Abstract
This study examined the antibacterial activities of two different cinnamon essential oil emulsions against Escherichia coli O157:H7 and Salmonella Typhimurium on basil leaves. Cinnamon oil (0.25%) treatments containing CPC (0.05%) exhibited greater effects on the pathogenic bacteria than cinnamon oil treatment without this emulsifier (p < 0.05). Treatment with cinnamon bark and leaf oil emulsions (CBE and CLE, respectively) reduced the populations of E. coli O157:H7 by 4.10 and 5.10 log CFU/g, and S. Typhimurium by 2.71 and 2.82 log CFU/g, respectively. Scanning electron micrographs showed morphological changes in the two pathogenic bacteria following emulsion treatment. In addition, there was no difference in the color or ascorbic acid content of the basil leaves by the emulsion treatment. These results suggest that CBE or CLE treatment can be an effective way to ensure the microbial safety of minimally processed vegetables and a good alternative to chlorination treatment in the fresh produce industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-quality, minimally processed vegetables are popular in fresh produce markets due to their high nutritional value and ease of consumption [1]. Non-thermal processing methods are usually used to deliver high-quality, minimally processed vegetables to consumers without health-related problems [2]. Chlorination, which is a commonly used non-thermal processing method, has the advantage of low processing cost [3]. However, there have been many studies aiming at replacing chlorination because of the harmful substances, such as trihalomethanes and chlorophenols, generated during this process [4].
Basil (Ocimum basilicum), which is frequently consumed in salads, is particularly vulnerable to foodborne bacteria such as Escherichia coli O157:H7 and Salmonella spp. [5, 6]. Due to difficulty in removing adherent bacteria and the nature of basil cultivation in the field, basil leaves can be contaminated during harvest [7], and contaminated basil-related foodborne illnesses have been reported [8]. Therefore, appropriate treatments are needed to ensure the microbial safety of basil.
Essential oils (EOs) are natural antimicrobial substances that are derived from plants [9]. Due to their strong antibacterial properties, EOs generally recognized as safe (GRAS) have been used as food preservatives [10, 11]. Burt [12] reported that, to have antimicrobial effects on fresh vegetables, the concentration of EOs should be higher than the minimum inhibitory concentration (MIC). However, application of EOs at concentrations that are too high can have a negative effect on food quality, such as an intense aroma of the EOs [13]. EOs exert antimicrobial effects by penetrating cell membranes and damaging cells, and the mechanism is explained by the main components of EOs [11, 14]. As the outer membrane present in gram-negative bacteria acts as a barrier against EOs, they are generally more resistant to EOs than gram-positive bacteria [11]. A solution to this problem is using an EO emulsion containing a surfactant; however, there have been few studies on EO emulsions [15].
Cinnamon (Cinnamomum zeylanicum) EOs are divided into two types based on their source; cinnamon bark (CB), which contains trans-cinnamaldehyde as the main component, and cinnamon leaf (CL), which contains eugenol as the major compound and very little trans-cinnamaldehyde [16, 17]. Both EOs have been used because they have excellent antibacterial effects due to penetrating cell membranes by the main component [14, 17].
In this study, cetylpyridinium chloride (CPC), a quaternary ammonium compound, was used as a surfactant to enhance the antibacterial effect of the cinnamon EOs. CPC has been widely used in oral hygiene products as a cationic surfactant that is known to have antibacterial properties [18]. CPC has been approved by the Food and Drug Administration (FDA) as GRAS, and its application has been extended to food industries [19]. However, there have been few studies on the use of CPC as an antimicrobial agent on fresh vegetables [20, 21]. In addition, its application as EO emulsion for fresh vegetables is limited [15]. Therefore, studies on EO emulsion with CPC are needed to improve the microbiological safety of fresh vegetables. The objective of this study was aimed to examine the antimicrobial activities of cinnamon EO/CPC emulsions against two major pathogenic bacteria on basil leaves.
Materials and methods
Materials
The basil (O. basilicum) leaves used for this study were harvested from a farm located in Pyeongtaek, Korea, right before the experiment. Upon harvest, the basil leaves were kept at 10 °C and were used within 24 h for the experiment. The cinnamon EOs (purity, 100%) and CPC were purchased from Gooworl Co. (Daegu, Korea) and Sigma-Aldrich Co. (St. Louis, MO, USA), respectively, before the experiment.
Preparation of bacterial culture
To examine the antibacterial activity of the EOs and EO emulsions, E. coli O157:H7 (ATCC 43889, NCTC 12079) and S. Typhimurium (KCTC 2421, ATCC 14028) were used as test bacteria (11). Both bacterial strains were streaked onto corresponding selective medium, i.e., MacConkey sorbitol agar (Difco Co., Detroit, MI, USA), and xylose lysine deoxycholate agar (Difco Co.), respectively. After streaking, the plates were incubated at 37 °C for 24 h, and colonies of E. coli O157:H7 and S. Typhimurium were added into tryptic soy broth (TSB) and incubated at 37 °C for 18 h. Cultures were then centrifuged at 2000×g for 15 min, washed twice with sterile 0.1% peptone water, and then re-suspended in the same. The density of each bacterial cocktail was approximately 8–8.5 log CFU/mL.
Minimum inhibitory concentration (MIC) measurement
The method used for measuring the MIC of the EOs in a 96-well plate was previously described by Bassanetti et al. [22]. Four EOs with different active ingredients were used: CB, CL, rosewood oil (RW), and tea tree oil (TT). First, 100 μL of sterile TSB was added to the plates. Then, each EO was dissolved in distilled water to a concentration of 8%, and 100 μL of each EO solution was added to the wells by twofold serial dilution. Finally, 100 μL of a tenfold diluted bacterial cocktail (approximately 7–7.5 log CFU/mL) was added. The total volume in each well was 200 μL, and the concentration range of the EOs was 2–4 × 10−7%. A well without EO was used as a control. All plates were incubated at 37 °C for 24 h, and then the optical density of each well was measured using a microplate reader (Bio-Rad, Hercules, CA, USA). The experiment was performed in triplicate.
Viable cell count assay
Viable cell counts were determined to assess the antimicrobial activity of CB, CL, CBE, and CLE. To obtain 7.5–8 log CFU/mL of each bacterial solution, the bacterial cocktail, which had been incubated for 18 h, was diluted fivefold with sterile 0.1% peptone water. For a single treatment, diluted inoculum and EO were mixed in a sterile tube to obtain a total volume of 10 mL. The CB and CL concentration used was 0.0625%, which was based on the MIC. CBE and CLE were applied to the diluted inoculum at a ratio of 5:1 (EO, 0.0625%; CPC, 0.0125%). All treatment solutions were homogenized with a sonicator (500 W, Sonics & Materials Inc., USA) for 10 min. The samples were then incubated at 37 °C for 0, 2, 5, 15, and 30 min, and 1-mL aliquots were removed and diluted serially. Then, a portion (100 μL) of each diluted sample was plated onto selective media, and the plates were incubated at 37 °C for 24–48 h.
Inoculation of pathogens on basil leaves
Fresh basil leaves, weighing about 1 g, were selected and treated with UV-C radiation (front and back, UV-340, Lutron Electronic Co., Taipei, Taiwan) for 10 min on a clean bench to reduce the background microflora on the leaves. After UV-C treatment, it was confirmed that there were no both pathogens used in this study on the leaves. Then, the leaves were spot-inoculated 5 times with 20 μL of each bacterial cocktail (100 μL total) and dried on a sterile aluminium foil for 40 min on a clean bench.
CB, CL, or CPC single treatment
The concentrations of the CB and CL solutions were set to 0.0625, 0.125, and 0.25% based on the MIC (0.0625%) of CL, and the concentrations of the CPC solution were 0.005, 0.01, and 0.05%. All solutions were homogenized in distilled water with ultrasonication (500 W) for 10 min. Inoculated samples (10 g) were immersed in each solution (500 mL) for 3 min and then dried for 30 min on a clean bench. The basil leaves (5 g) were transferred to a sterile bag containing 45 mL of sterile peptone water, and the bag was vigorously shaken by hand for 3 min. Then, the contents were placed in a sterile tube. The solution was diluted tenfold with sterile 0.1% peptone water, and the diluted solutions were plated onto selective media and incubated at 37 °C for 24–48 h.
Cinnamon EO emulsion treatment
CBE and CLE solutions were prepared by mixing CB and CL (0.25%) with CPC (0.05%), based on the single treatment results. First, 0.05% CPC was added to the distilled water and the mixture was stirred at 500 rpm for 10 min. Then, 0.25% of each cinnamon EO was added to a total volume of 500 mL and homogenized by ultrasonication (500 W) for 10 min. To determine the particle size, polydispersity index (PDI), and zeta (ζ) potential of both emulsions, particle size analyser (Malvern Instruments, Worcested, UK) was used. For the emulsions, the inoculated basil leaves were treated using the same method as described above for the single treatment.
Scanning electron microscopy (SEM) analysis
The morphology of the pathogens treated with CBE and CLE was examined with a focused ion beam SEM (Tescan, Warrendale, PA, USA). Each bacterial cocktail was diluted tenfold with 0.05 M phosphate buffered saline (PBS, pH 7.0), and the diluted cocktails were treated with CBE and CLE at 37 °C for 3 min. The treated samples were centrifuged at 3000×g for 15 min and washed three times with 0.05 M PBS. After washing the bacterial pellets, the samples were re-suspended in 0.05 M PBS containing 2.5% glutaraldehyde and incubated at 4 °C for 2 h. After incubation, the samples were centrifuged as described above, and then dehydrated in a graded series of ethanol (30, 50, 70, 95, and 100%). The final dehydrated samples were dropped onto a cover glass and dried. For the measurement, a cover glass was attached to the carbon tape and coated with osmium for 10 s.
Color measurement
The color changes in the treated basil leaves were determined using a colorimeter (Minolta Camera Co., Osaka, Japan). After drying, 30 points in the basil leaves were measured per treatment. The values are shown as Hunter value L, a, and b.
Ascorbic acid content measurement
The changes in the ascorbic acid content of the basil leaves were measured using 2, 6-dichlorophenol indophenol [23]. Each treated sample (1 g, each, including the control) was blended in 40 mL of extraction solution (pH 4.0) containing 5 g of oxalic acid and 0.75 g of EDTA in 1 L of distilled water for 1 min. The blended solution was centrifuged at 10,000×g for 15 min, and 1 mL of the supernatant was reacted with 5 mL of 2, 6-dichlorophenol indophenol (DIP) solution. The DIP solution was prepared by dissolving 100 mg of DIP in 1 L of distilled water at 80 °C, cooling the solution, and diluting 5 times with distilled water. The supernatant was reacted with the DIP solution, and the absorbance was measured at 520 nm (E1). Then, to bleach the pink colour, a drop of 1% ascorbic acid solution was added, and the solution was re-measured at the same wavelength (E2). The absorbance of a blank, containing the extraction solution and the DIP solution, was also measured at 520 nm (E0). The final absorbance was calculated as follows: [E0 − (E1 − E2)]. A standard curve was prepared using ascorbic acid. The ascorbic acid content of the basil leaves was expressed in ppm.
Statistical analysis
The experimental data were analysed using Duncan’s multiple range test in the Statistical Analysis System program version 9.4 (SAS Institute Inc., Cary, NC, USA), and P values less than 0.05 were considered statistically significant. All data were presented as mean ± standard deviation, and were the results of at least two independent experiments with triplicate assays.
Results and discussion
MIC of essential oils against E. coli O157:H7 and S. Typhimurium
CB and CL are typical EOs with antimicrobial activities. The MIC results were used to measure the antimicrobial activity of the EOs. As shown in Table 1, the MICs of CB and CL were lower than those of RW and TT and there were significant differences (p < 0.05). The MIC of CB was 0.125 and 0.0625% for E. coli O157:H7 and S. Typhimurium, respectively, whereas the MIC of CL was 0.0625% for both pathogens. These results indicate that CB and CL have superior antimicrobial effects against these two pathogens compared to those of the other two EOs, RW and TT. The antimicrobial activities of CB and CL come from the action of trans-cinnamaldehyde and eugenol [11]. However, trans-cinnamaldehyde and eugenol have different antimicrobial mechanisms, although they both penetrate and destroy bacterial cell membranes by the same principle [11, 24]. The MICs of CB and CL observed in this study are similar to those reported by Senhaji et al. [25] and Mazzarrino et al. [26].
Antimicrobial activity of cinnamon EOs and EO emulsions
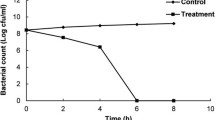
In the control, the populations of the two pathogens did not change during the first 30 min of incubation; however, treatment with CB or CL (single treatment) reduced the populations of the two pathogens by 2–3 log CFU/mL (Fig. 1). In addition, 2 min of treatment with CBE reduced the populations of E. coli O157:H7 and S. Typhimurium by 4.21 and 4.25 log CFU/g, respectively, and CLE treatment reduced them by 5.34 and 4.22 log CFU/g, respectively. And after 15 min of both treatments, neither pathogen was detected. The results of the viable cell count experiment showed that the antimicrobial effects of the emulsions on both pathogens were greater than those of the cinnamon EOs alone. Moghimi et al. [27] also reported that treatment with a sage oil emulsion had a greater inhibitory effect on E. coli O157:H7 than sage oil alone. Thus, it is apparent that the antimicrobial activity of EOs is increased when it is in the form of an emulsion.
Antimicrobial activity of Cinnamomum zeylanicum oil emulsions against pathogenic bacteria. (A) E. coli O157:H7, (B) S. Typhimurium (filled circle) control; (opened circle) cinnamon bark oil; (inverted filled triangle) cinnamon leaf oil; (opened triangle) cinnamon bark emulsion; (filled square) cinnamon leaf emulsion
Particle size, PDI, and zeta (ζ) potential of CBE and CLE
CBE and CLE had average sizes of 228.23 ± 9.83 and 169.17 ± 17.35 nm, with PDI of 0.161 ± 0.015 and 0.183 ± 0.026, respectively. The surfaces of the CBE and CLE had cationic charges with zeta (ζ) potential of 61.77 ± 1.47 and 74.63 ± 2.42 mV, respectively, mainly due to the property of CPC that is a cationic surfactant. These cationic charges make these emulsions penetrate easily the microbial cell membrane with negative charge [20].
Effects of washing treatment
The antimicrobial effects of CB and CL increased with increasing concentration [Fig. 2(A)], and at 0.25%, CB and CL reduced the population of E. coli O157:H7 by 2.27 and 2.67 log CFU/g, respectively, compared to the numbers of bacteria in the water washed samples. Likewise, treatment with 0.25% CB and CL reduced the S. Typhimurium population by 1.61 and 1.82 log CFU/g. Overall, S. Typhimurium was more resistant to the EOs than E. coli O157:H7. Our results are similar to those of Bhargava et al. [13], wherein E. coli O157:H7 was more sensitive to EO emulsion than S. Typhimurium. In comparison, CPC treatment at 0.05% decreased the populations of E. coli O157:H7 and S. Typhimurium by 0.87 and 0.56 log CFU/g, respectively [Fig. 2(B)]. Compared to the CB and CL as single treatments, treatment with CBE or CLE was more effective in terms of the log reduction of the two pathogens [Fig. 2(C)]. While CB and CL treatment reduced the population of E. coli O157:H7 by 2.07 and 2.39 log CFU/g, respectively, CBE and CLE treatment reduced the population of E. coli O157:H7 by 4.10 and 5.10 log CFU/g. Similarly, CBE and CLE treatment reduced the population of S. Typhimurium by 2.71 and 2.82 log CFU/g, respectively, whereas CB and CL treatment reduced it by 1.59 and 2.00 log CFU/g, respectively, suggesting a synergistic effect that has better antibacterial activity against pathogens than the sum of each single treatment.
Change in the populations of E. coli O157:H7 and S. Typhimurium on basil leaves by various treatments. (A) Cinnamon essential oil treatment, (B) cetylpyridinium chloride treatment, (C) cinnamon essential oil-cetylpyridinium chlroride emulsion treatment. filled square, E. coli O157:H7; opened square, S. Typhimurium
There are several limitations to the application of CB and CL as a washing agent for fresh produce, including the resistance of gram-negative bacteria to the EOs and their poor water solubility [15]. To resolve these issues, EOs can be mixed with emulsifiers to generate emulsions that can easily penetrate the cell membranes through the porins present in outer membrane of gram-negative bacteria [15]. The use of EO emulsions can increase the antibacterial activities of the EOs and decrease the resistance of gram-negative bacteria [15]. In this study, increased antimicrobial activities were clearly observed when CBE and CLE were applied to basil leaves inoculated with two pathogens compared to the effects of CB and CL. Similarly, it was reported that a carvacrol emulsion was effective for inhibition of E. coli O157:H7 on cabbage [28]. In addition, when 0.05% oregano oil and oregano oil emulsion were applied to lettuce inoculated with S. Typhimurium, a greater log reduction of S. Typhimurium was observed with the emulsion treatment [13, 29].
The use of EOs as EO emulsions enhances their wettability on the food surface and expands their coverage on fresh produce [13]. As a result, the antimicrobial activities of the EOs are increased [15]. In addition, the use of CPC as an emulsifier had a synergistic effect on the antimicrobial activities of CB and CL. CPC is a cationic surfactant that can penetrate a cell membrane with negative charges [20]. Chang et al. [30] reported that the antimicrobial activity of a thyme oil emulsion produced with a lauric arginate ester (LAE) emulsifier was greatly increased compared to the activity of the EO alone. Ruengvisesh et al. [31] also reported an increased antibacterial effect of a eugenol emulsion with LAE against foodborne pathogens inoculated on spinach.
The present study also demonstrated that CL showed enhanced antimicrobial activity against the two pathogens when compared the activity of CB. These results can be explained by the different major compounds in the EOs. Mattson et al. [32] reported that the antimicrobial activity of eugenol against Salmonella spp. was higher when compared to the same amount of trans-cinnamaldehyde. Kim and Rhee [33] also showed that eugenol led to a greater reduction of E. coli O157:H7 when compared to the reduction induced by trans-cinnamaldehyde with the same medium chain fatty acid.
SEM analysis and quality changes in basil leaves
Figure 3 shows the morphology of the two pathogens after treatment with CBE or CLE. SEM images of control pathogens showed that they were intact in terms of cellular morphology, whereas bacteria treated with CBE or CLE were severely damaged and wrinkled. This was similar to the morphological changes observed in EO-treated pathogens in previous studies [13, 34]. These results clearly demonstrate that treatment with CBE or CLE disrupts the cellular morphology. To monitor the quality changes in basil samples treated with cinnamon EOs and emulsions, the changes in the color and ascorbic acid contents of the basil leaves were determined after each treatment. There were no significant differences (p > 0.05) among the treatments, even when compared with the untreated control and water washed samples (Tables 2, 3). Therefore, these results clearly indicate that cinnamon EOs and emulsions do not affect the color and quality of the basil leaves.
In conclusion, the addition of CPC as an emulsifier enhanced the antimicrobial activities of CB and CL. EO emulsion containing CPC increased the solubility as well as the antibacterial effect of EO at relatively low concentration, indicating that the cost of washing process can be decreased and be more effective than chlorination treatment. Thus, CBE and CLE treatments are effective for improving microbiological safety and maintaining quality, including the color and ascorbic acid content of basil leaves. EO emulsions using cationic surfactants such as CPC are considered to be a suitable alternative to chlorine-based sanitizers to ensure the microbial safety of fresh produce. However, it should be noted that further studies are needed on the optimal combination of EO and cationic surfactants to control foodborne pathogens in minimally processed vegetables more effectively.
References
Patrignani F, Siroli L, Serrazanetti DI, Gardini F, Lanciotti R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Tech. 46: 311–319 (2015)
Rawson A, Patras A, Tiwari BK, Noci F, Koutchma T, Brunton N. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 44: 1875–1887 (2011)
Chun HH, Yu DJ, Song KB. Effects of combined nonthermal treatment on microbial growth and the quality of minimally processed yam (Dioscorea japonica Thunb) during storage. Int. J. Food Sci. Technol. 48: 334–340 (2013)
Gómez-López VM, Gil MI, Allende, Vanhee B, Selma MV. Water reconditioning by high power ultrasound combined with residual chemical sanitizers to inactivate foodborne pathogens associated with fresh-cut products. Food Control 53: 29–34 (2015)
Pezzoli L, Elson R, Little CL, Yip H, Fisher I, Yishai R, Mather H. Packed with Salmonella—investigation of an international outbreak of Salmonella senftenberg infection linked to contamination of prepacked basil in 2007. Foodborne Pathog. Dis. 5: 661–668 (2008)
Elviss NC, Little CL Hucklesby L, Sagoo S, Surman-Lee S, De Pinna E, Threlfall EJ. Microbiological study of fresh herbs from retail premises uncovers an international outbreak of salmonellosis. Int. J. Food Microbiol. 134: 83–88 (2009)
Tirawat D, Phongpaichit S, Benjakul S, Sumpavapol P. Microbial load reduction of sweet basil using acidic electrolyzed water and lactic acid in combination with mild heat. Food Control 64: 29–36 (2016)
Delbeke S, Ceuppens S, Jacxsens L, Uyttendaele M. Microbiological analysis of pre-packed sweet basil (Ocimum basilicum) and coriander (Coriandrum sativum) leaves for the presence of Salmonella spp. and Shiga toxin-producing E. coli. Int. J. Food Microbiol. 208: 11–18 (2015)
Jung SH, Song KB. Effects of lactic acid and lemongrass oil treatment on the pre-existing microorganisms and foodborne pathogens in Tatsoi (Brassica rapa var. rosularis) baby leaves. J. Food Sci. Technol. 52: 7556–7560 (2015)
Tzortzakis NG. Essential oil: Innovative tool to improve the preservation of fresh produce—a review. Fresh Produce 3: 87–97 (2009)
Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceut. 6: 1451–1474 (2013)
Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int. J. Food Microbiol. 94: 223–253 (2004)
Bhargava K, Conti DS, da Rocha SR, Zhang Y. Application of an oregano oil nanoemulsion to the control of foodborne bacteria on fresh lettuce. Food Microbiol. 47: 69–73 (2015)
Calo JR, Crandall PG, O’Bryan CA, Ricke SC. Essential oils as antimicrobials in food systems–a review. Food Control 54: 111–119 (2015)
Donsì F, Ferrari G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 233: 106–120 (2016)
Wang R, Wang R, Yang B. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov. Food Sci. Emerg. 10: 289–292 (2009)
Singh G, Maurya S, Catalan CA. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 45: 1650–1661 (2007)
Pohlman FW, Stivarius MR, McElyea KS, Johnson ZB, Johnson MG. The effects of ozone, chlorine dioxide, cetylpyridinium chloride and trisodium phosphate as multiple antimicrobial interventions on microbiological, instrumental color, and sensory color and odor characteristics of ground beef. Meat Sci. 61: 307–313 (2002)
Lim K, Mustapha A. Effects of cetylpyridinium chloride, acidified sodium chlorite, and potassium sorbate on populations of Escherichia coli O157:H7, Listeria monocytogenes, and Staphylococcus aureus on fresh beef. J. Food Prot. 67: 310–315 (2004)
Wang H, Li Y, Slavik MF. Efficacy of cetylpyridinium chloride in immersion treatment for reducing populations of pathogenic bacteria on fresh-cut vegetables. J. Food Prot. 64: 2071–2074 (2001)
Yang H, Cheng Y, Swem BL, Li Y. Efficacy of cetylpyridinium chlorine on Salmonella Typhimurium and Escherichia coli O157:H7 in immersion spray treatment of fresh‐cut lettuce. J. Food Sci. 68: 1008–1012 (2003)
Bassanetti I, Carcelli M, Buschini A, Montalbano S, Leonardi G, Pelagatti P, Rogolino D. Investigation of antibacterial activity of new classes of essential oils derivatives. Food Control 73: 606–612 (2017)
Pandey V, Patel A, Patra DD. Integrated nutrient regimes ameliorate crop productivity, nutritive value, antioxidant activity and volatiles in basil (Ocimum basilicum L.). Ind. Crop Prod. 87: 124–131 (2016)
Devi KP, Nisha SA, Sakthivel R, Pandian SK. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharm. 130: 107–115 (2010)
Senhaji O, Faid M, Kalalou I. Inactivation of Escherichia coli O157:H7 by essential oil from Cinnamomum zeylanicum. Braz. J. Infect Dis. 11: 234–236 (2007)
Mazzarrino G, Paparella A, Chaves-López C, Faberi A, Sergi M, Sigismondi C, Serio A. Salmonella enterica and Listeria monocytogenes inactivation dynamics after treatment with selected essential oils. Food Control 50: 794–803 (2015)
Moghimi R, Aliahmadi A, McClements DJ, Rafati H. Investigations of the effectiveness of nanoemulsions from sage oil as antibacterial agents on some food borne pathogens. LWT-Food Sci. Technol. 71: 69–76 (2016)
Sow LC, Tirtawinata F, Yang H, Shao Q, Wang S. Carvacrol nanoemulsion combined with acid electrolysed water to inactivate bacteria, yeast in vitro and native microflora on shredded cabbages. Food Control 76: 88–95 (2017)
Gündüz GT, Niemira BA, Gönül ŞA, Karapinar M. Antimicrobial activity of oregano oil on iceberg lettuce with different attachment conditions. J. Food Sci. 77: M412–M415 (2012)
Chang Y, McLandsborough L, McClements DJ. Fabrication, stability and efficacy of dual-component antimicrobial nanoemulsions: essential oil (thyme oil) and cationic surfactant (lauric arginate). Food Chem. 172: 298–304 (2015)
Ruengvisesh S, Loquercio A, Castell‐Perez E, Taylor TM. Inhibition of bacterial pathogens in medium and on spinach leaf surfaces using plant‐derived antimicrobials loaded in surfactant micelles. J. Food Sci. 80: M2522–M2529 (2015)
Mattson TE, Johny AK, Amalaradjou MAR, More K, Schreiber DT, Patel J, Venkitanarayanan K. Inactivation of Salmonella spp. on tomatoes by plant molecules. Int. J. Food Microbiol. 144: 464–468 (2011)
Kim SA, Rhee MS. Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) against Escherichia coli O157:H7. Food Control 60: 447–454 (2016)
Shen S, Zhang T, Yuan Y, Lin S, Xu J, Ye H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control 47: 196–202 (2015)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Park, JB., Kang, JH. & Song, K.B. Antibacterial activities of a cinnamon essential oil with cetylpyridinium chloride emulsion against Escherichia coli O157:H7 and Salmonella Typhimurium in basil leaves. Food Sci Biotechnol 27, 47–55 (2018). https://doi.org/10.1007/s10068-017-0241-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0241-9