Abstract
The objective of this study was to increase the bioavailability of Inula britannica (IB) through fermentation with probiotic Weissella cibaria D30, and to evaluate the chemical composition, viability, and anti-inflammatory effect of fermented I. britannica (FIB). IB was fermented with W. cibaria D30 at 37 °C for 24 h. FIB increased total phenolic content and decreased total flavonoid content of IB. 1-O-acetylbritannilactone and ergolide production, which are associated with the viability, increased from 1.38 to 4.13 μg/mg, and decreased from 5.24 to 0.94 μg/mg, in the control and FIB, respectively. In addition, the cell viability of RAW264.7 cells increased when pretreated with 400 μg/mL FIB. FIB inhibited the production of nitric oxide and proinflammatory cytokines by inhibiting NF-κB and MAPKs pathways. Therefore, FIB with W. cibaria D30 reduced the toxicity and increased the anti-inflammatory properties. These results indicate that FIB is a potential beneficial bioactive agent for functional foods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbs have been extensively used for medicinal and edible purposes, and their consumption has increased significantly over the decades (Hussain et al., 2016). They are easy to consume with tea or food and have health benefits after a long-term intake (Sharifi-Rad et al., 2015). The development of functional foods using medicinal herbs can create enormous economic value in a short time. Medicinal herbs are also stable and have few side effects (Hussain et al., 2016). However, the bioavailability of some medicinal herbs such as ginseng, depends on the intestinal microbiome. This finding has application in bioconversion technology which involves the use of heat, enzyme treatment, and fermentation (Lee and Paik, 2017).
Inula britannica (IB) is a traditional oriental herbaceous plant of East Asia used for the treatment of bronchitis, digestive disorders, and inflammation. Various studies have shown that IB extracts have antibacterial, antitumor, antihistamine, antioxidant, and anti-inflammatory activities (Bai et al., 2005; Han et al., 2001; Khan et al., 2010; Lee et al., 2013). The flowers of IB have been used in the manufacture of medicinal products, beverages, and cottage cheese (Lee et al., 2016a). Furthermore, the antibacterial effects of IB have been reported on methicillin-resistant Staphylococcus aureus and Helicobacter pylori strains (Lee et al., 2016b). IB contains various biologically active substance such as sesquiterpene lactones (1-O-acetylbritannilactone (ABL), 1,6-O,O-diacetylbritannilactone, and eroglide), taraxasteryl acetate, patuletin, axillarin, nepitrin, and quercetin (Khan et al., 2010). ABL has been reported to have multi-functional properties including anti-inflammatory, antibacterial, antihepatitis, and antidiabetic properties (Zhengfu et al., 2015). Sesquiterpene lactone ergolide, which is known as the main compound in IB, has anti-inflammatory, apoptotic, and cytotoxic effects (Khan et al., 2010). Ergolide has been shown to suppress inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), in addition to activating nuclear factor-kappa B (NF-κB) signaling (Han et al., 2001).
Probiotics are defined as useful microorganisms which can alter the human intestinal microbiota when ingested in a suitable amount (Lee et al., 2015). The metabolism of lactic acid bacteria (LAB) interacts with that of human nutrition in the intestine through β-glucosidase production. Plant metabolites are degraded by fermentation with probiotics or β-glucosidase treatment, leading to the enhancement of bioavailability and the reduction of bitterness through the production of aromatic compounds (Son et al., 2017). Foods that have been fermented using LAB include table olives, soybeans, ginseng, and wine. It has been reported that LAB eliminates bitterness and increase the production of isoflavone aglycone and aromatic compounds (Michlmayr and Kneifel, 2014). IB extract fermented with Lactobacillus plantarum KCCM 11613P has been shown to have an anti-microbial effect against S. aureus strains, and this antimicrobial effect was attributed to epicatechin (Bae et al., 2019).
Weissella cibaria is known as the main LAB involved in kimchi fermentation, and it has attracted increased attention as probiotic candidates (Lee et al., 2018). W. cibaria D30 was isolated from Korean kimchi, and its probiotic properties, antagonistic activity, and antioxidant effect have been demonstrated (Yu et al., 2019). In particular, W. cibaria D30 has been reported to have high β-glucosidase activity. In this study, IB was fermented with W. cibaria D30 (FIB), and its bioavailability, effect on cell viability, total phenolics, total flavonoids, and total sugar contents, and the production of ABL and eroglide were investigated. In addition, the viability and anti-inflammatory effects of FIB in RAW 264.7 cells were assessed.
Materials and methods
Preparation of IB and FIB extracts
IB was purchased from Kyungdong Market (Seoul, Korea). IB powder was diluted in 1000 mL of distilled water (1/10, w/v) and extracted by heating for 15 min at 121 °C. The extract was filtered through Whatman No. 2 paper (GE Healthcare, Chicago, IL, USA) using vacuum filtration. After lyophilization, the powder was stored at 4 °C.
Prior to fermentation, IB extract was adjusted to pH 6.5 using 5 M NaOH. W. cibaria D30 with probiotic potential was used for fermentation of IB (Yu et al., 2019). W. cibaria D30 was propagated in lactobacilli MRS broth (Becton, Dickinson and Company, Spark, MD, USA) for 24 h at 37 °C. The activated cultures were used for IB fermentation. IB extract (500 mL; 1/10, w/v) was inoculated with 3 mL of the inoculums (to 6 log CFU/mL), and cultures were incubated at 37 °C for 72 h in a shaking incubator at 50 rpm. The culture was then centrifuged at 10,000×g, 4 °C for 10 min. The supernatant was filtered through Whatman No. 2 paper using vacuum filtration, and lyophilized. The lyophilized extracts were stored at 4 °C and used for further study.
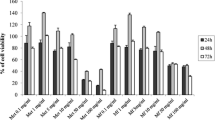
Cell viability of W. cibaria D30 during fermentation
The FIB was sampled at 0, 3, 6, 9, 12, 18, 24, 48, and 72 h during fermentation. The cell viability of W. cibaria D30 was determined by dilution of the cells, plating on MRS agar medium, and incubation at 37 °C for 24 h. Cell viability was presented as viable cell counts (log CFU/mL).
Total phenolic, flavonoid, and sugar contents
Total phenolic contents (TPC) were determined by the Folin-Ciocalteu assay (Singleton et al., 1999). Ninety microliters of 50% (v/v) Folin-Ciocalteu’s reagent (St. Louis, MO, USA), 90 μL of each extract, and 1.8 mL of 2% (w/v) Na2CO3 solution were mixed. After 30 min, the production of molybdenum oxide was measured at 752 nm. The contents of phenolic compounds were expressed as mg gallic acid equivalents (GAE)/g solid.
Total flavonoid contents (TFC) were determined by the aluminum chloride assay (Um et al., 2018). One hundred microliters of each extract, 20 μL of 5% NaNO2, and 800 μL of 60% ethanol were mixed and incubated for 6 min. The incubated mixture was added to 20 μL of 10% AlCl3 and 60 μL of 4% NaOH. After 30 min, the absorbance of the mixture was measured at 405 nm. The flavonoid content was expressed as mg quercetin equivalents (QE)/g of solid.
Total sugar contents (TSC) were determined by anthrone-sulfuric acid assay (Bae et al., 2019). Each extract (50 μL) was mixed with anthrone reagent and incubated in ice for 10 min. The mixtures were then boiled at 100 °C for 20 min and subsequently cooled at 25 °C. Next, 800 μL of water was added and absorbance measured at 620 nm. The reducing sugars content was expressed as mg sucrose equivalents (SE)/g of solid.
High-performance liquid chromatography (HPLC) of 1-O-acetylbritannilactone and ergolide
The lyophilized samples were dissolved in water and filtered through 0.20 μm membrane filters in preparation for HPLC. A liquid chromatograph (Waters 600 HPLC system, Waters Co., Milford, MA, USA) was used to analyze ABL (Chengdu Biopurify Phytochemicals Ltd., Chengdu, China) and ergolide (Chengdu Biopurify Phytochemicals Ltd.). The column used for the analysis was an Eclipse XDB-C18 (4.6 mm × 150 mm, 5 μm, Agilent Technologies Inc., Santa Clara, CA, USA). The mobile phase was composed of water with 0.1% ortho-phosphoric acid as eluent A and acetonitrile with 0.1% ortho-phosphoric acid as eluent B, and the elution gradient was as follows: 0–15 min: 90–48% A, 10–52% B; 15–20 min: 48% A, 52% B; 20–40 min: 48–20% A, 52–80% B; 40–50 min: 20–90% A, 80–10% B, at a flow rate of 0.5 mL/min. The detector was a Waters 2487 dual wavelength detector. ABL and ergolide were detected at 210 nm.
Cell culture
RAW 264.7 cells were purchased from the American Type Culture Collection (Rockville, MD, USA), and the cells were cultured in DMEM (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone) as well as 1% penicillin and streptomycin (Hyclone) at 37 °C in a 5% CO2 humidified incubator.
Measurement of cell viability of RAW 264.7 cells
Cell viability of RAW 264.7 cells was measured by using the MTT assay. The cells were seeded at 5 × 105 cells/well in 96 well plates. Seeded RAW 264.7 cells were pretreated with IB or FIB. After 2 h, the cells were incubated with lipopolysaccharide (LPS) (Sigma, St. Louis, MO, USA) at 37 °C in 5% CO2 for 24 h. After incubation at 37 °C for 2 h, MTT solution was removed and formazan crystals were dissolved in DMSO to obtain a colored solution. The absorbances of the samples were measured at 570 nm using a microplate reader and cell viability was calculated using the following equation. Negative control was defined treatment without LPS and sample.
Evaluation of anti-inflammatory effect
Measurement of nitric oxide (NO) production
NO concentration in the cultured medium was determined by using the Griess reaction assay (Yang et al., 2019). RAW 264.7 cells were seeded at 5 × 105 cells/well in 96-well plates. Seeded cells were pretreated with FIB at various concentrations. After 2 h, the cells were incubated with LPS at 37 °C in 5% CO2 for 24 h. The cultured medium (100 μL) was mixed with a 100 μL of Griess reagent and incubated for 15 min at room temperature. Absorbance was measured at 540 nm using a microplate reader. NO production was extrapolated from the standard curve generated from different concentrations of sodium nitrate.
Measurement of cytokine expression using quantitative real-time PCR
RAW 264.7 cells were seeded at 1 × 106 cells/well in 6-well culture plates and pre-incubated for 24 h. The cells were treated and incubated with various concentrations of FIB for 2 h. And then the wells were treated and incubated with or without LPS (1 μg/mL) for 24 h.
Total RNA was isolated using RNeasy mini kit according to the manufacturer (Qiagen, Hilden, Germany). The total RNA (2 μg) was converted to cDNA using RevertAid™ First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). The expression levels of induced cytokines associated with anti-inflammatory effects were determined by using the SYBR Green PCR Master mix with semi-quantitative real-time PCR (PikoReal 96, Scientific Pierce, Waltham, MA, USA). The primers used were β-actin (forward primer 5′-GTGGGCCGCCCTAGGCACCAG-3′ and reverse primer 5′-GGAGGAAGAGGATGCGGCAGT-3′), TNF-α (forward primer 5′-TTGACCTCAGCGCTGAGTTG-3′ and reverse primer 5′-CCTGTAGCCCACGTCGTAGC-3′), IL-1β (forward primer 5′-CAGGATGAGGACATGAGCACC-3′ and reverse primer 5′-CTCTGCAGACTCAAACTCCAC-3′), and IL-6 (forward primer 5′-GTACTCCAGAAGACCAGAGG-3′ and reverse primer 5′-TGCTGGTGACAACCACGGCC-3′). PCR was performed as follows: 95 °C for 2 min for polymerase activation, followed by 40 cycles of 95 °C for 5 s for denaturation and 60 °C for 15 s for annealing and extension. The results were analyzed by using the delta–delta Ct method. The melting curve was used to analyze the measurement of reaction specificity.
Western blot analysis
Western blot was carried out by the modified method of Kim et al. (2018). In NF-κB phosphorylation, RAW 264.7 cells were seeded at 5 × 105 cells/well in 6-well culture plates and pre-incubated for 24 h. Next, the cells were treated and incubated with various concentrations of FIB for 2 h. The wells were treated with or without LPS (1 μg/mL) for 24 h.
For MAPKs phosphorylation, RAW 264.7 cells were seeded at 1 × 106 cells/well in 6-well culture plates and pre-incubated for 24 h. Next, the cells were treated and incubated with various concentrations of FIB for 2 h. The wells were treated with or without LPS (1 μg/mL) for 2 h.
After washing the cells with ice-cold PBS, cellular proteins were obtained in RIPA buffer supplemented with protease and phosphatase inhibitors. Cell lysates were kept on an ice-bath for 2 min and sonicated at 10 Amps (pulse on, 5 s; pulse off, 5 s) thrice. After a further 20 min, cell lysates were centrifuged at 14,000×g, 4 °C for 20 min. The total protein concentration of harvested supernatants was measured by using DC ™ protein assay kit (Bio-Rad, Hercules, CA, USA). An aliquot (30 μg) of each lysate was separated with 8–12% SDS-PAGE and subsequently transferred onto PVDF membrane. The immunoblots were blocked with 5% skim milk in tris buffered saline with Tween 20 (TBS-T) buffer for 1 h at 25 °C. The immunoblots were incubated with appropriately diluted specific primary antibodies for 16 h at 4 °C. After brief washing with TBS-T, the immunoblots were incubated with secondary antibodies for 2 h at room temperature. Each blot was detected on x-ray blue film by using enhanced chemiluminescence (Bio-Rad). The intensity of blots was determined by using Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics version 18 software (IBM, New York, NY, USA). Data are shown as means ± standard deviations of three independent experiments. The mean values were compared using a one-way analysis of variance. Significant treatment means were separated by Tukey’s test; p < 0.05 was considered significant.
Results and discussion
Effect of fermentation of IB with W. cibaria D30
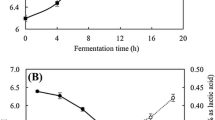
The cell viability and pH changes during fermentation of IB with W. cibaria D30 are described in Fig. 1A. The viable cell counts and pH changed from 6.05 to 9.33 log CFU/mL and 6.5 to 4.98 during fermentation, respectively. The viable cell counts increased until 9 h, and then decreased until 72 h. The decrease in the number of viable cells observed after 9 h was possibly due to the consumption of nutrients and production of antimicrobial metabolites such as organic acids. It has been reported that W. cibaria D30 produces organic acids including lactic acid and butyric acid as metabolites during fermentation (Yu et al., 2019).
Fermentaiton of IB with W. cibaria D30 and HPLC analysis. (A) Cell growth and pH of IB fermented with W. cibaria D30. ●, Total bacterial count in FIB; ■, pH value of FIB. (B) HPLC data of O-acetylbritannilactone (ABL) and ergolide contents of IB; FIB; and standard compound. (C) Concentration of ABL and ergolide. Peak identification: 1, ABL; 2, ergolide. ***p < 0.001, compared to IB
The phytochemical properties of IB and FIB are shown in Table 1. The TPC of IB and FIB was increased from 0.047 to 0.149 mg GAE/g (p < 0.001), whereas the TFC was decreased from 0.178 to 0.113 mg QE/g (p < 0.001) by fermentation. No significant difference was detected in the TSC of IB and FIB. IB contains steroids, terpenoids (sesquiterpene lactones, diterpenes, and triterpenoids), and phenolics (Khan et al., 2010). Fermentation of phenolic compounds of IB by LAB strains can lead to the production of the aglycone forms which are more bioactive (Đorđević et al., 2010; Lee and Paik, 2017). These results may lead to increase of TPC and decrease of TFC. IB can also be influenced by β-glucosidase or organic acids produced by W. cibaria D30 during fermentation.
ABL and ergolide as fermentation products of IB
ABL and sesquiterpene lactone ergolide produced by IB have been reported as cytotoxic agents to cancer cells (Song et al., 2005; Zhengfu et al., 2015). Ergolide induced apoptosis through DNA fragmentation, activation of caspase-3, cleavage of PARP, and cytochrome c release in Jurkat T cells (Song et al., 2002). Figure 1B–C shows the ergolide contents of IB and FIB, and the standard compounds identified by HPLC analysis. The regression curve of ABL was \({\text{y}} = 43,000\times {\text{x}} - 16,000 \left( {R^{2} = 0.9998} \right)\) and the retention time was 22.783 min. The regression curve of ergolide was \({\text{y}} = 32,300\times {\text{x}} + 910 \left( {R^{2} = 0.9999} \right)\) and the retention time was 27.49 min. ABL concentration significantly increased from 1.38 to 4.13 μg/mg by fermentation (p < 0.001). Fermentation significantly decreased the concentration of ergolide from 5.24 to 0.94 μg/mg (p < 0.001). The change in the concentrations of ABL and ergolide after fermentation could influence the viability and the anti-inflammatory effects of IB.
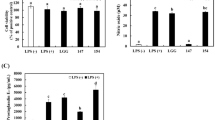
Effect of IB and FIB on cell viability in LPS-induced RAW 264.7 cells
The effect of IB and FIB (100–400 μg/mL) on the cell viability of RAW 264.7 cells was examined using the MTT assay (Table 2). IB treatment reduced the cell viability of RAW 264.7 cells in a dose-dependent manner. There was no significant difference in the viability of FIB-treated cells and control cells. Treatment with IB and FIB at 400 μg/mL resulted in cell viability of 31.73% (p < 0.001) and 94.53%, respectively. The concentration of IB should be kept below 200 μg/mL (< 80% viability) in in vitro anti-inflammatory assay; however, FIB could be used up to 400 μg/mL. Oh et al. (2012) also reported that the cytotoxicity of Oyaksungisan was lowered by fermentation with Lactobacillus spp. The increased viability of FIB could be attributed to the decreased concentration of ergolide. Further research is necessary to elucidate the mechanism behind the viability increment, as FIB contains various compounds that can interact to exert complementary effects.
Inhibitory effect of FIB on NO production and proinflammatory cytokines expression in LPS-induced RAW 264.7 cells
It has been demonstrated that the anti-inflammatory effects of medicinal herbs are due to the inhibition of expression of inflammatory cytokines (Han et al., 2011; Hussain et al., 2016). Han et al. (2011) reported the anti-inflammatory effects of non-fermented and fermented Sophora flavescens. IB effectively inhibits the production of NO at relatively low concentrations compared with other herbal plants (Lee et al., 2011). However, the anti-inflammatory effect of FIB has not been previously investigated. In this study, NO production was investigated in LPS-induced RAW 264.7 cells. FIB remarkably reduced NO production in a dose-dependent manner (Fig. 2A). In particular, FIB at 400 μg/mL inhibited NO production by more than 92.4% in treated cells compared with cells treated with LPS alone. These results indicate that FIB effectively suppressed the secretion of nitrite from LPS-stimulated RAW 264.7 cells. Lee et al. (2011) showed that IB dissolved in DMSO at 5 μg/mL inhibited NO production by 45.3%. The effect of IB is also influenced by extraction solvents.
Anti-inflammatory effects of FIB in lipopolysaccharide (LPS)-induced RAW 264.7 cells. (A) NO production (μM); relative intensity of (B) TNF-α; (C) IL-1β; (D) IL-6; (E) iNOS; (F) COX-2 expression. ***p < 0.001, compared to LPS only-treated cells; different letters indicate significant differences (p < 0.05) as determined by Tukey’s test
FIB influenced the expression of proinflammatory cytokines, TNF-α, IL-1β, IL-6, iNOS, and COX-2, produced by LPS stimulation (Fig. 2B–F). FIB effectively reduced mRNA expression of proinflammatory cytokines in a dose-dependent manner. In particular, 400 μg/mL of FIB showed a suppressive effect on the production of most proinflammatory cytokines. Of these cytokines, IL-1β, IL-6, and iNOS were inhibited at all the tested concentrations.
Effect of FIB on NF-κB activation in LPS-induced RAW 264.7 cells
NF-κB is a transcription factor that induces proinflammatory genes, such as iNOS, COX-2, TNF-α, IL-1β, and IL-6 (Kim et al., 2013). The influence of FIB on the expression of inflammatory mediators associated with the NF-κB pathway, p65 and IκBα were investigated (Fig. 3). Figure 3 shows the influence of FIB on NF-κB activated by phosphorylation upon LPS stimulation. LPS increased p-p65 and decreased IκBα. The expression level of p-p65 was markedly inhibited in the presence of FIB at a concentration of 400 μg/mL, and the inhibitory effect increased in a dose-dependent manner (Fig. 3B). p-p65 affected the levels of IκBα and p-IκBα in the cytoplasm (Fig. 3C–D). FIB significantly repressed IκBα phosphorylation in a dose–dependent manner, implying that FIB reduced IκBα degradation and NF-κB activation. These findings are consistent with the reports of other studies, indicating that NF-κB elements are present on the promoter of the iNOS, COX-2, TNF-α, IL-6, and IL-10 genes (Ahn et al., 2005; Barnes and Karin, 1997; Chen et al., 2000). Furthermore, ABL decreased NO production and iNOS expression in RAW 264.7 cells (Tavares and Seca, 2019).
Effect of FIB on protein expression level of NF-κB phosphorylation in LPS-treated RAW 264.7 cells. (A) NF-κB protein expression level was measured by western blot analysis. (B) p-p65 (normalized to p65) expressed. (C) p-IκBα (normalized to β-actin) expressed. (D) IκBα (normalized to β-actin) expressed.***p < 0.001, compared to only LPS-treated cells; different letters indicate significant differences (p < 0.05) as determined by Tukey’s test
Effect of FIB on the phosphorylation of MAPKs in LPS-induced RAW 264.7 Cells
MAPKs pathways are the major pathways induced by LPS in RAW 264.7 cells. Western blot analysis was carried out to investigate the effect of FIB on the phosphorylation of MAPKs induced by LPS stimulation (Fig. 4). FIB reduced the levels of p-JNK, p-ERK, and p-p38 (Fig. 4B–D). The expression levels of p-p38, p-ERK, and p-JNK were dramatically reduced by FIB at 400 μg/mL, in a dose-dependent manner. These results suggest that the inhibitory effect of FIB on the phosphorylation of MAPKs was involved in the decreased production of proinflammatory mediators in RAW 264.7 cells.
Effect of FIB on protein expression level of MAPKs phosphorylation in LPS-inducd RAW 264.7 cells. (A) MAPKs protein expression level was measured by western blot analysis. (B) p-JNK (normalized to JNK) expressed. (C) p-ERK1/2 (normalized to ERK1/2) expression. (D) p-p38 (normalized to p38) expressed. **p < 0.05, compared to LPS-only treated cells; ***p < 0.001, compared to LPS-only treated cells; different letters indicate significant differences (p < 0.05) as determined by Tukey’s test
In conclusion, the fermentation of IB with probiotic W. cibaria D30 were carried out, causing an increase in the levels of TPC and ABL but a decrease in the levels of TFC and ergolide. These results influenced the viability of RAW 264.7 cells and induced anti-inflammatory effects. FIB has higher viability than IB, which is mediated by its modified components. FIB at a higher concentration than that of IB exhibited anti-inflammatory effects at a lower toxicity by inhibiting the NF-κB and MAPKs pathways. Therefore, the fermentation of IB with W. cibaria D30 could lead to an improved anti-inflammatory medicinal herbal agent with higher viability.
References
Ahn KS, Noh EJ, Zhao HL, Jung SH, Kang SS, Kim YS. Inhibition of inducible nitric oxide synthase and cyclooxygenase II by Platycodon grandiflorum saponins via suppression of nuclear factor-κB activation in RAW 264.7 cells. Life Sci. 76: 2315-2328 (2005)
Bae WY, Kim HY, Kim KT, Paik HD. Inhibitory effects of Inula britannica extract fermented by Lactobacillus plantarum KCCM 11613P on coagulase activity and growth of Staphylococcus aureus including methicillin-resistant strains. J. Food Biochem. 43: e12785 (2019)
Bai N, Zhou Z, Zhu N, Zhang L, Quan Z, He K, Zheng QY, Ho CT. Antioxidative flavonoids from the flower of Inula britannica. J. Food Lipids 12: 141-149 (2005)
Barnes PJ, Karin M. Nuclear factor-κB—a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336: 1066-1071 (1997)
Chen YC, Yang LL, Lee TJ. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochem. Pharmacol. 59: 1445-1457 (2000)
Đorđević TM, Šiler-Marinković SS, Dimitrijević-Branković SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 119: 957-963 (2010)
Han CC, Wei H, Guo J. Anti-inflammatory effects of fermented and non-fermented Sophora flavescens: a comparative study. BMC Complem. Altern. M. 11: 100 (2011)
Han JW, Lee BG, Kim KY, Yoon JW, Jin HK, Hong S, Lee HY, Lee KR, Lee HW. Ergolide, sesquiterpene lactone from Inula britannica, inhibits inducible nitric oxide synthase and cyclo-oxygenase-2 expression in RAW 264.7 macrophages through the inactivation of NF-κB. Br. J. Pharmacol. 133: 503-512 (2001)
Hussain A, Bose S, Wang JH, Yadav MK, Mahajan GB, Kim H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 81: 1-16 (2016)
Khan AL, Hussain J, Hamayun M, Gilani SA, Ahmad S, Rehman G, Lee IJ. Secondary metabolites from Inula britannica L. and their biological activities. Molecules 15: 1562-1577 (2010)
Kim MJ, Kim KBWR, Jeong DH, Ahn DH. Anti-inflammatory activity of ethanolic extract of Sargassum sagamianum in RAW 264.7 cell. Food Sci. Biotechnol. 22: 1113-1120 (2013)
Kim HS, Yu HS, Lee JH, Lee GW, Choi SJ, Chang PS, Paik HD. Application of stabilizer improves stability of nanosuspended branched-chain amino acids and anti-inflammatory effect in LPS-induced RAW 264.7 cells. Food Sci. Biotechnol. 27: 451-459 (2018)
Lee NK, Han KJ, Son SH, EomSJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT-Food Sci. Technol. 64: 1036-1041 (2015)
Lee NK, Paik HD. Bioconversion using lactic acid bacteria: ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechn. 27: 869-877 (2017)
Lee NK, Jeewanthi RKC, Park EH, Paik HD. Physicochemical and antioxidant properties of Cheddar-type cheese fortified with Inula britannica extract. J. Dairy Sci. 99: 83-88 (2016a)
Lee NK, Lee JH, Lee YJ, Ahn SH, Eom SJ, Paik HD. Antimicrobial effect of Inula britannica flowers extract against methicillin-resistant Staphylococcus aureus. Korean J. Microbiol. Biotechnol. 41: 335-340 (2013)
Lee SE, Lee J H, Kim JK, Kim GS, Kim YO, Soe JS, Kim SY. Anti-inflammatory activity of medicinal plant extracts. Korean J. Med. Crop Sci. 19: 217-226 (2011)
Lee YH, Lee NK, Paik HD. Antimicrobial characterization of Inula britannica against Helicobacter pylori on gastric condition. J. Microbiol. Biotechn. 26: 1011-1017 (2016b)
Lee YJ, Lee A, Yoo HJ, Kim M, Noh GM, Lee JH. Supplementation with probiotic strain Weissella cibaria JW15 enhances natural killer cell activity in nondiabetic subjects. J Funct. Foods 48: 153-158 (2018).
Michlmayr H, Kneifel W. β-Glucosidase activities of lactic acid bacteria: mechanisms, impact on fermented food and human health. FEMS Microbiol. Lett. 352(1): 1-10 (2014)
Oh YC, Cho WK, Oh JH, Im GY, Jeong YH, Yang MC, Ma JY. Fermentation by Lactobacillus enhances anti-inflammatory effect of Oyaksungisan on LPS-stimulated RAW 264.7 mouse macrophage cells. BMC Complem. Altern. M. 12: 17 (2012)
Sharifi-Rad J, Hoseini-Alfatemi SM, Sharifi-Rad M, Da Silva JAT. Antibacterial, antioxidant, antifungal and anti-inflammatory activities of crude extract from Nitraria schoberi fruits. 3 Biotech 5: 677-684 (2015)
Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 299: 152-178 (1999)
Son SH, Jeon HL, Jeon EB, Lee NK, Park YS, Kang DK, Paik HD. Potential probiotic Lactobacillus plantarum Ln4 from kimchi: Evaluation of β-galactosidase and antioxidant activities. LWT-Food Sci. Technol. 85: 181-186 (2017)
Song QH, Kobayashi T, Hong T, Cyong JC. Effects of Inula britannica on the production of antibodies and cytokines and on T cell differentiation in C57BL/6 mice immunized by ovalbumin. Am. J. Chin. Med. 30: 297-305 (2002)
Song YJ, Lee DY, Kang DW, Kim YK, Kim SN, Lee KR, Lee HY. Apoptotic potential of sesquiterpene lactone ergolide through the inhibition of NF-κB signaling pathway. J. Pharm. Pharmacol. 57: 1591-1597 (2005)
Tavares WR, Seca AML. Inula L. secondary metabolites against oxidative stress-related human diseases. Antioxidants 8: 122 (2019)
Um M, Han TH, Lee JW. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa Thunb.) fruit. Food Sci. Biotechnol. 27: 375-382 (2018)
Yang SJ, Lee JE, Lim SM, Kim YJ, Lee NK, Paik HD. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 28: 491-499 (2019)
Yu HS, Jang HJ, Lee NK, Paik HD. Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi. LWT-Food Sci. Technol. 112: 108229 (2019)
Zhengfu H, Hu Z, Huiwen M, Zhijun L, Jiaojie Z, Xiaoyl Y, Xiujun C. 1-O-acetylbritannilactone (ABL) inhibits angiogenesis and lung cancer cell growth through regulating VEGF-Src-FAK signaling. Biochem. Biophys. Res. Commun. 464: 422-427 (2015)
Acknowledgements
This study was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through Agri-Bio industry Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (#116136-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, HY., Bae, WY., Yu, HS. et al. Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells. Food Sci Biotechnol 29, 569–578 (2020). https://doi.org/10.1007/s10068-019-00690-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00690-w