Abstract
Fish protein hydrolysates (FPHs) were prepared from freshwater carps (Catla catla, Labeo rohita, and Cirrhinus mrigala) using flavorzyme at different degrees of hydrolysis (DHs) ranging from 5 to 20%. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free-radical-scavenging activity of the FPHs prepared from the three species were in the range of 50–82%; the ferric reducing power of the FPHs prepared from catla was the highest. The linoleic acid peroxidation inhibition activity of the prepared FPHs varied from 71 to 91%. The emulsion activity index of the FPHs prepared from catla and rohu decreased significantly with an increase in the DH (p < 0.05). The emulsion stability index of the FPHs prepared from the three species was the highest at 20% DH. FPHs prepared from freshwater carps possess good antioxidant and surface-active properties and are therefore suitable to be used as natural antioxidants in health-food formulation and as water-soluble antioxidants in the food-processing industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, fish protein hydrolysates (FPHs) have received significant attention owing to their bioactive properties. FPHs are mainly derived using food-grade proteases from various sources. Application of enzymatic hydrolysis to FPH production is a highly convenient technology and an easy approach for improving the quality and usability of FPHs [1]. FPHs comprise amino acids and peptides of varying sizes depending on the extent of hydrolysis and nature of proteases [2]. The peptides present in FPHs have been reported to exhibit bioactive properties, such as antioxidant properties, angiotensin-I converting enzyme (ACE) inhibitory activity, immune modulation, antithrombotic and antidiabetic properties [3,4,5,6]. The peptides responsible for bioactivity have a molecular mass of less than 6000 Da, and the number of amino acid residues varies from 2 to 20 [7].
The properties of FPHs are mainly governed by the amino acid sequence and the composition of released peptides; they also depend on the nature of the substrate used for hydrolysis, the type of enzyme, and the extent of hydrolysis [8]. Among the bioactive properties of FPHs, antioxidant activity has been studied extensively considering its applications in food processing. Prevention of lipid oxidation is essential to minimize nutritional and economical losses in the food-processing sector and to protect consumers against oxidative-stress-mediated diseases [9]. Long-term use of synthetic antioxidants is suspected to have toxic effects. There is a growing interest in nontoxic natural antioxidative compounds as an alternative to synthetic antioxidants. FPHs prepared from different species such as grass carp (Ctenopharyngdon idella), mackeral (Scomber austriasicus), sardinella (Sardinella aurita), and red snapper (Lutjanus vitta) have been reported to exhibit antioxidative properties [10,11,12,13]. In addition, the FPH provides higher nutritional benefits due to the presence of low molecular weight peptides and free amino acids [14].
India occupies the second position in the world in terms of aquaculture production, and carps are the major species cultured here, especially Catla catla, Labeo rohita, and Cirrhinus mrigala (Indian major carps). The utilization of these carps is mainly in fresh and iced forms. Preparing FPHs with nutraceutical value may strengthen the link between their production and utilization. Studies on the bioactive properties of protein hydrolysates from the muscles of Indian major carps limited or rarely found in the literature. Hence, the objectives of the present investigation are to prepare FPHs from freshwater carps using flavorzyme and to evaluate their emulsion and antioxidant properties. Flavorzyme is a mixture of proteases obtained from Aspergillus oryzae, and it exhibits both endoprotease and exopeptidase activities. Consequently, it can cause the formation of a wide range of peptides. In addition, it is known to minimize the bitterness of hydrolysates. It is one of the most studied proteolytic enzymes to produce protein hydrolysates with potential bioactive properties, including antioxidant properties. Our research interest is to produce protein hydrolysates from freshwater carps as a functional food ingredient with bioactive potential. With this rationale, we have chosen flavorzyme in the present study. This study supports the possibility of using freshwater carps for the preparation of muscle protein hydrolysates as a base for the development of functional food ingredients.
Materials and methods
Fish
Freshwater carps, viz. C. catla, L. rohita, and C. mrigala, were procured from a private farm at Shimoga, Karnataka, India. The fish were fed using the conventional feed (rice bran and groundnut oil cake) during the culture period. Harvesting was performed in February, and the fish were brought to the laboratory under iced conditions. The length and weight of the three species of the Indian major carps used were between 30 and 40 cm and 550 and 900 g, respectively.
Chemicals
Flavorzyme (from A. oryzae, activity >500 U/g, density 1.10–1.30 g/mL), 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals, iron (II) chloride, butylated hydroxy anisole (BHA), butylated hydroxyl toluene (BHT), α-tocopherol, linoleic acid, Sephadex G-25, aprotinin, vitamin B12, and tryptophan were purchased from Sigma–Aldrich, Inc. (St. Louis, MO, USA). All the other chemicals and reagents used were of analytical grade.
Preparation of substrate
The fish were washed with chilled water and dressed. The meat was separated using a meat–bone separator (SG, a model of Toyo Seikan Kaisha Limited, Tokyo, Japan) and washed with chilled potable water (4 ± 1 °C). The quantity of water used for washing was 1:3 (meat:water, w/v). The slurry was agitated for 3 min and allowed to settle for 7–10 min. Water was decanted and filtered through a muslin cloth. Excess water was removed manually by squeezing the mince. The water-washed meat was frozen at −35 °C using an air-blast freezer (Armfield, Armfield Limited, Ringwood Hampshire, England) and then stored at −20 °C until further use.
Preparation of fish protein hydrolysates
FPHs were prepared from the three freshwater carps using flavorzyme according to the procedure described by Elavarasan and Shamasundar [5]. The frozen mince was thawed at 4 ± 2 °C and used for the preparation of hydrolysates. The fish mince was mixed with water in a 1:2 ratio (fish mince:water). The following conditions were used for hydrolysis: temperature = 50 °C, pH 6.5 ± 0.2, and time of hydrolysis = 60 min. The mixture was pre-incubated at 50 °C, and the hydrolysis reaction was initiated by adding the enzyme. The concentration of the enzyme added was derived from the plot of the log of the enzyme to substrate concentration versus degree of hydrolysis (DH) to achieve DHs of 5, 10, 15, and 20% (data not given). The DH was calculated as the ratio of α-amino nitrogen released during hydrolysis to total protein nitrogen in the sample taken for the hydrolysis reaction. Total protein nitrogen was estimated using the Kjeldahl method [15], and α-amino nitrogen was determined according to the method described by Taylor [16]. The hydrolytic reaction was deactivated by heating the reaction mixture in a boiling water bath for 15 min. It was filtered using Grade 4 Whatman filter paper (GE Healthcare UK Ltd, Amersham Place Little Chalfont, Bucking Hampshire, UK), and the filtrate was oven dried at 80 ± 2 °C till the moisture content was less than 5%. Hydrolysates were designated based on the species used for hydrolysis, i.e., as HCF (catla), HRF (rohu), and HMF (mrigal), and stored under desiccation at ambient temperature.
Analyses
DPPH free-radical-scavenging activity
The DPPH free-radical-scavenging activities of the FPHs were determined according to the method described by Yen and Wu [17] at a protein concentration of 10 mg/mL. The DPPH free-radical-scavenging activity was calculated as:
Appropriate control was maintained using double-distilled water instead of sample. BHA and BHT were used at a concentration of 200 ppm as positive controls to compare the activity. The analysis was performed in triplicate, and the mean values with standard deviation were reported.
Ferric reducing antioxidant power (FRAP) assay
The ferric reducing power of FPHs was determined using the method described by Oyaiza [18] at a protein concentration of 40 mg/mL. Color development was measured at 700 nm using a double-beam spectrophotometer. BHA and BHT were used at a concentration of 200 ppm as positive controls. The higher absorbance of the reaction mixture indicated a higher reducing power. The test was performed in triplicates, and the mean values with standard deviation were reported.
Peroxidation inhibition activity in the linoleic acid model system
The linoleic acid peroxidation inhibition (LAPI) activity of the FPHs was measured according to the method described by Osawa and Namiki [19] at a protein concentration of 10 mg/mL. The degree of oxidation of linoleic acid was measured using the ferric thiocyanate method, which was reported by Mitsuda et al. [20]. A natural antioxidant α-tocopherol was used as the internal standard. The experiments were performed in triplicate. The ability of the FPHs to inhibit peroxide formation in linoleic acid model system was calculated using a formula mentioned below and expressed as the percentage of the inhibition.
Emulsion properties
Emulsion properties of the FPHs, such as the emulsion activity index (EAI) and emulsion stability index (ESI), were studied. EAI was determined according to the method described by Pearce and Kinsella [21]. The turbidity of the emulsion prepared was measured at 500 nm. The absorbance values measured immediately (A0) and 10 min after emulsion formation (A10) were used to calculate the EAI and ESI as follows:
where ∆A = A0 − A10, ∆t = 10 min, A0 = absorbance at 0 min, A10 = absorbance after 10 min.
Fractionation of HCF-DH 5% using size-exclusion chromatography
The FPHs obtained from catla with a 5% DH (HCF-DH 5%) were used for fractionation using size-exclusion chromatography. The choice of HCF-DH 5% for fractionation was based on the experimental results obtained for the antioxidant properties. Approximately, 0.5 g of HCF-DH 5% was dissolved in 5 mL of distilled water and filtered using a syringe filter made of nylon with a 0.2-μm pore size. An aliquot of 2 mL of a hydrolysate solution was loaded into a Sephadex G-25 gel filtration column (1.6 cm × 96 cm) pre-equilibrated with distilled water. Elution was performed with distilled water, and fractions (3 mL each) were collected at a flow rate of 30 mL/h. Moreover, the absorbance at 280 nm was recorded. The different fractions collected were pooled separately, freeze dried (Edward Freeze Dryer, Super Modulyo, UK), and stored under desiccated conditions at ambient temperature. The DPPH free-radical-scavenging activity and ferric reducing power of the fractions were analyzed at a protein concentration of 3 mg/mL, as described earlier. The standard molecular weight markers used were aprotinin (6512 Da), Vitamin B12 (1356.37 Da), and tryptophan (204.22 Da). The molecular weight was determined by the plot of Ve/Vo versus log10 molecular weight; Ve is the elution volume and Vo is the void volume.
Amino acid composition in the fraction of HCF-DH 5%
The amino acid composition in the fraction of HCF-DH 5% was analyzed. The amino acid content was determined after derivatization with phenylisothiocyanate (PITC), according to the Waters Pico-Tag method reported by Bidlingmeyer et al. [22] using the Waters Pico-Tag HPLC amino acid analyzer (Water Model 712 WISP, Waters, Watford, Herts, UK).
Statistical analysis
Statistical analysis was performed to test the effect of DH and the type of species on the antioxidative properties. The data were subjected to analysis of variance (ANOVA). The significant difference between the means was determined by Duncan’s multiple range comparison test using a statistics program (SPSS.16.0 for windows, SPSS Inc., Chicago, IL).
Results and discussion
In the present investigation, FPHs prepared from freshwater carps were produced at different DHs using flavorzyme and evaluated for their antioxidant and surface active properties.
Antioxidant properties
In general, the in vitro antioxidant properties of bioactive substances are assayed using more than one method. In the present study, we used the DPPH free-radical-scavenging assay, the ferric reducing antioxidant power (FRAP) assay, a fatty acid model system-the LAPI assay (LAPI). The DPPH assay indicates the ability of the test sample to provide hydrogen (proton), and the FRAP assay indicates the ability of the sample to donate electron. The LAPI assay imitates the situation where the oxidation of lipids can be minimized using the studied material. Hence, in the present study, assays working on different mechanisms, i.e., electron transfer and proton transfer, and a model system that uses hydrolysates to inhibit lipid/fatty acid oxidation were employed.
DPPH free-radical-scavenging activity
The DPPH free-radical-scavenging activities of HCF, HRF, and HMF are shown in Fig. 1(A). The DPPH free-radical-scavenging assay has been widely used to investigate the scavenging activities of antioxidant compounds. DPPH is a stable free radical that shows maximum absorbance at 517 nm in ethanol. In the presence of a proton/electron-donating substance, the DPPH radical is reduced and forms yellow color [23]. The results of the present study indicate that the peptides present in FPHs could donate protons/electrons that could interact with the DPPH free radical and reduce it to a stable form. With increasing DH, there was a marginal increase in the scavenging activities of HCF and HMF, whereas, HRF showed a radical-scavenging activity independent of DH. The difference in the capacity of the FPHs to reduce the stable nitrogen free radical (DPPH) could be due to different amino acid compositions, sequences, and sizes of the peptides present in them. The DPPH free-radical-scavenging activities of the FPHs prepared with 20, 30, and 40% DH from brown stripe red snapper using alcalase and flavourzyme were significantly different [13]. The protein hydrolysates prepared from round scad muscle using flavorzyme and alcalase showed an increase in the DPPH free-radical-scavenging activity with an increase in the DH [24]. The antioxidant properties of the FPHs prepared from yellow stripe trevally using flavorzyme and alcalase showed a decrease in the DPPH activity with an increase in the DH [25]. These results suggest that the nature of substrate is an important factor that affects the DPPH free-radical-scavenging activity. In the present study, all hydrolysates showed significantly lower radical-scavenging activities than BHA (p < 0.05).
DPPH free-radical-scavenging activity (A), ferric reducing antioxidant power (B), and linoleic acid peroxidation inhibition activity (C) of the FPHs from C. catla (HCF), L. rohita (HRF), and C. mrigala (HMF), influenced by the DH. Two synthetic antioxidants, namely butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT), were used for comparison. The error bars represent the standard deviations from triplicates. Different capital letters on the error bars within the same DH indicate that the results are significantly different (p < 0.05). Different small letters on the error bars within the same species indicate that the results are significantly different (p < 0.05)
Ferric reducing antioxidant power (FRAP)
The reducing power assay is often used to evaluate the ability of a natural antioxidant to donate electron or hydrogen [26]. Figure 1(B) shows the ability of the FPHs prepared from freshwater carps using flavorzyme to reduce Fe3+ to Fe2+. The higher the reducing power of the samples, the better their abilities to donate an electron. The presence of antioxidants in the FPHs results in the reduction of the ferric cyanide complex to a ferrous form, which was monitored by measuring the formation of Perl’s Prussian blue at 700 nm. HCF showed a higher reducing power than HRF and HMF at any given DH. The reducing power of HCF decreased marginally with an increase in the DH. A similar trend was observed in the HRF samples. The FRAP activity of HMF did not show any significant difference (p > 0.05) with changes in the DH. However, there was a significant difference in the activity between 10 and 20% DH (p < 0.05).
There was no correlation between the DPPH free-radical-scavenging activity and FRAP of the FPHs studied herein. This could be due to the different mechanisms of action, i.e., the DPPH assay works on the proton-transfer mechanism, whereas the FRAP assay works on the electron-transfer mechanism.
Linoleic acid peroxidation inhibition (LAPI) activity
The ability of the FPHs to inhibit the oxidation of linoleic acid is shown in Fig. 1(C). HRF showed superior peroxidation inhibition ability compared with HCF and HMF. The peroxidation inhibition ability of HRF was uniform, irrespective of DH (p < 0.05). The same trend was observed for HCF. Among the hydrolysates, HMF–DH 10% showed the lowest activity. All the hydrolysates (at 10 mg/mL) showed better activity than α-tocopherol (61.16% inhibition). Synthetic peptides with branched chain residues (valine, leucine, isoleucine) exert better antioxidative activity on lipid oxidation than the peptides with non-branched amino acids [27]. The reaction of oxygen with unsaturated lipids involves the initiation of free-radical formation, propagation, and termination. Initiation results from the loss of a hydrogen radical in the presence of trace elements, light, or heat. The resulting lipid free radicals react with oxygen to form peroxy radicals. In the propagation process, peroxy radicals react with more lipids to form lipid hydroperoxides, which are the primary products of autoxidation. The peptides containing basic amino acids are electron acceptors from the radicals formed during the oxidation process of unsaturated fatty acids [28]. FPHs may terminate the free radicals which promote the oxidation of unsaturated fatty acids. The hydrophobic amino acids present in the sequence of the peptides contribute greatly to their potential antioxidant activity [29]. The peptides derived from fish proteins have strong antioxidant properties in different oxidative systems [30]. However, the exact mechanism of peptides acting as antioxidants is not clearly known; however, aromatic amino acids and histidine contribute significantly to the antioxidant activity. Most of the marine food-derived bioactive peptides show a higher antioxidative potential when compared with α-tocopherol. For example, the peptide (Glu-Ser-Thr-Val-Pro-Glu-Arg-Thr-His-Pro-Ala-Cys-Pro-Asp-Phe-Asn), which is isolated from the peptic hydrolysate of hoki (Johnius belengerii) frame protein, inhibits lipid peroxidation more effectively than α-tocopherol and efficiently quenches different sources of free radicals [31].
Emulsion properties
Bioactive substances can be used as functional ingredients. To use FPHs as an antioxidant/emulsifier in emulsion-based food systems, it is essential to understand their emulsion properties. Thus, in the present study, the EAI and ESI of the FPHs were studied on the basis of the DH. The emulsion properties were assayed at a single concentration (1% protein solution).
EAI
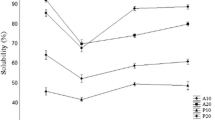
The EAI and ESI of the FPHs from freshwater carps were studied as functions of the DH, and the results are presented in Fig. 2(A, B), which show that there is no clear relationship between the EAI and DH of the FPHs obtained from catla and mrigal. However, the EAI of the FPHs from rohu (HRF) decreased with an increase in the DH. It is generally accepted that an increase in the DH results in the formation of smaller peptides that are usually hydrophilic in nature [25]. Hydrophilic peptides stay in the aqueous phase and may not migrate to the oil–water interface. Thus, an increase in the DH results in a lower EAI. The EAI of the HCF and HMF samples were not related to the DH. This indicates that the EAI depends not only on DH but also on the relative distribution and amphiphilicity of the peptides present in hydrolysates.
Emulsion activity index (A) and emulsion stability index (B) of the FPHs from C. catla (HCF), L. rohita (HRF), and C. mrigala (HMF), influenced by the DH. The error bars represent the standard deviations from triplicates. Different capital letters on the error bars within the same DH indicate that the results are significantly different (p < 0.05). Different small letters on the error bars within the same species indicate that the results are significantly different (p < 0.05)
ESI
The ESI of the FPHs from rohu (HRF) with a 20% DH yielded a value of 67 min. The ESI value of HRF increased with the DH and was found to be significant. The ESI of HCF and HMF did not show any relation with the DH. The results imply that the ESI was mainly affected not only by the DH but also by the nature of peptide chains. In the present study, it is evident that the FPHs have peptides with varying sizes and sequences. The interfacial properties of proteins and peptides are mainly determined by the amino acid composition and the structural conformation conferred in the oil–water interface [32].
Approximate molecular weight and antioxidant activity of the peptide fractions in HCF-DH 5%
FPHs contain peptides with various sizes and some free amino acids depending on the DH. Size-exclusion chromatography techniques separate the peptides into different fractions with various molecular-weight ranges. In the present study, based on the overall antioxidant activity, HCF-DH 5% was chosen for fractionation using size-exclusion chromatography. Fractionation of HCF-DH 5% yielded three different peptide fractions (Fig. 3). The approximate molecular weights of the peptides of Fractions 1, 2, and 3 were >7020–2546, 2379–987, and 923–658 Da, respectively. The antioxidative properties of the fractions assessed are given in Table 1. Fraction 2 showed the highest DPPH free-radical-scavenging activity, followed by Fractions 1 and 3, and Fraction 3 showed the highest FRAP. The FRAP of the peptide fractions increased with a decrease in the molecular-weight range. From the results, it is evident that the size may not be the factor in determining the antioxidant activity of peptides and that peptide composition and sequence play an important role [33].
Size-exclusion chromatography profile of HCF-DH 5%. Inset standard curve obtained from the plot of Ve/Vo versus log10 molecular weight of the standards (aprotinin-6512 Da; vitamin b12-1356.37 Da; tryptophan-204.22 Da). The standard curve was used for approximating the molecular weights of the peptides distributed in HCF-DH 5%
Amino acid composition of Fraction 2 from HCF-DH 5%
The amino acid composition of Fraction 2 of HCF-DH 5% is presented in Table 2. Fraction 2 was chosen to obtain the amino acid profile because it showed a high radical-scavenging activity and moderate FRAP. Amino acid residues such as histidine, cysteine, tyrosine, proline, hydroxyproline, and tryptophan as well as their positions play a crucial role in determining the antioxidant properties. In the present study, Fraction 2 contained a high proportion of glycine, proline, and tyrosin. Amino acids such as histidine and cysteine were not detected in the sample. It has been reported by Chen et al. [34] that peptides exhibit stronger antioxidant properties if they are rich in alanine, valine, leucine, and arginine. In the present study, these amino acids are present in moderate quantity and may be responsible for the stronger antioxidant properties. The ratio of total hydrophobic amino acids to the total amino acid content in Fraction 2 was 42.31%. The hydrophobic to total amino acid content ratio is believed to play an important role in determining the antioxidant potential of the fractions/hydrolysates [34, 35].
To conclude, in the present study, FPHs obtained from water-washed mince of three freshwater carps, namely catla, rohu, and mrigal were prepared using flavorzyme at four different DHs (5, 10, 15, and 20%). The antioxidative properties of the FPHs were varied in different species and influenced by the DH. The molecular weight of the FPH fraction from catla with 5% DH varied from 7020 to 658 Da. The FPHs from freshwater carps have the potential to be used as an antioxidant in functional food formulation. The FPHs prepared herein can find applications in the development of peptide-based natural healthcare products.
References
Pires C, Clemente T, Batista I. Functional and antioxidative properties of protein hydrolysates from Cape hake by-products prepared by three different methodologies. J. Sci. Food Agric. 93: 771–780 (2013)
Ovissipour M, Rasco B, Shiroodi SG, Modanlow M, Gholami S, Nemati M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J. Sci. Food Agric. 93: 1718–1726 (2013)
Nasri R, Amor IB, Bougatef A, Nedjar-Arroume A, Dhulster P, Gargouri J, Chaabouni MK, Nasri, M. Anticoagulant activities of goby muscle protein hydrolysates. Food Chem. 133: 835-841 (2012)
Elavarasan K, Naveen Kumar V, Shamasundar BA. Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the nature of enzyme. J. Food Process. Pres. 38: 1207–1214 (2014)
Elavarasan K, Shamasundar BA. Angiotensin I-converting enzyme inhibitory activity of protein hydrolysates prepared from three freshwater carps (Catla catla, Labeo rohita and Cirrhinus mrigala) using flavorzyme. Int. J. Food Sci. Technol. 49: 1344–1350 (2014)
Chalamaiah M, Hemalatha R, Jyothirmayi T, Prakash VD, Uday Kumar P, Nimgulkar C, Dinesh Kumar B. Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg (roe) in BALB/c mice. Food Res. Int. 62: 1054-1061 (2014)
Meisel H, Fitzgerald RJ. Biofunctional peptides from milk proteins: mineral binding and cytomodulatory effects. Curr. Pharm. Design. 9: 1289-1295 (2003)
Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 40: 43-81 (2000)
Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 113: 71s-88s (2002)
Wu HC, Chen HM, Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 36: 949-957 (2003)
Ren J, Zhao M, Shi J, Wang J, Jiang Y, Cui C, Kakuda Y, Xue SJ. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 108: 727–736 (2008)
Bougatef A, Arroume NN, Manni L, Ravellac R, Barkia A, Guillochon D, Nasri M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 116: 559-565 (2010)
Khantaphant S, Benjakul S, Kishimura H. Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brownstripe red snapper prepared using pyloric caeca and commercial proteases. Process Biochem. 46: 318-327 (2011)
Liaset B, Lied E, Epse M. Enzymatic hydrolysis of by-products from the fish filleting industry, chemical, characterization and nutritional evaluation. J. Sci. Food Agric. 80: 581-589 (2000)
AOAC. Official Methods of Analysis. Method No. 920.153. Association of Official Analytical Chemists, Arlington VA, USA (2000)
Taylor WH. Formol titration: an evaluation of its various modifications. Analyst. 82: 488-498 (1957)
Yen GC, Wu JY. Antioxidant and radical scavenging properties of extract from Ganoderma tsugae. Food Chem. 65: 375–379 (1999).
Oyaiza M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 44: 307–315 (1986)
Osawa T, Namiki M. Natural antioxidants isolated from Eucalyptus leaf waxes. J. Agric. Food Chem. 33: 777-780 (1985)
Mitsuda H, Yasumoto K, Iwami K. Antioxidation action of indole compounds during the autoxidation of linoleic acid. Jpn. Soc. Nutr. Food Sci. 19: 210-214 (1996)
Pearce KN, Kinsella JE. Emulsifying properties of proteins: evaluation of a turbidimetric technique. J. Agric. Food Chem. 26: 716-723 (1978)
Bidlingmeyer BA, Cohen SA, Tarvin TL, Frost B. A new, rapid, high-sensitivity analysis of amino acids in food type samples. J Assoc. Off. Anal. Chem. 70: 241–247 (1987)
Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 40: 945–948 (1992)
Thiansilakul Y, Benjakul S. Shahidi F. Antioxidative activity of protein hydrolysate from round scad muscle using alcalase and flavorzyme. J. Food Biochem. 31: 266-287 (2007)
Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 102: 1317–1327 (2007)
Dorman HJD, Peltoketo A, Hiltunen R Tikkanen MJ. Characterisation of the antioxidant properties of deodourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 83: 255–262 (2003)
Kawashima K, Itoh H, Miyoshi M, Chibata I. Antioxidant properties of branched-chain amino acid derivatives. Chem. Pharm. Bull. 27: 1912–1918 (1979)
Chan KM, Decker EA. Endogenous skeletal muscle antioxidants. Crit. Rev. Food Sci. Nutr. 34: 403–426 (1994)
Mendis E, Rajapakse N, Kim SK. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 53: 581 − 587 (2005)
Jun SY, Park PJ, Jung WK, Kim SK. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 219: 20 − 26 (2004)
Kim SY, Je JY, Kim SK. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 8: 31 − 38 (2007)
Pacheco-Aguilar R, Mazorra-Manzano MA, Ramirez-Suarez JC. Functional properties of fish protein hydrolysates from Pacific whiting (Merluccius productus) muscle produced by a commercial protease. Food Chem. 109: 782-789 (2008)
Imelda WYC, Lennie KYC, Nina YT, Eunice CYL. The role of molecular size in antioxidant activity of peptide fractions from Pacific hake (Merluccius productus) hydrolysates. Food Chem. 134: 1297-1306 (2012)
Chen HM, Muramoto K, Yamauchi F, Nokihara K. Antioxidant activity of designed peptides based on the antioxidative peptide isolated from digests of a soybean protein. J. Agric. Food Chem. 44: 2619–2623 (1996)
Song R, Wei R, Zhang B, Yang Z, Wang D. Antioxidant and antiproliferative activities of heated sterilized pepsin hydrolysate derived from half-fin anchovy (Setipinna taty). Mar. Drugs. 9: 1142–1156 (2011)
Acknowledgements
The funding support provided by Indian Council of Agricultural research (ICAR), New Delhi, India, under the National Agricultural Innovative Project (NAIP), Component II, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Elavarasan, K., Shamasundar, B.A. Antioxidant and emulsion properties of freshwater carps (Catla catla, Labeo rohita, Cirrhinus mrigala) protein hydrolysates prepared using flavorzyme. Food Sci Biotechnol 26, 1169–1176 (2017). https://doi.org/10.1007/s10068-017-0154-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0154-7