon SR, Nes FI, Yoon SS. Novel Podoviridae family bacteriophage infecting Weissella cibaria isolated from kimchi. Applied and Environmental Microbiology 78: 7299-7308 (2012)
Kong SJ, Park JH. Acid tolerance and morphological characteristics of five Weissella cibaria bacteriophages isolated from kimchi. Food Science and Biotechnology 29: 873-878 (2020)
Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870-1874 (2016)
Kyung KH, Pradas EM, Kim SG, Kim KH, Choi, JJ, Cho JH, Chung CH, Barrangou R, Breidt F. Microbial ecology of watery kimchi. Journal of Food Science 80: M1031-1038 (2015)
Ledormand P, Desmassures N, Dalmasso M. Phage community involvement in the fermented beverages: An open door to technological advances? Critical Reviews in Food Science and Nutrition https://doi.org/https://doi.org/10.1080/10408398.2020.1790497 (2020)
Lee M, Song JH, Jung MY, Lee SH, Chang JY. Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiology 66: 173-183 (2017)
Lee ME, Jang JY, Lee JH, Park HW, Choi HJ, Kim TW. Starter cultures for kimchi fermentation. Journal of Microbiology and Biotechnology 25: 559-568 (2015a)
Lee SH, Jung JY, Jeon CO. Source tracking and succession of kimchi lactic acid bacteria during fermentation. Journal of Food Science 80: 1871-1877 (2015b)
Lee YD, Kim JY, Park JH. Characteristics of coliphage ECP4 and potential use as a sanitizing agent for biocontrol of Escherichia coli O157: H7. Food control 34: 255-260 (2013)
Lin WH, Hwang CF, Chen LW, Tsen HY. Viable counts, characteristic evaluation for commercial lactic acid bacteria products. Food Microbiology 23: 74-81 (2006)
Lu Z, Breidt F, Plengvidhya V, Fleming HP. Bacteriophage ecology in commercial sauerkrauft fermentations. Applied and Environmental Microbiology 69: 3192-3202 (2003)
Lu Z, Perez-Diaz IM, Hayes JS, Breidt F. Bacteriophage ecology in a commercial cucumber fermentation. Applied and Environmental Microbiology 78: 8571-8578 (2012)
Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Folign B, Ganzle M, Kort R, Pasin G, Pihlanto A. Health benefits of fermented foods: Microbiota and beyond. Current Opinion in Biotechnology 44: 94-102 (2017)
Mheen TI, Kwon TW. Effect of temperature and salt concentration on kimchi fermentation. Korean Journal of Food Science and Technology 16: 443-450 (1984)
Modi SR, Lee HH, Spina CS, Collins JJ. Antibiotic treatment expands the resistance reservoir and ecological network of the phage metagenome. Nature 499: 219-222 (2013)
Nam YD, Yi SH, Lim SI. Bacterial diversity of Cheonggukjang, a traditional Korean fermented food, analyzed by barcoded pyrosequencing. Food Control 28: 135-142 (2012)
Ortman AC, Suttle CA. Determination of virus abundance by epifluorescence microsopy. Vol. 1, pp. 87–95. In: Bacteriophages Methods and Protocols. Clokie MRJ, Kropinski AM (eds). Humana Press, New York, USA (2009)
Park EJ, Kim KH, Abell GCJ, Kim MS, Roh SW, Bae JW. Metagenomic analysis of the viral communities in fermented foods. Applied and Environmental Microbiology 77: 1284-1291 (2011)
Park SH, Lee JH. 2005. The correlation of physico-chemical characteristics of kimchi with sourness and overall acceptability. Korean Journal of Food and Cookery Science 21: 103-109 (2005)
Park SJ, Chang JH, Cha SK, Moon GS. Microbiological analysis of Dongchimi, Korean watery radish kimchi, at the early and mid-phase fermentation. Food Science and Biotechnology 17: 892-894 (2008)
Park WJ. Succession of lactic acid bacteria and bacteriophage during Dongchimi fermentation and bacteriophage characterization. MS thesis, Gachon University, Seongnam, Korea (2017)
Park WJ, Lim GY, Park JH. Enumeration of Weissella cibaria phage with cytometry, epiflurorescence microscopy, and plaque assay. Korean Journal of Food Science and Technology 50: 244-247 (2018)
Parsley LC, Consuegra EJ, Thomas SJ, Bhavsar J, Land AM, Bhuian NN, Mazher MA, Waters RJ, Wommack KE, Harper WF, Lies MR. Census of the viral metagenome within an activated sludge microbial assemblage. Applied and Environmental Microbiology 76: 2673-2677 (2010)
Pringsulaka O, Patarasinpaiboon N, Suwannasai N, Atthakor W, Rangsiruji A. Isolation and characterisation of a novel Podoviridae-phage infecting Weissella cibaria N 22 from Nham, a Thai fermented pork sausage. Food Microbiology 28: 518-525 (2011)
Pujato SA, Guglielmotti DM, Martinez-Garcia M, Quiberoni A, Mojica JM. Leuconostoc mesenteroides and Leuconostoc pseudomesenteroides bacteriophages: Genomics and cross-species host ranges. International Journal of Microbiology 257: 128-137 (2017)
Raya RLR, H’bert EM. Isolation of phage via induction of lysogens. pp. 23–32. In: Bacteriophages: Methods and Protocols Vol. 1: Isolation, Characterization, and Interactions. Clokie MRJ, Kropinski AM (eds). Human Press, New York, USA (2009)
Schillinger U, Boehringer B, Wallbaum S, Caroline L. Gonfa A, Huch M, Holzapfel WH, Franz CMAP. A geuns-specific PCR method for differentiation between Leuconostoc and Weissella and its application in identification of heterofermentative lactic acid bacteria from coffee fermentation. FEMS Microbiololgy 286: 222–226 (2008)
Weinbauer MG. 2004. Ecology of prokaryotic viruses. FEMS Microbiology Reviews 28: 127-181 (2004)
Weitz JS, Poisot T, Meyer JR, Flores CO, Valverde S, Sullivan MB, Hochberg ME. Phage-bacteria infection networks. Trends in Microbiology 21: 82-91 (2012)
Wommack KE, Colwell RR. Virioplankton: Viruses in aquatic ecosystems. Microbiology and Molecular Biology Reviews 64: 69-110 (2000)
Zhao YW, Wu ZF, Shen XQ, Weng PF, Chen JJ. Bacteria community analysis by quantitative real-time PCR of fermenting wax gourd and its changes of organic acids. Journal of Food Processing and Preservation 38: 1653–1659 (2013)
Acknowledgements
This research was supported by the National Research Foundation of Korea (Grant # 2020R1F1A107000111).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, WJ., Kong, SJ. & Park, JH. Kimchi bacteriophages of lactic acid bacteria: population, characteristics, and their role in watery kimchi. Food Sci Biotechnol 30, 949–957 (2021). https://doi.org/10.1007/s10068-021-00930-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-021-00930-y