Abstract
Conjugated linoleic acid (CLA) isomers, c9, t11-CLA and t10, c12-CLA, have been proved to exhibit excellent biomedical properties for potential use in anti-cancer applications and in reducing obesity. Acer truncatum Bunge (ATB), which is rich in unsaturated fatty acids, including oleic acid, linoleic acid, and nervonic acid, is a new resource for edible oil. In the present study, we developed a new method for producing two CLA isomers from ATB-seed oil by fermentation using Lactobacillus plantarum CGMCC8198 (LP8198), a novel probiotics strain. Polymerase chain reaction results showed that there was a conserved linoleate isomerase (LIase) gene in LP8198, and its transcription could be induced by ATB-seed oil. Analyses by gas chromatography–mass spectrometry showed that the concentration of c9, t11-CLA and t10, c12-CLA in ATB-seed oil could be increased by about 9- and 2.25-fold, respectively, after being fermented by LP8198.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conjugated linoleic acid (CLA) is defined as a naturally occurring group of conjugated diene acid isomers derived from linoleic acid, and the most common positional and geometric isomers are those with conjugated double bonds at C10 and C12 or at C9 and C11 [1, 2]. In 1987, CLA was firstly isolated and identified in fried ground beef [3]. In all of the possible cis and trans combinations of CLAs, c9, t11-CLA and t10, c12-CLA have been implicated as the most valuable isomers with noteworthy biological activities such as anti-carcinogenic, anti-obese, anti-diabetic and anti-hypertensive activities [4]. For example, it has been reported that c9, t11-CLA could inhibit the proliferation of estrogen receptor positive breast cancer cells by hormone-mediated mitogenic pathways, and t10, c12-CLA could ameliorate disorders of glucose and lipid metabolism through PPARγ and some other signal pathways [5,6,7,8]. Therefore, how to elevate the production of c9, t11-CLA and t10, c12-CLA has become a hot spot.
Acer truncatum Bunge (ATB) seed oil was approved as a New Resource Food by the National Health and Family Planning Commission of the People’s Republic of China in 2011, and this novel edible oil is richer with oleic acid, linoleic acid, and nervonic acid than other edible oils including rapeseed, peanut, grape and sunflower oils [9]. In addition, it was reported that the ATB extract might reduce weight and inhibit tumor cell proliferation by inhibiting fatty acid synthesis [10].
In this study, a conserved linoleate isomerase (LIase) gene in Lactobacillus plantarum CGMCC8198 (LP8198), a novel probiotics strain isolated in our previous study [11], was identified and analyzed. Subsequently, the effect of ATB-seed oil on the transcription of this LIase gene was examined via RT–PCR, and the bioconversion of c9, t11-CLA and t10, c12-CLA in the fermentation of LP8198 supplemented with ATB-seed oil was finally detected by gas chromatography–mass spectrometry (GC–MS).
Materials and methods
Plant materials, strains, media, and growth conditions
ATB seeds were obtained from Jindao Seed Company in Yangling, Shaanxi province, in October 2013, and the seeds were stored at − 80 °C until further use. The strain of Lactobacillus plantarum CGMCC8198 (LP8198) isolated from fermented herbage was cultured in de Man, Rogosa and Sharpe (MRS) medium comprising 1% tryptone, 0.5% meat extract, 0.5% yeast extract, 2% glucose, 0.1% Tween 80, 0.2% K2HPO4, 0.5% sodium acetate, 0.2% triammonium citrate, 0.02% MgSO4·7H2O, and 0.005% MnSO4·H2O (pH 6.2 ± 1) under anaerobic conditions at 30 °C for 24 h.
Total RNA extraction and RT–PCR
Prior to extraction of the total RNA of LP8198 using a Trizol reagent, the seeds were ground in liquid nitrogen. Then 2 μg total RNA was reverse-transcribed using M-MLV reverse transcriptase (Promega, BJ, CA) according to the manufacturer’s instructions with N6 primers (Invitrogen, BJ, CA).
Semi-quantitative PCR (semi-PCR) was performed using Applied Biosystems thermocycler (Applied Biosystems, Foster City, CA, USA). The PCR amplifications included an initial 5 min denaturation incubation at 95 °C followed by 30 cycles of denaturation (95 °C), annealing (52 °C), and elongation (72 °C) for 20 s by using 1.25 U of Taq DNA polymerase (TransGen Biotech, BJ, CA). Besides, an additional 72 °C final extension was performed for 10 min. PCR products were visualized on 2% agarose gels stained with ethidium bromide under UV transillumination. The gene of 16 s rRNA was used as an internal control to show equal loading of the cDNA samples. Besides, quantitative real-time PCR (qPCR) was further performed using a StepOne™ real-time PCR system (Applied Biosystems, Foster City, CA, USA). Bestar® SybrGreen qPCR Mastermix was obtained from DBI® Bioscience. The thermal profiles were 95 °C for 10 s and 60 °C for 1 min. Melting curve analysis was performed for each PCR to confirm the specificity of amplification. At the end of each phase, fluorescence was measured and quantified. Data was shown as a relative expression level of mRNA after normalization to 16 s rRNA. The primers for semi-PCR and qPCR analyses were as follows: LIase, 5′-CAACACGCCTGCTCCTGAA (forward), 5′-TGGGTGGTG ATCCGAACGA (reverse); 16S: AAGGCTGAAACTCAAAGG (forward), AACCCAACAT CTCACGAC (reverse).
Lipid extraction
The oil from ATB seeds was extracted by the Soxhlet extraction method. Prior to lipid extraction of the seeds, all experimental material was dried for 12 h at 45 °C. About 4 g of the seed powder was placed in a 250 mL distillation flask, and 150 mL of anhydrous diethyl ether was added. Extraction was conducted at 45 °C for 12 h, and the residual solvent of the extraction was dried under nitrogen. The obtained ATB-seed oil was added with a 30 mg mL−1 stock solution containing 2/3 (w/w) Tween 80 and filter sterilized through a 0.22 μM Minisart filter (Agilent) and stored in the dark at − 20 °C before use.
Fermentation of ATB-seed oil by LP8198
LP8198 was inoculated (1%) in MRS broth with or without 0.5 mg mL−1 ATB-seed oil and then incubated anaerobically at 30 °C with a mixture of 80% nitrogen, 10% carbon dioxide, and 10% hydrogen. After 24-h fermentation, the cultures were centrifuged at 5000 g for 10 min at room temperature. The lipid of the culture supernatant fluid was extracted by using a hexane/methanol (2:1, v/v) solution at room temperature and then centrifuged at 5000 g for 10 min at 4 °C after being shocked fully. The chloroform phase was finally dried under nitrogen.
GC–MS analysis of fatty acid in total lipid extracts
Fatty acids were converted to the corresponding methyl esters before GC–MS analysis. In brief, the total lipid extracts were reacted with 1 mL 0.5 M NaOH-CH3OH at 65 °C for 30 min, and then 1 mL BF3-CH3OH was added to the reaction liquid at 70 °C for 2 min after cooling down. Subsequently, the esterified products were extracted with n-hexane by oscillating, and then a saturated NaCl aqueous solution was added to the entire mixture. After being agitated for 2 min, the fatty acid methyl esters (FAMEs) were removed from the upper layer and stored at − 20 °C.
FAMEs were analyzed by an Agilent 7890A GC with an Agilent 5975B Inert XL mass selective detector using an HP-5 column (Agilent 19091 J-416, CA; 60 m × 320 μm × 0.25 μm) with the following temperature program: initial temperature 50 °C, increased to 200 °C at 10 °C/min and 230 °C at 2 °C/min, and then raised at 8 °C/min to 270 °C and held for 15 min. Besides, the inlet temperature was 270 °C with constant flow of nitrogen (N2) at 1 mL/min in split mode (50:1). The transfer lines were set to 280 °C, and the temperature of quadrupole and the MS ion source were 230 and 150 °C, respectively. MS detection mode was set as electron impact ionization, scanning from 35 amu to 800 amu masses. Characteristic peaks were identified by comparing with the NIST08 MS library and retention time of external c9, t11-CLA analytical standard (Sigma 16413, CA) and t10, c12-CLA analytical standard (NU-CHEK-PREP, INC. UC-61-A, USA).
The c9, t11-CLA and t10, c12-CLA standard curves were constructed using the concentration gradients of the corresponding methyl esters. The methods of methyl esterification and GC–MS were the same as above.
Bioinformatics analysis
Firstly, the amino acid sequence homology comparison was performed by NCBI BLASTP, and 9 LIase gene sequence (Table 1) alignment was analyzed by CLUSTAL-X. Subsequently, a phylogenetic tree was constructed with MEGA6. Furthermore, the subcellular localization analysis was performed using TargetP 1.1 Server (http://www.cbs.dtu.dk/services/TargetP/), the transmembrane segment prediction was performed using the TMpred Server (http://www.ch. embnet.org/software/TMPRED_form.html), and the tertiary structure of this protein was established by SWISS-MODEL (http://swissmodel.expasy.org/) and visualized by Swiss-PDB-Viewer based on homology modeling.
Statistical analysis
Statistical evaluations were performed using GraphPad PRISM 5.0, with three independent experiments. The statistics were analyzed using Student’s t test. Differences at P < 0.05 were considered statistically significant.
Results and discussion
Identification, analysis, and phylogenetic analysis of LIase in LP8198
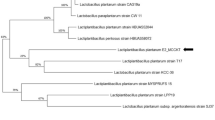
As shown in Fig. 1, the full-length cDNA of LIase is 1710 bp, comprising a 5′ untranslated region of 15 bp and an uninterrupted open reading frame (ORF) of 1695 bp, and the complete CDS region was submitted to GenBank by BankIt tool and acquired GenBank ID as KU555936. The predicted ORF of the cDNA encodes a protein of 564 amino acids with a molecular weight of 64.23 kDa and a theoretical pI of 5.36. Besides, it was predicted as a stable protein by the ProtParam tool (http://web.expasy.org/protparam/). Furthermore, a phylogenetic tree of the obtained LIase in LP8198 was constructed, and the results indicated that the gene is most closely related to the linoleic acid isomerase gene of L. plantarum strains lp15-2-1 and ZS2058 [Fig. 2(A)].
Further bioinformatics analyses of LIase in LP8198 by TargetP 1.1 Server indicated that it is a secretory pathway signal peptide (Table 2). Subsequently, the possible transmembrane helices structure performed by the TMpred Sever indicated that the N-terminal region includes 18 amino acids (from 6 th aa to 23 th aa) which were predicted as an inside to outside helices structure [Fig. 2(B)]. Besides, the tertiary structure of this protein was also established by SWISS-MODEL and visualized by Swiss-PDB-Viewer based on homology modeling [Fig. 2(C)].
ATB-seed oil induced the transcription of LP8198 LIase
Since the content of linoleic acid was up to about 34% in ATB-seed oil (Fig. 3), we speculated whether ATB-seed oil could affect the transcription of LP8198 LIase. To confirm this issue, the transcriptional level of LP8198 LIase was detected by semi-PCR and qPCR with different fermentation times and substrate concentrations. As shown in Fig. 4, the mRNA level of LIase was upregulated depending on time and dose was time-dependently and dose-dependently upregulated by ATB-seed oil. When the lactobacilli were treated by 1 mg/mL ATB-seed oil for 24 h, the mRNA level of LIase could attain a value nearly 15 fold of that of the control group.
The effects of ATB-seed oil on transcriptional level of LP8198 LIase. (A) The semi-PCR analysis of the transcriptional level of LIase in LP8198 without ATB-seed oils. Lanes 1–3 represent fermentation for 12, 24, and 36 h, respectively. (B) The semi-PCR analysis of the transcriptional level of LIase in LP8198 treated with 0.5 mg/mL ATB-seed oils for different times. Lanes 1–3 represent fermentation for 12, 24, and 36 h, respectively. (C) The semi-PCR analysis of the transcriptional level of LIase in LP8198 treated with different concentrations of ATB-seed oils for 24 h. Lanes 1–4 represent the treatments of ATB-seed oil at 0, 0.25, and 0.5 1 mg/mL, respectively. (D), (E), and (F) were detected by qPCR and the treatment was consistent with that of (A), (B), and (C)
c9, t11-CLA and t10, c12-CLA could be biotransformed by LP8198 fermentation with ATB-seed oil
Accumulating evidence has demonstrated that c9, t11-CLA and t10, c12-CLA, two major isomers of CLA, have excellent biomedical properties for potential use in anti-cancer applications and for improving immunity, preventing inflammation, reducing obesity by different pathways such as Wnt/beta-catenin pathway, hormone-mediated mitogenic pathway, PPARγ, 5-lipoxygenase (5-LOX) pathway and NF-κB pathway [6,7,8, 12,13,14,15]. Although there was tremendous potential for the application of CLA isomers, their source of human daily intake was too limited to reach the recommended dosage, 3 g/d, which would be required to observe health benefits in human subjects [16]. Thus, the development of production technology of these CLA isomers is very necessary.
Although CLA could be chemically synthesized, this method would produce mixed products, which might contain some unsafe ingredients [12]. Therefore, selective synthesis of CLA isomers by microbial transformation has received great interest. Nowadays, biosynthesis of CLA isomers, especially c9, t11-CLA and t10, c12-CLA, by linoleate isomerase in Butyrivibrio fibrisolvens and Propionibacterium acnes, has been well studied [17, 18]. Besides, a series of lactobacillus have also become the protagonist to produce c9, t11-CLA and t10, c12-CLA in the recent years. It has been reported that some L. plantarum strains could convert linoleic acid to c9, t11-CLA and t10, c12-CLA by LIase [18, 19]. Li and his colleagues analyzed CLA bioconversion by six L. plantarum strains cultured in MRS broth supplemented with sunflower oil, and the results showed that the production of CLA was increased by adding high concentration of substrate in sunflower oil, and L. plantarum IMAU60042 produced the highest CLA [20]. Besides, the study of Elaheh Sadat Hosseini had also shown that both sunflower oil and castor oil could be used as substrates for the production of c9, t11-CLA and t10, c12-CLA by Lactobacillus fermentation [21]. Here, to validate whether LP8198 could biotransform linoleic acid from ATB-seed oil into c9, t11-CLA and t10, c12-CLA, the concentration of these two CLA isomers in 0.5 mg/mL ATB-seed oil before and after LP8198 fermentation was detected by GC–MS. As shown in Fig. 5, according to the standard curves, the results showed that the concentration of c9, t11-CLA could be increased from 0.23 to 2.06 mg/mL by about ninefold and that of t10, c12-CLA could be increased from 1.68 to 3.79 mg/mL by about 2.25-fold.
The concentration of c9, t11-CLA and t10, c12-CLA in ATB-seed oil before and after being fermented using LP8198. (A) The standard curve of c9, t11-CLA detected by GC–MS. (B) The concentration of c9, t11-CLA in a 0.5 mg/mL ATB-seed oil emulsion before and after being fermented using LP8198 for 24 h. (C) The standard curve of t10, c12-CLA detected by GC–MS. (D) The concentration of t10, c12-CLA in a 0.5 mg/mL ATB-seed oil emulsion before and after being fermented using LP8198
In summary, here we discovered a new lactobacillus strain which might produce c9, t11-CLA and t10, c12-CLA during fermentation with ATB-seed oil, a kind of valuable edible oil which has noteworthy health benefits and has been authorized as a New Resource Food in China [9, 10]. To the best of our knowledge, this study was applied for the first time to ATB-seed oil for producing c9, t11-CLA and t10, c12-CLA by microbial fermentation. These findings might provide some new theoretical basis to develop a new resource for CLA isomers, and meanwhile, it also might contribute to new applications of ATB.
References
Demir AS, Talpur FN. Chemoenzymatic conversion of linoleic acid into conjugated linoleic acid. J. Agric. Food Chem. 58: 1646–52 (2010)
Sun C, Black BA, Zhao YY, Ganzle MG, Curtis JM. Identification of conjugated linoleic acid (CLA) isomers by silver ion-liquid chromatography/in-line ozonolysis/mass spectrometry (Ag + -LC/O3-MS). Anal. Chem. 85: 7345–52 (2013)
Ha YL, Grimm NK, Pariza MW. Anticarcinogens from fried ground beef: heat-altered derivatives of linoleic acid. Carcinogenesis 8: 1881–7 (1987)
Koba K, Yanagita T. Health benefits of conjugated linoleic acid (CLA). Obes. Res. Clin. Pract. 8: e525–32 (2014)
Brown JM, Boysen MS, Jensen SS, Morrison RF, Storkson J, Lea-Currie R, Pariza M, Mandrup S, McIntosh MK. Isomer-specific regulation of metabolism and PPARgamma signaling by CLA in human preadipocytes. J. Lipid. Res. 44: 1287–300 (2003)
Durgam VR, Fernandes G. The growth inhibitory effect of conjugated linoleic acid on MCF-7 cells is related to estrogen response system. Cancer Lett. 116: 121–30 (1997)
Yeganeh A, Taylor CG, Poole J, Tworek L, Zahradka P. Trans10, cis12 conjugated linoleic acid inhibits 3T3-L1 adipocyte adipogenesis by elevating beta-catenin levels. Biochim. Biophys. Acta. (2016)
Lehnen TE, da Silva MR, Camacho A, Marcadenti A, Lehnen AM. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J. Int. Soc. Sports Nutr. 12: 36 (2015)
Zhao WH, Zhang JF, Zhe W, Zhang YX, Tian WX. The extract of leaves of Acer truncatum Bunge: A natural inhibitor of fatty acid synthase with antitumor activity. J. Enzyme Inhib. Med. Chem. 21: 589–96 (2006)
Zhao WH, Gao LF, Gao W, Yuan YS, Gao CC, Cao LG, Hu ZZ, Guo JQ, Zhang YX. Weight- reducing effect of Acer truncatum Bunge may be related to the inhibition of fatty acid synthase. Nat. Prod. Res. 25: 422–31 (2011)
Gu XC, Luo XG, Wang CX, Ma DY, Wang Y, He YY, Li W, Zhou H, Zhang TC. Cloning and analysis of bile salt hydrolase genes from Lactobacillus plantarum CGMCC No. 8198. Biotechnol. Lett. 36: 975–83 (2014)
Kelley NS, Hubbard NE, Erickson KL. Conjugated linoleic acid isomers and cancer. J. Nutr. 137: 2599–607 (2007)
Rosberg-Cody E, Stanton C, O’Mahony L, Wall R, Shanahan F, Quigley EM, Fitzgerald GF, Ross RP. Recombinant lactobacilli expressing linoleic acid isomerase can modulate the fatty acid composition of host adipose tissue in mice. Microbiology 157: 609–15 (2011)
Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobesity effect of trans-10,cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J. Appl. Microbiol. 103: 1140–6 (2007)
Shokryzadan P, Rajion MA, Meng GY, Boo LJ, Ebrahimi M, Royan M, Sahebi M, Azizi P, Abiri R, Jahromi MF. Conjugated Linoleic Acid: A Potent Fatty Acid Linked to Animal and Human Health. Crit. Rev. Food Sci. Nutr: 0 (2015)
Mushtaq S, Heather Mangiapane E, Hunter KA. Estimation of cis-9, trans-11 conjugated linoleic acid content in UK foods and assessment of dietary intake in a cohort of healthy adults. Br. J. Nutr. 103: 1366–74 (2010)
Gorissen L, Leroy F, De Vuyst L, De Smet S, Raes K. Bacterial production of conjugated linoleic and linolenic Acid in foods: a technological challenge. Crit. Rev. Food Sci. Nutr. 55(11): 1561–74 (2015)
Yang B, Chen H, Gu Z, Tian F, Ross RP, Stanton C, Chen YQ, Chen W, Zhang H. Synthesis of conjugated linoleic acid by the linoleate isomerase complex in food-derived lactobacilli. J. Appl. Microbiol. 117: 430–9 (2014)
Niu XY, Chen W, Tian FW, Zhao JX, Zhang H. Bioconversion of conjugated linoleic acid by resting cells of Lactobacillus plantarum ZS2058 in potassium phosphate buffer system. Acta Meteorol. Sin. 47: 244–8 (2007)
Li H, Liu Y, Bao Y, Liu X, Liu X, Zhang H. Conjugated linoleic acid conversion by six Lactobacillus plantarum strains cultured in MRS broth supplemented with sunflower oil and soymilk. J Food Sci 77: M330–6 (2012).
Hosseini, E.S, Kermanshahi, R.K, Hosseinkhani, S, Shojaosadati, S.A, Nazari, M. Conjugated linoleic acid production from various substrates by probiotic Lactobacillus plantarum. Ann. Microbiol. 65: 27–32 (2015).
Acknowledgements
This study was financially supported by the National Key R&D Program of China (2017YFD0400303), the 863 (Hi-tech research and development program of China) program under contract NO. 2012AA022108, the Tianjin Research Program of Application Foundation and Advanced Technology (14JCZDJC33200, 15JCQNJC45800), the State Key Laboratory of Tree Genetics and Breeding (Chinese Academy of Forestry) (TGB2016002), and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT1166).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, DJ., Yan, LH., Li, Q. et al. Bioconversion of conjugated linoleic acid by Lactobacillus plantarum CGMCC8198 supplemented with Acer truncatum bunge seeds oil. Food Sci Biotechnol 26, 1595–1611 (2017). https://doi.org/10.1007/s10068-017-0218-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0218-8