Abstract
Egg products are widely consumed in Korea and continue to be associated with risks of Staphylococcus aureus-induced food poisoning. This prompted the development of predictive mathematical models to understand growth kinetics of S. aureus in egg products in order to improve the production of domestic food items. Egg products were inoculated with S. aureus and observe S. aureus growth. The growth kinetics of S. aureus was used to calculate lag-phase duration (LPD) and maximum specific growth rate (µmax) using Baranyi model as the primary growth model. The secondary models provided predicted values for the temperature changes and were created using the polynomial equation for LPD and a square root model for µmax. In addition, root mean square errors (RMSE) were analyzed to evaluate the suitability of the mathematical models. The developed models demonstrated 0.16–0.27 RMSE, suggesting that models properly represented the actual growth of S. aureus in egg products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of the economy and the food industry has raised the public’s standard of living and increased interest in health, and efforts to secure food hygiene and safety have been continuously carried out globally. Changes in eating habits, which are focused on economic efficiency and convenience, have promoted the consumption of ready-to-eat foods and have led to the growth of the meal service industry. In particular, the use of egg products, which are universal and high-protein nutritional foods, is increasing among consumers. In a recent studies, egg products are the most likely to cause food poisoning among domestic livestock products (Hong et al., 2015). Various nutritional elements of egg products provide favorable conditions for microbial growth and internal contamination of eggs. In addition, the bacteria causing food poisoning are present in egg shells and may grow and increase the level of cross contamination during distribution. Grilled eggs that have been contaminated through distribution and storage at room temperature are expected to be at higher risk. Shells of quail eggs are thin, and most of these eggs are distributed as peeled quail egg products; therefore, risk of microbial growth is high. For whole egg liquid, the processing steps, such as heat treatment, are often inadequate and their shelf life is short; therefore, sufficient sanitation control is required (Jo et al., 2015; Jones et al., 1995).
Bacteria that cause food poisoning in egg products include Salmonella Enteritidis and Escherichia coli, among others. In addition, because eggs provide good nutrient source for growth of most microbes, they are very likely to be contaminated by S. aureus, which is widely distributed in nature and resistant to various environments. Also, S. aureus was presented in various foods and was detected in Kimbap, lunch box, rice cakes, bread, and seafood in Korea (Park et al., 2010). S. aureus enterotoxin (SE) contained in foods has a very high heat resistance and increases the potential for food poisoning (Dinges et al., 2000; Wieneke et al., 1993).
Predictive microbiology, a technology that assesses food contamination or its potential contamination, has a significant impact on risk assessment. It is a field of study that secures food safety by applying various factors of food to equations to quantitatively predict changes in the microbial growth and death, from raw materials to consumption stages, and blocks the risk factors in advance. Research utilizing predictive microbiology has attracted worldwide attention (Pal et al., 2008; Van Impe et al., 2005). Models predict the growth and survival of microorganisms by measuring changes in microorganisms over time where the resulting parameters (Lag phase duration (LPD) and growth rate) are measured across various environmental conditions. The primary models mostly used are the Baranyi model and the Gompertz model, and the secondary model used include the square root model, polynomial equation, Arrhenius model, etc. A third model integrates these two models and applies it to software commercially (Walls and Scott, 1997; Yoon, 2010). This growth prediction model is considered to be effective in preventing food poisoning and is being developed continually for various food groups. US Food Safety Inspection Service (FSIS) and WHO are also studying risk assessment using predictive microbiology (Park et al., 2007).
According to statistics from the Korea Ministry of Agriculture, Food and Rural Affairs and the Korea Rural Economic Institute, egg consumption per capita in Korea has increased from 184 in 2000, to 236 in 2010, to 254 in 2014. Grilled eggs are not only widely used as diet foods, but, especially in Korea, they are also highly consumed in places like a jjimjilbang where bacteria can grow easily. For quail egg and whole egg liquid products, it is necessary to manage the safety of egg products because of the high possibility of large-scale food poisoning, as they are used widely in the meal service industry. Therefore, this study aims to provide data for microbial risk assessment to be utilized in food safety management and to prevent food poisoning by developing a growth prediction model of S. aureus, whose potential risk is high in grilled eggs, ready-to-eat peeled quail egg, and whole egg liquid products, whose demand is increasing.
Materials and methods
Standard strain
The standard strains used in this study were S. aureus ATCC 14458, ATCC 27664, ATCC 23235, ATCC 13565, and ATCC 19095 obtained from the American Type Culture Collection (ATCC). After incubating in a tryptic soy broth (TSB, Difco Laboratories, Detroit, MI, USA), we added 1 mL of TSB and 50% glycerol to a vial tube. Cultures were frozen and stored at − 70 °C to be used in tests.
Test solution
A single colony of the standard strain was inoculated in 10 mL of tryptic soy broth (TSB), incubated at 35 °C for 24 h, and centrifuged at 4 °C with 1912×g for 15 min to obtain the cell pellet. It was washed twice with phosphate buffer solution (PBS), mixed with the cultured broth and diluted to 5 CFU/mL with PBS.
Sample preparation
Predictive models were developed for grilled egg, peeled quail egg, and whole egg liquid products purchased from local grocery stores. After purchase, samples were stored at 4 °C, and 25 g of each samples were placed in vacuum packs until used (for quail eggs, weight includes both the quail egg and the filling water). The diluted test solution was then inoculated to make the final level of 3 log CFU/g. For grilled egg products, S. aureus was inoculated using a syringe to keep the original shape. Inoculated samples were stored at each testing storage temperature to measure the bacterial count.
Measurement of bacterial growth
In developing a predictive model, temperature is the most closely related factor for S. aureus growth. Therefore, inoculated grilled egg, peeled quail egg, and whole egg liquid were stored for a maximum of 30 days at 4, 10, 20, 30, and 37 °C. The bacterial growth in the samples was analyzed 20–30 times for each time period at each temperature. Each sample was homogenized for 2 min using a homogenizer and diluted tenfold. Then it was smeared on a selective medium for S. aureus (Baird-Parker RPF agar) to determine changes in bacterial counts.
Development of the primary model
Based on the results of the temperature-dependent growth pattern of S. aureus using the Baranyi model (Eq. 1) (Baranyi and Roberts, 1994), a primary model was developed that shows the lag phase duration (LPD, h), maximum specific growth rate (µmax, log CFU/g/h), initial bacterial counts (N0, log CFU/g), final bacterial counts (Nmax, log CFU/g), and R-square value.
µmax: maximum specific growth rate, N0: the initial bacterial counts, Nmax: the final bacterial counts, q0: a parameter defining the initial physiological state of the cells, t: time.
Development of the secondary model
We developed a secondary model that shows the effect of temperature changes on the parameters (µmax, LPD) of S. aureus in samples. The model for the LPD (lag phase duration, h) used a polynomial equation (Eq. 2), and the model for the maximum specific growth rate (µmax, log CFU/g/h) used a square root model (Eq. 3) (Ratkowsky et al., 1982).
LPD: lag phase duration, a, b, c: regression coefficients, T: storage temperature (°C).
µmax: maximum specific growth rate, a: the slopes of the regression lines for µmax, T: storage temperature (°C), Tmin: theoretical minimum temperature for cell growth (°C).
Validation of suitability
The suitability of the S. aureus growth prediction model equation was verified by obtaining RMSE (root mean square error), Af (accuracy factors), Bf (bias factors), and MRE (median relative error). To do so, we compared our predictions of the parameters calculated using the secondary model to the parameter estimates that were analyzed after measuring growth with the conventional method (not using temperature for model development) (25 °C).
n: number of observation.
RMSE (Eq. 4) is a value obtained by comparing the predicted value of the growth prediction model with the change of the microorganism through the test at the same temperature. The closer it is to 0, the more suitable the model (Baranyi et al., 1996).
n: number of observation.
Af (Eq. 5) is the absolute value of the difference between the value of a parameter obtained from the test and the predicted value measured in the secondary model. The farther the calculated value is from 1, the more inaccurate the model. The model is most suitable when the value is 1.3–1.5 (Ross, 1996; Ross, 1999).
n: number of observation.
Bf (Eq. 6) is the relative deviation between the experimental and predicted values. The closer it is to 0, the more accurate it is. If the calculated value is outside the range of 0.7–1.5, the model is not interpreted to be suitable (Ross, 1996; Ross, 1999).
MRE (Eq. 7) estimates a safe prediction of a model if the measured relative error is negative, but a positive value is a poorly predicted model. However, the closer MRE is to 0, the more suitable the model (Delignette-Muller et al., 1995).
Results and discussion
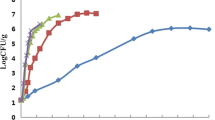
In order to predict the probability of food poisoning caused by microbiological hazards present in foods, and to systematically manage them, there is a need for risk assessment studies. Many advanced countries are making efforts to reduce food poisoning by establishing a food safety system through risk assessment. Models that predict the growth and death of bacteria, especially for bacteria likely to cause food poisoning, must be considered for risk assessment (Yoon, 2013). Therefore, a predictive model was developed through verification of growth patterns of S. aureus, which is highly adaptable to various environments. The model focused on egg products (grilled egg, peeled quail egg, and whole egg liquid) that are widely consumed and pose high risks for food poisoning (Kadariya et al., 2014). S. aureus proliferated in egg products at all temperatures (10, 20, 30, and 37 °C) except 4 °C. For grilled eggs, bacterial growth started at 10 °C after 50 h, and at 20, 30, 37 °C, growth began after 8, 4, and 2 h, respectively. For peeled quail eggs, growth was observed after 76 h at 10 °C and after 7, 3, and 1 h at 20, 30, and 37 °C, respectively. For whole egg liquid, bacterial growth began after 72, 14, 10, and 6 h at 10, 20, 30, and 37 °C (Figs. 1, 2, and 3), showing a slower growth rate in whole egg liquid than in grilled eggs or peeled quail eggs. A linear relationship with temperature was confirmed by analyzing the growth of S. aureus in the samples. Bacterial growth patterns were similar to the growth of S. aureus across different storage temperatures of milk, which is a high-protein food (Kim et al., 2014). In addition to the original experiment, S. aureus growth was measured only in the filling water of peeled quail eggs, and similarly the bacterial growth was also proportional to temperature. So it is considered that the filling water of peeled quail eggs has no effect on the growth of S. aureus.
Based on these results, a primary model was developed based on temperature by applying the growth pattern of S. aureus to the Baranyi model equation. The temperature (4 °C) was not included in the model, because bacterial growth was inhibited at this temperature. The primary models widely used for developing the growth prediction model are Gompertz model, Baranyi model, logistic model, and exponential model. The Gompertz model was originally used for the census, but because it could be expressed in a sigmoid form, it was used to predict microbial growth. Recently, a modified Gompertz model has been used that has a slightly modified equation compared to the existing model (Ratkowsky and Ross, 1995). The Baranyi model was developed in the 1990s and is dynamic growth model using a differential equation based on microbial growth that reflects ‘bottlenecks’ (Buchanan et al., 1997). The Gompertz and logistic models are used widely as primary models (change of microbial growth over time). In recent years, however, the Baranyi model has been used most widely, because it can apply various environmental factors appropriately and interpret the calculated parameters biologically, and the DMFIT program provided in a PC version makes it more convenient to use (Grijspeerdt and Vanrolleghem, 1999; Yilmaz, 2011). The developed model computed the lag phase duration (LPD), the maximum specific growth rate (µmax), and the r2 value, which indicates the statistical fit of the primary model. The LPD of S. aureus in the grilled egg, peeled quail egg, and whole egg liquid showed the highest value at 10 °C, being inversely proportional to the temperature, and µmax was the highest at 37 °C, being proportional to the temperature (Table 1). This is a similar result to the previous report, which shows that the maximum growth rate increases proportionally with increasing temperature up to the storage temperature (30 °C), and LPD decreases and is inversely proportional to the temperature (Kang et al., 2010; Park et al., 2010). Also the r2 for the primary model was 0.97–0.99, showing a high fit of the parameter values in the secondary model (Duffy et al., 1994). In this study, a primary model was developed using only the Baranyi model. However, because the efficiency of models applied to the growth prediction model development is different based on the food, environmental conditions, etc., it is important to select the most appropriate model by using various methods (Yoon, 2010). In a study on predictive models of Listeria monocytogenes growth in fresh vegetables, a growth prediction model was developed using the Gompertz model and the Baranyi model. Comparing the fit of the two models showed that the Gompertz model was more suitable (Cho et al., 2011a). When developing a growth prediction model of S. aureus in boiled meat, the Baranyi model was a more suitable model (Park et al., 2010). As shown above, even if a growth prediction model is developed using the same data, different results may be obtained depending on the primary model that has been applied. Therefore, it is important to find the most appropriate model after developing the models using various primary models.
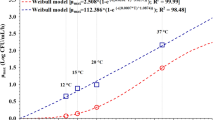
The mathematical formulas used to develop secondary models are square root, Davey, polynomial, and response surface models, but it is known that various equations are used based on the fit with the primary model. The polynomial equation is the most general secondary model and is useful for developing a model in accordance with various environmental factors. The square root model is known to represent the LPD and the maximum growth rate by showing the relationship with temperature (Ross, 1993). The secondary model equations for LPD and µmax which calculated from the primary model were developed using the polynomial equation and the square root model, respectively (Fig. 4). In the case of the polynomial equation, it is possible to use linear, quadratic, and cubic equations depending on the study results. A quadratic equation was used in this study. The secondary model that was developed can analyze the predicted values of the parameters (LPD, µmax) according to the temperature changes, and Tmin in the µmax model equation indicates the minimum temperature of bacterial growth. The minimum growth temperatures of S. aureus in grilled eggs, peeled quail eggs, and whole egg liquid were estimated to be 3.3, 4.5, and 5.2 °C, respectively. This result was similar to that of a growth prediction model for S. aureus in processed cheese that uses the square root (mozzarella: 6.3 °C, cheddar: 5.7 °C) (Kim et al., 2013). Also, the results of the growth prediction model for S. aureus in dried herring, which uses the square root, showed that the minimum growth temperature about 10 °C, indicating that bacterial growth was different depending on the food (Kang et al., 2013). S. aureus is generally known to be capable of growing at temperature of 7–48 °C, and in particular, at temperatures below 4 °C, it is known that the active transport system is degraded to inhibit the bacterial growth (McClure et al., 1997).
In order to verify the suitability of the developed model, the growth pattern of S. aureus at 25 °C was analyzed, and the LPD and µmax values were expressed through the Baranyi model. The RMSE, Af, Bf, and MRE values were calculated by comparing the predicted values at 25 °C in the secondary model equation (Table 2). RMSE, Af, and Bf are widely known for verifying the suitability of growth prediction models. The closer the RMSE and RME values are to zero, the better the suitability of the model is. And the closer Af and Bf are to 1, the better the suitability of the model is. In recent years, RMSE values are generally used to verify growth prediction models. However, each validation method is slightly different in terms of the accuracy (RMSE), validity (RME), inaccuracy (Af), and safety (Bf) (Bharathi et al., 2001; Moon et al., 2016) of the predicted model while evaluating the suitability of the model at the same time. Therefore, if various verification methods are used, it will be possible to accurately judge the suitability of a growth prediction model. The RME of the LPD and µmax values that have been computed in this study for the growth prediction models for grilled eggs and whole egg liquid were close to zero. Af was 1.1–1.2, which was close to 1.3, and Bf was 0.8–1.1, showing a value between 0.7 and 1.5, indicating a proper statistical fit. Similar results were obtained when compared to other studies (Cho et al., 2011b) on the growth prediction model of L. monocytogenes in smoked salmon (Af: 1.03–1.58, Bf: 1.01–1.55), a study (Kang et al., 2010) on the growth prediction of S. aureus and Bacillus cereus in RTE foods (Af: 1.04–1.37, Bf: 0.90–1.11), the growth prediction model (Park et al., 2009) of S. aureus in green-bean sprouts provided in school meals (Af: 1.10–1.31, Bf: 0.97–1.03, MSE: 0.002–0.02), and the suitability verification value (Yun et al., 2013) of the growth prediction model for pathogenic E. coli in paprika (Af: 1.04–1.18, Bf: 0.98–1.00, MRE: − 1.03, − 0.04). RMSE for the growth prediction model for grilled eggs, peeled quail eggs, and the whole egg liquid was 0.16, 0.26, and 0.27, respectively, showing a value that is closer to 0 than 0.300–0.5344, the RSME value measured in the study (Kim et al., 2013) on the prediction of growth of S. aureus in cheese. It is similar to the RMSE value of 0.09–0.24 in the predictive model (Park et al., 2010) of S. aureus growth in boiled meat, showing a high suitability.
S. aureus is a facultative anaerobe, which has a high potential for food poisoning due to its high proliferation potential in various foods. However, based on the results of the growth prediction model developed using the Baranyi model, safe consumption of egg products would be possible if the egg products are kept at ≤ 5 °C during distribution and storage. However, unlike peeled quail eggs and whole egg liquid that are distributed and stored at a refrigerated temperature, grilled eggs are heat treated, and stored at room temperature during sale. Thus, if the microorganisms penetrate into the egg shell due to cross contamination during distribution, there is a high possibility that the bacteria will proliferate in a short period of time. If the egg shell is broken, it should not be consumed, and the product should be discarded to prevent food poisoning.
At present, there has been no study on the risk assessment of food poisoning bacteria except Salmonella spp. in egg products. This study is expected to contribute to the food safety management through industrial applications, such as establishment of expiration dates and publication of risk assessment reports.
References
Baranyi J, Roberts TA. A dynamic approach to prediction bacterial growth in food. Int. J. Food Microbiol. 23: 277–294 (1994)
Baranyi J, Ross T, Roberts TA, MeMeekin TA. Effect of parameterization on the performance of empirical models used in ‘predictive microbiology’. Food Microbiol. 13: 83–91 (1996)
Bharathi S, Ramesh MN, Varadaraj MC. Predicting the behavioural pattern of Escherichia coli in minimally processed vegetables. Food Control. 12: 275–284 (2001)
Buchanan RL, Whiting RC, Damert WC. When is simple good enough: a comparison of the Gompertz, Baranyi, and three-phase linear models for fitting bacterial growth curves. Food Microbiol. 14: 313–326 (1997)
Cho JI, Lee SH, Lim JS, Kwak HS, Hwang IG. Development of a Predictive model describing the growth of Listeria Monocytogenes in fresh cut vegetable. J. Food Hyg. Saf. 26: 25–30 (2011)
Cho JI, Lee SH, Lim JS, Kwak HS, Hwang IG. Predictive mathematical model for the growth kinetics of Listeria monocytogenes on smoked salmon. J. Food Hyg. Saf. 26: 120–124 (2011)
Delignette-Muller ML, Rosso L, Flandrois JP. Accuracy of microbial growth predictions with square root and polynomial models. Int. J. Food Microbiol. 27: 139–146 (1995)
Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin. Microbiol Rev. 13: 16–34 (2000)
Duffy LL, Vanderline PB, Grau FH. Growth of Listeria monocytogenes on vaccum-packed cooked meats: effects of pH, aw, nitrite and ascorbate. Int. J. Food Microbiol. 23: 377–390 (1994)
Grijspeerdt K, Vanrolleghem P. Estimating the parameters of the Baranyi model for bacterial growth. Food Microbiol. 16: 593–605 (1999)
Hong SH, Park NY, Jo HJ, Ro EY, Ko YM, Na YJ, Park KC, Choi BG, Min KJ, Lee JK, Moon JS, Yoon KS. Risk ranking determination of combination of foodborne pathogens and livestock or livestock products. J. Food Hyg. Saf. 30: 1–12 (2015)
Jo HJ, Choi BG, Yan W, Moon JS, Kim YJ, Yoon KS. Microbiological quality and growth and survival of foodborne pathogens in ready-to-eat egg products. J. Food Hyg. Saf. 30: 178–188 (2015)
Jones FT, Rives DV, Carey JB. Salmonella contamination in commercial eggs and an egg production facility. Poult. Sci. 74: 753–757 (1995)
Kadariya J, Smith TC, Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. Biomed. Res. Int., 2014:827–965. https://doi.org/10.1155/2014/827965 (2014)
Kang HS, Ha SD, Jeong SW, Jang M, Kim JC. Predictive modeling of Staphylococcus aureus growth on Gwamegi (semidry Pacific saury) as a function of temperature. J. Korean Soc. Appl. Biol. Chem. 56: 731–738 (2013)
Kang KA, Kim YW, Yoon KS. Development of Predictive growth models for Staphylococcus aureus and Bacillus cereus on various food matrices consisting of ready-to-eat (RTE) foods. Korean J. Food Sci. Anim. Resour. 30: 730–738 (2010)
Kim KH, Park BY, Oh MH, Kim HW. Effect of storage temperature on growth and toxin production of Staphylococcus aureus in milk. Korean J. Dairy Sci. Technol. 32: 105–109 (2014)
Kim KM, Lee HY, Moon JS, Kim YJ, Heo EJ, Park HJ, Yoon Yh. Mathematical models to predict Staphylococcus aureus growth on processed cheeses. J. Food Hyg. Saf. 28: 217–221 (2013)
McClure PJ, Beaumont AL, Sutherland JP, Roberts TA. Predictive modeling of growth of Listeria monocytogenes: the effect on growth of NACL, pH, storage temperature and sodium nitrate. Int. J. Food Microbiol. 34: 221–232 (1997)
Moon HJ, Lim JG, Yoon KS. Comparative study of change in Salmonella Enteritidis and Salmonella Typhimurium populations in egg white and yolk. J. Food Hyg. Saf. 31: 342–348 (2016)
Pal A, Libuza TP, Diez-Gonzalez F. Comparison of primary predictive models to study the growth of Listeria monocytogenes at low temperatures in liquid cultures and selection of fastest growing ribotypes in meat and turkey product slurries. J. Food Microbiol. 25: 460–470 (2008)
Park HS, Bahk GJ, Park KH, Pak JY, Ryu K. Predictive model for Growth of Staphylococcus aureus in suyuk. Korean J. Food Sci. Anim. Resour. 30: 487–494 (2010)
Park HS, Kim MY, Jeong HS, park KH, Ryu K. Development of a predictive growth model of Staphylococcus aureus and shelf-life estimation of cooked mung bean sprouts served in school foodservice operations. J. Korean Soc Food Sci Nutr. 38: 1618–1624 (2009)
Park SY, Choi JW, Chung DH, Kim MG, Lee KH, Kim KS, Bahk GJ, Bae DH, Park SK, Kim KY, Kim CH, Ha SD. Development of a predictive mathematical model for the growth kinetics of Listeria monocytogenes in sesame leaves. Food Sci Biotechnol. 16: 238–242 (2007)
Ratkowsky DA, Olley J, McMeekin TA, Ball A. Relationship between temperature and growth rate of bacterial cultures. J. Bacteriol. 149: 1–5(1982)
Ratkowsky DA, Ross T. Modelling the bacterial growth/no growth interface. Lett Appl Microbiol. 20: 29-33 (1995)
Ross T. Belehradek-type models. J. Ind. Microbiol. Biotechnol. 12: 180–189 (1993)
Ross T. Indices for performance evaluation of predictive model in food microbiology. J. Appl. Bacteriol. 81: 501–508 (1996)
Ross T. Predictive food microbiology models in the meat industry. Meat and Livestock Australia, Sydney, Australia. pp. 196 (1999)
Van Impe JF, Poschet F, Geeraerd AH, Vereecken KM. Towards a novel class of predictive microbial growth models. Int. J. Food Microbiol. 100: 97–105 (2005)
Walls I, Scott VN. Use of predictive microbiology in microbial food safety risk assessment. Int. J. Food Microbiol. 36: 97–102(1997)
Wieneke AA, Roberts D, Gilbert RJ. Staphylococcal food poisoning in the United Kingdom 1969-90. Epidemiol. Infect. 110: 519–531 (1993)
Yilmaz MT. Identifiability of Baranyi model and comparison with empirical models in predicting effect of essential oils on growth of Salmonella typhimurium in rainbow trout stored under aerobic, modified atmosphere and vacuum packed conditions. Afr J Biotechnol. 10: 7468–7479 (2011)
Yoon KS. Application of Korea pathogen modeling program (KPMP) for food safety management. Food Sci Ind. 46: 2–12 (2013)
Yoon YH. Principle theory and application of predictive microbiology. Food sci Ind. 43: 70–74 (2010)
Yun HJ, Kim JH, Park KH, Ryu KY, Kim BS. Development and validation of predictive models of Escherichia coli O157:H7 growth in paprika. J. Food Hyg. Saf. 28: 168–173(2013)
Acknowledgements
This research was supported by the Korea Ministry of Food and Drug Safety (15161MFDS647). Later, this study will be used as a scientific basis for establishing food microbiological standards.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, WS., Son, N., Cho, JI. et al. Predictive model of Staphylococcus aureus growth on egg products. Food Sci Biotechnol 28, 913–922 (2019). https://doi.org/10.1007/s10068-018-0529-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-018-0529-4