Abstract
This study was performed to investigate the effects of highly bioavailable curcumin as Theracurmin® (TC) in rats with monosodium iodoacetate (MIA)-induced osteoarthritis (OA). Seventy-seven male Wistar rats were divided into six groups: normal, negative control (MIA only), positive control (Cerebrex), and three experimental groups treated with 500, 1300, or 2600 mg/kg of TC for 5 weeks. MIA injection-induced OA caused 30% weight-bearing imbalance whereas weight bearing imbalance was significantly improved in the TC groups. Mankin scores revealed TC treatment had significantly ameliorated cartilage damage and chondrocyte decrease. The expressions of nitrotyrosine, tumor necrosis factor-α, phosphorylated nuclear factor kappa B cells, and cleaved caspase-3 were markedly increased in rat with MIA-induced OA, but the TC-treated groups exhibited a significant reduction in the number of immunoreactive cells in a dose-dependent manner. In conclusion, administration of TC contributes to the anti-arthritic effect in rat with MIA-induced OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is an age-related degenerative disease causing the most common joint disorders. Symptomatic knee OA occurs globally in 10% of men and 18% of women over 60 years of age (Nelson, 2018), and in an estimated 22.6% of the elderly population in Korea (Lee, 2014). It is characterized by an increase in cartilage degradation, the disappearance of the normal skeletal structure, cartilage damage, and ligament stiffness (Watt, 2018). This joint degeneration in OA is mainly due to inflammation, although the extent of the inflammation is lower compared to rheumatoid arthritis (RA). A patient with early OA exhibits significantly higher immunohistological measures of inflammation, such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), nuclear factor kappa B cells (NF-κB), and cyclooxygenase 2 (COX-2) in synovial tissue (Watt, 2018). Another study by Sohn et al. (2012) showed that 108 proteins including Gc-globulin, and alpha 1- and alpha 2-microglobulins were differentially expressed in the synovial fluid of osteoarthritic patients compared to normal patients. Moreover, these proteins can promote the secretion of inflammatory cytokines associated with chondrolysis and the breakdown of the extracellular matrix.
Although targeted medication for osteroarthritis has not been developed, analgesics, anti-inflammatory drugs and non-steroidal anti-inflammatory drugs (NSAID) are widely used. Currently, treatment of osteoarthritis is mainly through agents such as acetaminophen, ibuprofen, nabumetone, cyclooxygenase-2 (COX-2) selective inhibitor, and tramadol (Hochberg et al., 2012). However, NSAID have common side effects including gastrointestinal toxicity, cardiovascular adverse effect, and nephrotoxicity and COX-2 inhibitors have cardio-nephrotoxic adverse effects (Bjarnason, 2013). In addition, long-term use or abuse can cause side effects of the digestive system and the blood coagulation mechanism, and may result in immunoresistance, which may worsen the arthritis. Therefore, it is important to develop natural materials that inhibit cartilage degradation with better safety.
Curcumin is a yellow natural polyphenol compound extracted from the roots of Curcuma longa (Zingiberaceae) belonging to the ginger family. It is mainly distributed in India and Southeast Asia and is traditionally used in food, coloring matter, and traditional medicine. Many studies have reported that curcumin has a variety of effects such as antioxidant, anti-inflammatory, and anti-carcinogenic (Panahi et al., 2015; Sharma et al., 2001). Especially in the mechanism, curcumin increases the phosphorylation and DNA-binding activity of nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor regulating the antioxidant response (Cui et al., 2016). However, curcumin’s natural form cannot often achieve its optimum therapeutic effectiveness in vivo or in human studies due to its low solubility and poor gastrointestinal absorption (Sharma et al., 2001; Yang et al., 2007). After ingestion, curcumin, its metabolites such as curcumin glucuronide, curcumin sulfate, and hexahydrocurcumin have rarely been detected in blood or urine (Sharma et al., 2001). An animal study in rats has revealed that a single oral dose of 500 mg/kg curcumin resulted in a maximum serum concentration of 0.06 μg/mL at 41.7 min, which is equivalent to an oral bioavailability of 1% (Yang et al., 2007). Various methods for increasing the bioabsorbability of curcumin have been carried out with a form of curcumin microparticulate (200–600 nm) through high pressure homogenization (Wan et al., 2012) and liquid-type liposomes, which enabled more than five times higher absorption in an animal model (Takahashi et al., 2009). Sasaki et al. (2011) reported curcumin absorption rate by Theracurmin® (TC), in which curcumin was micronized through wet milling followed by high pressure homogenizing, was around 27 times as high as that by curcumin powder.
In recent years, studies on the usefulness of highly concentrated curcumin form such as nanoparticles and liposomes have been carried out on diabetes (El-Far et al., 2017) and prostate cancer (Feng et al., 2017). Trivedi et al. (2017) also reported the immunomodulatory potential of a nanocurcumin-based formulation, which could be used against various inflammatory disorders such as allergies, asthma, and autoimmune diseases. For OA, nanoparticles encapsulating curcumin suppressed mRNA expression of pro-inflammatory mediators IL-1β and TNF-α and slowed OA disease progression in mouse model (Zhang et al., 2016). Niazvand et al. (2017) also reported that curcumin-loaded poly lactic-co-glycolic acid nanoparticles prevented the structural changes of articular cartilage in monosodium iodoacetate (MIA) model of OA in rats. In case of TC, the highly bioavailable curcumin, TC containing 180 mg/day of curcumin showed modest potential for treatment of human knee osteoarthritis (Nakagawa et al., 2014). However, there was no significant change in the disease assessment score determined by Japanese Knee Osteoarthritis Measure between the treatment and the placebo group. A large sample size and a longer duration of treatment were warranted to validate the findings of the study. Additionally, to the best of our knowledge, the therapeutic effects of this compound and its effects on the molecular mechanisms of OA have not been investigated in vivo. Therefore, in the present study, we investigated the anti-osteoarthritic effects of highly bioavailable curcumin and its underlying mechanism in rats with MIA-induced OA.
Materials and methods
Preparation of curcumin
The test material TC (kindly provided by Theravalues, Tokyo, Japan) had a curcumin content of 300 mg/g and was prepared by mixing gum ghatii, maltose, citric acid, and dextrin. Turmeric raw material (Curcuma longa L.) was extracted with hexane and acetone. Then the solvent was filtered and concentrated to obtain the turmeric oleoresin curcumin. Gum ghatti, maltose, citric acid, and dextrin were dissolved in water, and the primary obtained turmeric oleoresin curcumin was added, mixed, and ground. The mixture was filtered, dried through a spray dryer, and final powdered.
Animals
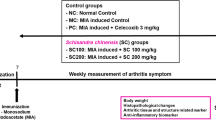
Seven-week-old male Wistar rats were purchased from Chungang Inc. (Seoul, Korea). The animals were provided with food and water ad libitum and four of them were housed per polycarbonate cage at 23 ± 3 °C under a 12 h light/12 h dark photoperiod. Experiments were performed according to the guidelines for the animal care and use of laboratory animal protocols approved by the Institutional Animal Care and Research Advisory Committee of Kyunggi Bio Center, Suwon, Korea (IRB number: 2016-06-0004). During the study period, there were no abnormal symptoms or deaths from the administration of the test substance and there were no significant changes in body weight, so it was concluded that the test substance did not cause weight change or general symptoms.
Induction of OA with MIA injection and administration with TC
Rats were randomly assigned to one of the six groups as follows: normal (injection of saline, n = 12); negative control (injection of 1.0 mg MIA, n = 13); MIA (1.0 mg) + TC (500 mg/kg/day, n = 13, TC-500); MIA (1.0 mg) + TC (1300 mg/kg/day, n = 13, TC-1300); MIA (1.0 mg) + TC (2600 mg/kg/day, n = 13, TC-2600); MIA (1.0 mg) + Celebrex (100 mg/kg/day, n = 13, positive control). They acclimatized for seven days with the basal diet. On day 7, MIA (Sigma-Aldrich, St. Louis, MO, USA) was injected in a 1 mL syringe at a dose of 50 μL (60 mg/mL) in the right knee joint to induce OA. After injection of MIA, each experimental group was administered orally with either saline, 500, 1300 or 2600 mg/kg TC, 100 mg/kg Celebrex (Pfizer, Inc., New York, NY, USA) once daily for 5 weeks.
Percent weight bearing on right hind paw
Changes in weight distribution between the left and right hind paws were determined using incapacitance testers (Stoelting Co., Wood Dale, IL, USA) on days 8, 14, 21, 28, and 35 after administration of TC. Rats were placed in an angled plexiglas chamber so that each hind limb was positioned on a separate force plate. The rats were allowed to acclimate to the apparatus and when stationary, readings were taken. The downward force (measured in grams) applied by each hind limb was assessed and averaged over a three second period (each data point was the average of three readings). For weight bearing measurements, the percent weight (in grams) borne on the right hind paw was determined using
Assessment of pain behavior and joint thickness measurement
Nociceptive testing was performed using an esthesiometer (BIO-EVF4, Bioseb®, Paris, France), which is an automated version of the von Frey hair assessment procedure, before the MIA injection (Day 6) and on given days after MIA injection. After 6, 13, 20, 27, and 34 days of TC administration, the animals were placed on a metal mesh surface in an acrylic chamber in a temperature-controlled room and allowed to rest for 15 min before testing. A touch stimulator unit was oriented beneath each animal, and when the instrument was activated, a fine plastic monofilament advanced at a constant speed and touched the paw in the proximal metatarsal region. The filament exerted a gradual increasing force on the plantar surface, starting below the threshold of detection and increasing until the stimulus became painful, which was indicated by the removal of its paw. The force required to elicit a paw withdrawal reflex was recorded automatically and measured in g.
The thickness of the knee joint was measured once a week using digital calipers after TC administration.
Histopathological examination of the joints
Histological changes were assessed to confirm the effects of TC on cartilage degeneration in the knee joints of the rats with MIA-induced OA. After the sacrifice of a rat at 5 weeks, each knee joint was resected, fixed in 10% formalin (Sigma-Aldrich, St. Louis, MO, USA) for 24 h at 4 °C, and then decalcified with 5% hydrochloric acid (Sigma-Aldrich, St. Louis, MO, USA) for 4 days at 4 °C. After decalcification, the specimens were embedded in paraffin and sections were stained with hematoxylin and eosin, and safranin-O staining was performed for cartilage testing of the joints. All of the stained slides were histologically evaluated and statistically graded on a scale of 0–12 according to a modified Mankin scoring system (Choi et al., 2015). The surface was scored on a scale of 0–3, where 0 = normal, 1 = irregular surface, 2 = fibrillation/vacuoles, and 3 = blisters and erosion. Cellular abnormalities were scored on a scale of 0–3, where 0 = normal, 1 = small decrease in chondrocytes, 2 = large decrease in chondrocytes, and 3 = no cells. Clone/osteophyte formation was scored on a scale of 0–3, where 0 = normal, 1 = occasional duos, 2 = duos or trios, and 3 = multiple nested cells. Staining with safranin-O for cartilage was scored on a scale of 0–3, where 0 = normal, 1 = small decrease in color, 2 = large decrease in color, and 3 = no color. Histopathological examination items were mean osteophyte numbers (osteophytes, osteophytes/mm2 of synovial cavity), mean osteophyte diameter (osteophyte diameter, μm/osteophyte), remaining articular cartilage percentage (%/knee joint), mean synovial membrane (SM) epithelial thickness (μm/SM), and mean number of inflammatory cells that had infiltrated the SM (cell/mm2 of SM). The same professor of clinical pathology performed the entire histological evaluation using a computer based automated image analyzer (iSolution FL ver 9.1, IMT i-solution Inc., Vancouver, Canada).
Immunohistochemical analysis
All knee joint paraffin blocks were serially re-sectioned as 3 μm-thick, individually. Immunoreactivities for NT, TNF-α, cleaved caspase-3, and phosphorylated NF-κB on the femur and tibia AC, and SM were considered using purified primary antibodies with avidin–biotin–peroxidase complex (ABC) and peroxidase substrate kit (Vector Labs, Burlingame, CA, USA), respectively. Briefly, endogenous peroxidase activity was blocked by incubation in methanol and 0.3% H2O2 for 30 min, and non-specific binding of immunoglobulin was blocked with normal horse serum blocking solution for 1 h in humidity chamber after epitope retrievals by pretreatment of trypsin (Sigma-Aldrich, St. Louis, MO, USA) and 2 N HCl as previously described (Choi et al., 2015). Primary antisera were treated for overnight at 4 °C in humidity chamber, and then incubated with biotinylated universal secondary antibody and ABC reagents for 1 h at room temperature in humidity chamber. Finally, sections were reacted with peroxidase substrate kit for 3 min at room temperature. All sections were rinse in 0.01 M PBS for 3 times, between each step. To observe more detail immunohistopathological changes, the mean numbers of immunoreactive cells on the tibia and femur AC, and also on SM were calculated using a computer based automated image analyzer (iSolution FL ver 9.1, IMT i-solution Inc., Vancouver, Canada) under a light microscope (Model Eclipse 80i, Nikon, Tokyo, Japan).
Statistical analysis
Data are expressed as mean ± standard error (SE) for each group. The differences between the groups were examined for statistical significance using one-way ANOVA or Student t test with IBM SPSS statistics 22 (Armonk, NY, USA). A value of p < 0.05 was considered as significant.
Results and discussion
TC ameliorated the MIA-induced knee pains in rats
MIA-induced OA is primarily remarked in the weight-bearing joint, and the changes in % weight distribution were signified as an index of joint discomfort. As for percent weight bearing, a statistically significant reduction was observed in the negative control compared to the normal (p < 0.01, Fig. 1A). Percentage weight bearing was significantly increased in the TC-1300 (p < 0.01) and TC-2600 (p < 0.05) compared to negative control. A statistically significant % weight bearing increase was observed at 1, 2, 3, 4, and 5 weeks in the positive control (p < 0.01).
Percent weight bearing (A), paw withdrawal threshold of the right hind paw (B), and knee joint thickness (C) of rats with monosodium iodoacetate-induced osteoarthritis. **Significantly different between normal and negative control (p < 0.01). +Significantly different from negative control (p < 0.05). ++Significantly different from negative control (p < 0.01). Normal (saline, 20 mL/kg/day), negative control [monosodium iodoacetate (MIA), 20 mL/kg/day], TC-500 [Theracurmin® (TC) 500 mg/kg/day], TC-1300 (TC 1300 mg/kg/day), TC-2600 (TC 2600 mg/kg/day), and positive control (Celebrex 100 mg/kg/day)
Mechanical sensitivity was measured by von Frey test, which is also represented as biomarker of knee pain. In the von Frey hair assessment test, the paw withdrawal threshold (PWT) were prolonged in rats given oral TC (TC-1300 or TC-2600) compared to the negative control (Fig. 1B). Furthermore, a statistically significant increase in PWT was observed in the TC-2600 from 3 to 5 weeks (p < 0.05 and p < 0.01, respectively) and in the positive control between 4 and 5 weeks (p < 0.05 and p < 0.01, respectively). These results demonstrate the anti-nociceptive property of TC.
As shown in Fig. 1C, knee joint thickness in the negative control increased significantly after an intra-articular injection of MIA as compared with the normal from week 1 to week 5. However, TC treatment significantly reduced the joint thickness at 4 weeks (p < 0.01) in the TC-2600 compared to the negative control. There was no statistically significant change in the other test substance-administered groups. A statistically significant decrease in joint thickness was observed in the positive control at 4 and 5 weeks (p < 0.01). Articular inflammation directly affects joint edema, which is related to increase joint thickness (Dias et al., 2017).
In the negative group, the joint thickness was significantly increased, the weight bearing and PWT were significantly decreased compared with the control, and the MIA-induced osteoarthritis model was confirmed in this study. In addition, TC significantly ameliorated the MIA-induced knee pains in rats, the thickness of joint was significantly inhibited in TC-2600 and positive control.
TC reduced the articular damage in rats with MIA-induced OA
Mankin score of the rat knee joint tissues was presented in Table 1. There was a statistically significant increase in Mankin score in the negative control compared to the normal, which showed decreased articular cartilage surface damage, chondrocyte decrease, and proliferative clone/osteophyte formation (p < 0.01). There was a statistically significant decrease (p < 0.01) in osteophyte number and diameter, residual synovial thickness, number of inflammatory cells, and residual articular cartilage ratio compared with the normal. Mankin scores of articular cartilage surface damage, chondrocyte reduction, and proliferative clone/osteophyte were significantly decreased in the TC-1300 and TC-2600 (p < 0.05 and p < 0.01, respectively). The number of osteophytes and the average diameter of the remaining cartilage and synovial membrane of the femur and tibia, the thickness of the synovial membrane, the number of infiltrated inflammatory cells, and the number of osteophytes and their average diameter were also significantly improved in the TC-administered groups (p < 0.05). A significant decrease in Mankin score and significant increases in femoral and tibial remnant cartilage, the thickness of the synovial membrane, and the number of infiltrated inflammatory cells were observed in the positive control compared to the negative control (p < 0.05 or p < 0.01). There was no statistically significant decrease in osteophyte count and mean diameter measurement in the positive control compared with the negative control.
In this experiment, the results of histopathological examination were noticeably improved by oral treatment of all three different dose levels of TC, and cartilage surface damage dramatically inhibited by oral treatment of positive control, Celebrex 100 mg/kg, which is a prescription drug. Overall, Theracurmin® 500 mg/kg showed favorable inhibitory effects against MIA-induced increases of Mankin scores and decreases of remaining AC percentages as comparable to Celebrex 100 mg/kg, in this observation. These results demonstrate that TC has the effect of reducing the pain of OA caused by MIA, and thus a positive chondroprotective effect in the MIA-induced OA rat model.
TC reduced the expression of nitrotyrosine and TNF-α in rats with MIA-induced OA
Nitrotyrosine (NT) is a product of tyrosine nitration mediated by reactive nitrogen species such as the peroxynitrite anion and nitrogen dioxide. Oxidative damage and nitration of cartilage is associated with misfolding and aggregation of proteoglycan–collagen network surrounding chondrocytes and matrix degradation (Hardin et al., 2015), which is identified as an indicator or marker of cell damage. NT immunohistochemical evaluation was shown in Fig. 2. NT expression, which indicates oxidative damage, was markedly increased in the rats with MIA-induced OA (p < 0.01), and treatment with TC reduced the expression of NT in the femur articular cartilage, tibial articular cartilage, and synovial membrane (p < 0.01). In the positive control, a significant decrease in the number of immune response cells was observed in the femoral and tibial articular cartilage and synovial membrane (p < 0.01). Increased protein nitration of cartilage has long been reported as damaging in the joint (Kaur and Halliwel, 1994; Richardot et al., 2009). Moreover, there have been few studies on protein nitration in the early stages of OA. Ahmed et al. (2016) reported that nitrated amino acid levels provided a plasma-based biochemical test of relatively high sensitivity and specificity for the early stage of arthritic disease. Our results showed that NT was highly expressed in MIA-induced rats, but TC treatment significantly ameliorated their expression in the femoral and tibial articular cartilage and synovial membrane.
Nitrotyrosine immunohistochemical staining (A) and immunolabeled cell numbers (B) of the rat knee joint tissues. **Significantly different between normal and negative control (p < 0.01). ++Significantly different from negative control (p < 0.01). Normal (saline, 20 mL/kg/day), negative control (monosodium iodoacetate (MIA), 20 mL/kg/day), TC-500 (Theracurmin® (TC) 500 mg/kg/day,), TC-1300 (TC 1300 mg/kg/day), TC-2600 (TC 2600 mg/kg/day), and positive control (Celebrex 100 mg/kg/day). AC, Articular cartilage; SM, Synovial membrane. Scale bars = 40 μm
TNF-α is produced mostly by macrophages and, alone or in combination with other cytokines or chemokines, recruits inflammatory cells such as B and T cells and neutrophils, and induces production of cytokines, chemokines, matrix metalloproteinases, or receptor activator of NF-κB ligand. TNF-α have been shown to pro-inflammatory signals triggering events and many studies have reported that increased activities of TNF-α in human OA cartilage as well as experimental animal models of OA (Chin, 2016). TNF-α is also known to be produced by arthritis synovium and is persistently elevated after knee joint injury (Watt, 2018). In addition, murine experimental arthritis is attenuated in TNF-α-deficient mice (Ji et al., 2002) and neutralization of TNF-α ameliorates murine collagen-induced arthritis (Williams et al., 1992). Our results showed that the number of chondrocytes stained positive for TNF-α increased in the MIA-induced OA cartilage (Fig. 3A, B). These TNF-α-positive chondrocytes were significantly lower in the TC-treated groups than in the negative control (p < 0.01). In the positive control, a significant decrease in the number of immune response cells was observed in the femoral and tibial articular cartilage and synovial membrane (p < 0.01).
TNF-α immunohistochemical staining (A) and immunolabeled cells (B) of the rat knee joint tissues. **Significantly different between normal and negative control (p < 0.01). ++Significantly different from negative control (p < 0.01). Normal (saline, 20 mL/kg/day), negative control [monosodium iodoacetate (MIA), 20 mL/kg/day], TC-500 [Theracurmin® (TC) 500 mg/kg/day], TC-1300 (TC 1300 mg/kg/day), TC-2600 (TC 2600 mg/kg/day), and positive control (Celebrex 100 mg/kg/day). AC, Articular cartilage; SM, Synovial membrane. Scale bars = 40 μm
TC decreased the expression of NF-κB in rats with MIA-induced OA
NF-κB signaling pathway can be activated by ROS or inflammatory mediators (Roman-Blas and Jimenez, 2006) and has crucial roles in inflammation, immunity, cell proliferation, and apoptosis. It plays a prominent role in the catabolism of the articular cartilage following stimulation with pro-inflammatory cytokines. Therefore, an increasing number of NF-κB inhibitors including anti-inflammatory drugs and anti-oxidant drugs have been reported (Roman-Blas and Jimenez, 2006). In our study, NF-κB expression was shown in Fig. 4. Phosphorylated NF-κB expression increased after MIA injection (p < 0.01), whereas treatment with TC ameliorated this in the TC-treated groups (p < 0.05 or p < 0.01). There was a dose-dependent decrease in the number of immune response cells in the femoral and tibial articular cartilage and synovial membrane (p < 0.05 or p < 0.01). In the positive control, a significant decrease in the number of immune response cells was observed in the femoral and tibial articular cartilage and synovial membrane (p < 0.01). Therefore, TC has been shown to inhibit the expression of NF-κB in the rat knee joint tissue and TC has been shown to act as an NF-κB inhibitor.
Phosphorylated NF-κB immunohistochemical staining (A) and immunolabeled cell numbers (B) of the rat knee joint tissues. **Significantly different between normal and negative control (p < 0.01). ++Significantly different from Negative control (p < 0.01). Normal (saline, 20 mL/kg/day), negative control [monosodium iodoacetate (MIA), 20 mL/kg/day], TC-500 [Theracurmin® (TC) 500 mg/kg/day], TC-1300 (TC 1300 mg/kg/day), TC-2600 (TC 2600 mg/kg/day), and positive control (Celebrex 100 mg/kg/day). AC, Articular cartilage; SM, Synovial membrane. Scale bars = 40 μm
TC decreased the expression of cleaved-caspase 3 in rats with MIA-induced OA
The expression of cleaved caspase-3 was increased in the osteoarthritic joint (Fig. 5, p < 0.01). Treatment with TC after MIA injection abrogated the elevated expression in a dose-dependent manner (p < 0.01). In the positive control, a significant decrease in the number of immune response cells was observed in the femoral and tibial articular cartilage and synovial membrane (p < 0.01). Chondrocyte apoptosis has also been associated with OA, in which the maintenance of cells and cell apoptosis serves an important role in the regulation of the cellular pathology process. Especially, caspase-3 is an executioner caspase causing cell death. Suppression of caspase-3, inferring apoptotic inactivation, may also be among the causes of cell growth. MIA-induced chondrocyte apoptosis, which is mechanism of cartilage degradation, was recently reported (Jiang et al., 2013). In the present study, the expression of cleaved caspase-3 was increased in the negative control, which was suppressed by TC treatment.
Cleaved caspase-3 immunohistochemical staining (A) and immunolabeled cell numbers (B) of the rat knee joint tissues. **Significantly different between Normal and Negative control (p < 0.01). ++Significantly different from negative control (p < 0.01). Normal (saline, 20 mL/kg/day), negative control [monosodium iodoacetate (MIA), 20 mL/kg/day], TC-500 [Theracurmin® (TC) 500 mg/kg/day], TC-1300 (TC 1300 mg/kg/day), TC-2600 (TC 2600 mg/kg/day), and positive control (Celebrex 100 mg/kg/day). AC articular cartilage, SM synovial membrane. Scale bars = 40 μm
OA is a progressive joint disease caused by cartilage damage, often referred to as degenerative joint disease. Inflammation and oxidative stress plays a central role in OA pathology (Watt, 2018). Many medications have been developed for the treatment of OA that act as antioxidants and anti-inflammatories, but side effects have been reported simultaneously (Bjarnason, 2013). As for curcumin, which has both anti-inflammatory and antioxidant properties, this natural agent has been reported as curative. In a study conducted in patients with metabolic syndrome, serum levels of inflammatory cytokines such as TNF-α, IL-6, TGF-β, and monocyte chemoattractant protein-1 were significantly decreased with a treatment of curcumin (Panahi et al., 2016). Induced macrophage foam cell formation and oxidative damage were weakened after treatment with curcumin powder (Soltani et al., 2017). TNF-α as mediator of inflammation plays an important role in OA progression (Chin, 2016). Our result also showed that TC ameliorated the TNF-α expression in an OA animal model, which means TC could be a therapeutic candidate to modulate inflammation.
Continuous and low-grade oxidative stress to cells and matrix contribute to OA pathogenesis (Moon et al., 2014). Recent clinical study reported that vitamin E is an effective antioxidant that can improve clinical symptoms and reduce oxidative stress conditions in patients with late-stage knee osteoarthritis (Tantavisut et al., 2017). Therefore, reducing oxidative stress could be a therapeutic strategy to inhibit cartilage degeneration. Another study has suggested that NT is implicated in OA progression (Lee et al., 2015) and animal studies showed that nitric oxide donor significantly increased subchondral bone sclerosis during subchondral remodeling suggesting that oxidative stress could contribute to pain generation in OA (Moon et al., 2014). Our study showed that TC treatment could significantly reduce the express of NT in rat knee joint tissues. The results suggest that TC could be a therapeutic candidate to control progression of pathophysiology in early stages of OA.
Apart from clinical benefits of TC, the pathophysiological mechanism underlying OA remained uncertain in this study. However, TC suppressed the phosphorylation of NF-κB proteins and reduced OA related markers such as NT, TNF-α, and cleaved caspase-3. Interestingly, Roman-Blas and Jimenez (2006) reported that NF-κB signaling pathways mediate critical events in the inflammatory response by chondrocytes, leading to progressive extracellular matrix damage and cartilage destruction. Moreover, Marcu et al. (2010) focused that NF-κB signaling in OA chondrocytes contributes to cartilage degeneration in OA by affecting a number of downstream processes, which particularly in response to extrinsic stress and inflammatory signals. Altogether, TC has a diverse effect on OA related biomarker, and may be an effective treatment of NF-κB activation. The inhibition of NF-κB expression could be thought of as affecting other biomarker and it help to ameliorate MIA-induced osteoarthritic pain and damage. However, further studies on the NF-κB signal pathway are needed.
In conclusion, 5 weeks of repeated oral administration of TC in the MIA-induced OA rat model incurred chondroprotective effects such as percent weight bearing increase, joint thickness reduction, PWT increase, and histopathological OA improvement. As for Mankin score and immunohistopathological observation, Theracurmin® 500 mg/kg showed similar effects to Celebrex 100 mg/kg. Therefore, we consider that TC has wide potential as a general food and biomedical medicinal material that incurs preventive medical aspects toward OA.
References
Ahmed U, Anwar A, Savage RS, Thornalley PJ, Rabbani N. Protein oxidation, nitration and glycation biomarkers for early-stage diagnosis of osteoarthritis of the knee and typing and progression of arthritic disease. Arthritis Res. Ther. 18: 250-260 (2016)
Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int. J. Clin. Pract. Suppl. 178: 37-42 (2013)
Chin KY. The spice for joint inflammation: anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des. Dev. Ther. 10: 3029-3042 (2016)
Choi JS, Shin HS, Kim KY, Ku SK, Choi IS, Kim JW. Effect of polycalcium, a mixture of polycan and calcium lactate-gluconate in a 1:9 weight ratio, on rats with surgery-induced osteoarthritis. Exp. Ther. Med. 9: 1780-1790 (2015)
Cui Q, Li X, Zhu H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol. Med. Rep. 13: 1381-1388 (2016)
Dias RG, Sampaio SC, Sant’Anna MB, Cunha FQ, Gutiérrez JM, Lomonte B, Cury Y, Picolo G. Articular inflammation induced by an enzymatically-inactive Lys49 phospholipase A2: activation of endogenous phospholipases contributes to the pronociceptive effect. J. Venom. Anim. Toxins Incl. Trop. Dis. 23: 18-31 (2017)
El-Far YM, Zakaria MM, Gabr MM, El Gayar AM, Eissa LA, El-Sherbiny IM. Nanoformulated natural therapeutics for management of streptozotocin-induced diabetes: potential use of curcumin nanoformulation. Nanomed. (Lond.) 12: 1689-1711 (2017)
Feng T, Wei Y, Lee RJ, Zhao L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 12: 6027-6044 (2017)
Hardin JA, Cobelli N, Santambrogio L. Consequences of metabolic and oxidative modifications of cartilage tissue. Nat. Rev. Rheumatol. 11: 521-529 (2015)
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 64: 465-474 (2012)
Ji H, Pettit A, Ohmura K, Ortiz-Lopez A, Duchatelle V, Degott C, Gravallese E, Mathis D, Benoist C. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J. Exp. Med. 196: 77-85 (2002)
Jiang L, Li L, Geng C, Gong D, Jiang L, Ishikawa N, Kajima K, Zhong L. Monosodium iodoacetate induces apoptosis via the mitochondrial pathway involving ROS production and caspase activation in rat chondrocytes in vitro. J. Orthop. Res. 31: 364-369 (2013)
Kaur H, Halliwel B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 350: 9-12 (1994)
Lee DG, Park SY, Chung WS, Park JH, Hwang E, Mavlonov GT, Kim IH, Kim KY, Yi TH. Fucoidan prevents the progression of osteoarthritis in rats. J. Med. Food. 18: 1032-1041 (2015)
Lee HS. Prevalence of osteoarthritis and related risk factors in the elderly: data from the fifth Korea National Health and Nutrition Examination Survey, 2010–2012. J. Korean Diet. Assoc. 20: 99-109 (2014)
Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: multiple angles to target OA. Curr. Drug Targets 11: 599-613 (2010)
Moon SJ, Jeong JH, Jhun JY, Yang EJ, Min JK, Choi JY, Cho ML. Ursodeoxycholic Acid ameliorates pain severity and cartilage degeneration in monosodium iodoacetate-induced osteoarthritis in rats. Immune Netw. 14: 45-53 (2014)
Nakagawa Y, Mukai S, Yamada S, Matsuoka M, Tarumi E, Hashimoto T, Tamura C, Imaizumi A, Nishihira J, Nakamura T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 19: 933-939 (2014)
Nelson AE. Osteoarthritis year in review 2017: clinical. Osteoarthritis Cartil. 26: 319-325 (2018)
Niazvand F, Khorsandi L, Abbaspour M, Orazizadeh M, Varaa N, Maghzi M, Ahmadi K. Curcumin-loaded poly lactic-co-glycolic acid nanoparticles effects on monoiodoacetate-induced osteoarthritis in rats. Vet. Res. Forum 8: 155-161 (2017)
Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin. Nutr. 34: 1101-1108 (2015)
Panahi Y, Hosseini MS, Khalili N, Naimi E, Simental-Mendía LE, Majeed M, Sahebkar A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 82: 578-582 (2016)
Richardot P, Charni-Ben Tabassi N, Toh L, Marotte H, Bay-Jensen AC, Miossec P, Garnero P. Nitrated type III collagen as a biological marker of nitric oxide-mediated synovial tissue metabolism in osteoarthritis. Osteoarthritis Cartil. 17: 1362-1367 (2009)
Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartil. 14: 839-848 (2006)
Sasaki H, Sunagawa Y, Takahashi K, Imaizumi A, Fukuda H, Hashimoto T, Wada H, Katanasaka Y, Kakeya H, Fujita M, Hasegawa K, Morimoto T. Innovative preparation of curcumin for improved oral bioavailability. Biol. Pharm. Bull. 34: 660-665 (2011)
Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 7: 1894-1900 (2001)
Sohn DH, Sokolove J, Sharpe O, Erhart JC, Chandra PE, Lahey LJ, Lindstrom TM, Hwang I, Boyer KA, Andriacchi TP, Robinson WH. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 14: R7 (2012)
Soltani B, Bodaghabadi N, Ghaemi N, Sadeghizadeh M. Radiation-induced surge of macrophage foam cell formation, oxidative damage, and cytokine release is attenuated by a nanoformulation of curcumin. Int. J. Radiat. Biol. 93: 303-314 (2017)
Takahashi M, Uechi S, Takara K, Asikin Y, Wada K. Evaluation of an oral carrier system in rats: bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 57: 9141-9146 (2009)
Tantavisut S, Tanavalee A, Honsawek S, Suantawee T, Ngarmukos S, Adisakwatana S, Callaghan JJ. Effect of vitamin E on oxidative stress level in blood, synovial fluid, and synovial tissue in severe knee osteoarthritis: a randomized controlled study. BMC Musculoskelet. Disord. 18: 281-289 (2017)
Trivedi MK, Mondal SC, Gangwar M, Jana S. Immunomodulatory potential of nanocurcumin-based formulation. Inflammopharmacology 25: 609-619 (2017)
Wan S, Sun Y, Qi X, Tan F. Improved bioavailability of poorly water-soluble drug curcumin in cellulose acetate solid dispersion. AAPS PharmSciTech. 13: 159-166 (2012)
Watt FE. Osteoarthritis biomarkers: year in review. Osteoarthritis Cartil. 26: 312-318 (2018)
Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA. 89: 9784-9788 (1992)
Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 853: 183-189 (2007)
Zhang Z, Leong DJ, Xu L, He Z, Wang A, Navati M, Kim SJ, Hirsh DM, Hardin JA, Cobelli NJ, Friedman JM, Sun HB. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 18: 128-139 (2016)
Acknowledgements
The authors gratefully acknowledge Prof. Ku, Sae Kwang for histopathological and immunohistochemistrical analysis.
Author information
Authors and Affiliations
Contributions
This study was partially designed and planned by Jee-Hye Yun and Ahsa Lee.
Corresponding author
Ethics declarations
Conflict of interest
This study was supported by research fund from Handok Inc. Jee-Hye Yun and Ahsa Lee were an employment at Handok Inc.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, HJ., Lee, CK., Song, SH. et al. Highly bioavailable curcumin powder suppresses articular cartilage damage in rats with mono-iodoacetate (MIA)-induced osteoarthritis. Food Sci Biotechnol 29, 251–263 (2020). https://doi.org/10.1007/s10068-019-00679-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00679-5