Abstract
Secondary autoimmune inner ear disease (AIED) is often bilateral and asymmetric in patients presenting with audiovestibular symptoms due to a systemic autoimmune disease. This systematic review and meta-analysis are aimed at identifying and highlighting patterns in prevalence of vestibular dysfunction, symptom presentation, and diagnostic methods in extant literature by combining clinical context from case reports with quantitative analyses from cohort studies. Screening of articles by title, abstract, and full text was completed by four reviewers (K.Z., A.L., S.C., and S.J.). In this study, we grouped secondary AIED and systemic autoimmune diseases by pathophysiologic mechanism: (1) connective tissue disease (CTD), (2) vasculitides (VAS), (3) systemic inflammatory disorders (SID), and (4) other immune-mediated disorders (OIMD). The search for AIED disease identified 120 articles (cohorts and case reports) that met the final inclusion criteria. All 120 were included in the qualitative review, and 54 articles were included for meta-analysis. Of these 54 articles, 22 included a control group (CwC). Ninety individual cases or patient presentations from 66 articles were included for analysis in addition to the 54 cohort articles. Secondary AIED does not have a diagnostic algorithm for managing vestibular symptoms. The management of audiovestibular symptoms requires close collaboration between otolaryngologists and rheumatologists to preserve end-organ function of the ear. To improve our ability to understand the impact on the vestibular system, vestibular clinicians need to develop a standardized reporting method. Clinical presentation should frequently be paired with vestibular testing to contextually investigate symptom severity and provide higher quality care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary autoimmune ear disease (AIED) due to a systemic autoimmune disease is often bilateral and asymmetric and presents with sensorineural hearing loss (SNHL) [1]. Harris et al. initially proposed a classification of AIED based on five principal causes (see Table 1) [2]. Secondary AIED typically occurs concurrently with rheumatic diseases (e.g., systemic lupus erythematosus and rheumatoid arthritis), vasculitis (e.g., Behcet disease), systemic inflammatory diseases (SID), or other autoimmune pathologies (OIMD). This article will focus on audiovestibular outcomes in secondary AIED according to the Harris classification.
In McCabe’s initial 1979 report, unsteadiness and ataxia were more frequently reported than vertigo in his cohort of patients [6]. This finding corroborated a similar progressive, bilateral, and simultaneous decline of vestibular end-organs to hearing loss [6]. Since then, diagnosis has been dependent on history and limited clinical findings [7].
Generally, AIED accounts for less than 1% of all cases of hearing impairment or dizziness [9]. In addition to SNHL, concurrent conductive hearing impairment may also be present, as found in otitis media with effusion or with presence of granulation tissue in autoimmune pathologies (i.e., GPA) [11, 12]. Per the CDC, more than 95% of hip fractures are a result of falls, typically sideway falls. Balance dysfunction can lead to 2.6-fold increase in the odds of falling, while clinically symptomatic patients (i.e., reported dizziness or vertigo) had a 12-fold increase in falls [10]. Thus, vestibular symptoms secondary to autoimmune diseases are important to identify and define to prevent high morbidity and mortality from increased fall-risk in the short as well as long term.
For patients suffering from secondary AIED, hearing loss can range from slowly progressive to rapidly progressive, as opposed to primary AIED, which is usually rapidly progressive and bilateral [3]. Damage due to AIED can occur directly from an uncontrolled, immune response toward inner ear antigens (i.e., ECM protein cochlin) or indirectly by immune-complex deposition in the inner ear apparatus, causing an array of otologic and vestibular symptoms secondary to endocochlear injury [4, 5].
Ralli et al. [11] published a comprehensive review on audiovestibular symptoms in systemic autoimmune disease describing various clinical presentations of AIED patients and postulated that identifying audiovestibular symptoms early could help with diagnosis and tracking of disease progression and can prove crucial to the prognosis of the inner ear function. However, this review did not encompass vestibular testing methods and diagnostic work-up [11].
Due to the varying presentations of secondary AIED in the setting of their systemic rheumatologic disorders, there is little consensus in the diagnostic algorithm and treatment [5]. Research has placed a larger emphasis on audiologic deterioration and outcomes, often overlooking vestibular presentations.
Ciobra et al.’s [5] review of diagnostic approaches to AIED determined that there are no standard diagnostic criteria or pathognomonic tests for a diagnosis of AIED; rather, the diagnosis is based on clinical symptoms, laboratory findings, and responsiveness to treatment with steroids, without the need for vestibular testing [5]. This systematic review and meta-analysis are aimed at highlighting patterns in prevalence of vestibular dysfunction and symptom presentation and identifying employed and effective diagnostic methods in extant literature.
Methods
Search strategy
This study was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A detailed search strategy was developed in the following four databases: PubMed (National Library of Medicine, National Institutes of Health), Scopus (Elsevier), CINAHL (EBSCO), and Cochrane Library (Wiley). Databases were searched from conception to February 6, 2022, using a combination of medical subject headings and keywords. The full search strategy with a list of systemic autoimmune diseases is shown in the Appendix.
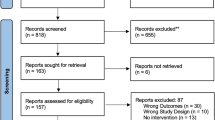
Screening of articles by title, abstract, and full text was completed by four reviewers (K.Z., A.L., S.C., and S.J.), and any disagreements were resolved by consensus. To identify additional studies, the reference lists of relevant articles were manually searched. A flow diagram detailing selection of studies with inclusions and exclusion criteria is shown in PRISMA Fig. 1 [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118].
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) schematic for vestibular manifestations in AIED [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118]
AIED classification rationale
In this study, we chose to classify secondary AIED and systemic autoimmune diseases by pathophysiologic mechanism (Table 2): (1) connective tissue disease (CTD), (2) vasculitides (VAS), (3) systemic inflammatory disorders (SID), and (4) other immune-mediated disorders (OIMD). We hope this classification helps highlight patterns in vestibular presentations and diagnostic work-up in vestibular testing [8].
Vestibular testing data were extracted for nystagmus (central positional, gaze-evoked, abnormal optokinetic, head-shaking induced, peripheral positional, spontaneous, and positional not otherwise specified (NOS)), caloric response (decreased/diminished/paresis, absent, and total/unspecified (NOS), canal paresis (%), and directional preponderance (%)), Dix-Hallpike testing, oculographic testing (abnormal saccadic movement, smooth pursuit), cVEMP (absent, total abnormal/NOS), cVEMP amplitude ratio, and electronystagmography (ENG)/videonystagmography (VNG). Central positional and peripheral positional nystagmus were included in the positional total/(NOS) categories.
While there is an American National Standard Procedure highlighting specific procedures for conducting Basic Vestibular Function Test Battery (spontaneous, gaze-evoked, positional/positioning nystagmus, saccade test, pursuit testing, and caloric testing), there is no standardized procedure for reporting these results. Given the heterogeneity of reporting methods, we organized the results according to Table 3. When available, data for the following audiologic testing modalities was extracted: pure tone audiometry (PTA), auditory brainstem response, tympanometry, word recognition testing, speech recognition thresholds, auditory recruitment, and reflex decay. Any discrepancies were resolved by consensus between the authors.
Quality assessment
Level of evidence for each selected article was evaluated with the Oxford Center for Evidence-Based Medicine [119]. The risk of bias for non-randomized studies was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions [120]. The Joanna Briggs Institute (JBI) critical appraisal checklist (8 questions) was used to assess the quality of case reports.
Statistical analysis and synthesis of results
The cohorts in this study were analyzed in two distinct groups: (1) all AIED cases and (2) AIED cases with controls (CwC). Each group was further split into the classification detailed in the above methods to ascertain subgroup trends and variations (group 1: CTD, group 2: VAS, group 3: SID, group 4: OIMD).
Statistical analyses were performed on case reports data with SPSS 28.0.1.0 (IBM Corporation, Armonk, NY). Continuous variables were summarized by mean ± standard deviation (SD) or median and interquartile range (IQR 25–75th) when appropriate. All continuous variables were tested for normal distribution as determined by the Kolmogorov–Smirnov test. Comparisons between continuous variables were performed with a t-test or Mann–Whitney test as appropriate. Categorical variables were summarized by frequency (N) and percentage (%). Comparisons between categorical variables were performed with a Fisher’s or chi-square test. The strength of association in cohorts with AIED cases and controls was estimated using odds ratios (ORs) and 95% confidence intervals (CIs) by the exact method.
In addition, meta-analysis of continuous measures (comparison of means and standard deviations) and odds ratios (comparison of events) between treatment and control were performed with Cochrane Review Manager (RevMan) version 5.4 (The Cochrane Collaboration, 2020). If median and range were provided for a continuous variable, a mean and SD were estimated using the quantile estimation method [129]. Due to the homogeneity of the studies, I2 < 50%, the fixed-effects model was used in this study. If homogeneity of studies or I2 > 50%, the random-effects model was used. Finally, the Sterne and Egger tests were performed for further assessment of risk of publication bias. Potential publication bias was evaluated by visual inspection of the funnel plot [130, 131]. In a funnel plot, treatment effect is plotted on the horizontal axis, and the standard error is on the vertical axis. The vertical line represents the summary estimate derived using fixed-effect meta-analysis. Two diagonal lines represent (pseudo) 95% confidence limits (effect ± 1.96 standard error) around the summary effect for each standard error on the vertical axis. These show the expected distribution of studies in the absence of heterogeneity or selection bias. In the absence of heterogeneity, 95% of the studies should lie within the funnel defined by these diagonal lines. Publication bias results in asymmetry of the funnel plot. A p value of < 0.05 was used to indicate a statistically significant difference for all statistical tests.
Results
Overview
The search for AIED disease identified 3810 unique abstracts; 758 articles underwent full-text review of which 120 articles (cohorts and case reports) met the final inclusion criteria. All 120 were included in the qualitative review, and 54 articles were included for meta-analysis. Of these 54 articles, 22 included a control group (CwC). The remaining 32 studies did not have a control group. Most of the included articles were either prospective cohort studies (N = 42) or retrospective cohort studies (N = 12). Ninety individual cases or patient presentations from 66 articles were included for analysis in addition to the 54 cohort articles. A complete list of cohort studies and case reports with bibliographic information is available in the Appendix. Critical appraisal of studies indicated an acceptably low risk of bias for the majority of included studies (Appendix). A funnel plot with Egger test (4.48, 95%CI 1.58 to 7.39, p = 0.04) demonstrated that most studies were within the funnel with little asymmetry, suggesting little publication bias (Fig. 2).
Demographics
A total of 54 cohort studies were included with 2195 patients. Demographic information by subgroup is detailed in the Appendix for all AIED cases (cohort studies). Of note, the AIED CwC (n = 814) are included in all AIED cases included in the Appendix (n = 2195). The youngest cohort of patients was the VAS group with mean age of 42.5 ± 3.47 (18–81) years old. With the most included studies, CTD and VAS cohorts yielded sufficient data for a case vs. control analysis (CwC). CTD patients were significantly more likely to be female (90.7%) when compared to control patients (65.0%) (p < 0.001). Patients with VAS pathologies had a shorter disease duration at the time of the study at 1.87 ± 0.63 years in comparison to CTD patients at 5.15 ± 1.06 years.
Vestibular symptoms
The presence of any vestibular symptom was the highest in the VAS cohort in 466 (63.0% [47.4–77.4%]) patients, followed by 245 (34.1% [21.7–47.6%]) patients in CTD, 43 (20.3% [4.7–43.2%]) patients in SID, and 44 (17.5% [13.1–22.3%]) patients in the OIMD cohort as shown in Table 4. The VAS subgroup had the highest prevalence of vertigo in 220 (50.3% [35.4–65.1%]) patients, dizziness/lightheadedness in 112 (34.3%) patients, and imbalance in 161 (54.8%) patients among the subgroups. In the SID subgroup, 43 (20.3% [4.7–43.2%]) and 51 (24.2% [34.9–52.0%]) patients screened for vertigo and imbalance, respectively, reported symptoms. The proportion of patients presenting with imbalance is significantly higher in the VAS cohort 162 patients (54.8% [49.0–60.5%]) compared to the CTD cohort (24 patients (19.0% [2.9–44.3])). The most significant findings between CwC and controls were presence of vertigo for CTD cohort (OR = 69.4 [23.5, 205.3]) and imbalance in the VAS cohort (OR = 61.6 [8.2, 460.3]) indicating a greater than 60 times likelihood for both CTD and VAS compared to controls.
Vestibular testing
Scarce and inconsistent reporting was notable when obtaining nystagmus, caloric testing, cVEMP, ENG/VNG results, and the rotational chair exam. The VAS cohort had the highest prevalence of after headshake nystagmus (AHSN) with 24.2% [1.7, 61.6] patients affected. Spontaneous and positional (NOS) nystagmus was the most prevalent in the OIMD subgroup with 22 (32.3%) of patients positive, followed by 35 (16.2%) patients in VAS and was rare in CTD with 2 (0.8%) patients. Positional nystagmus in the OIMD cohort was seen in 12 (29.5%) patients in the OIMD subgroup, followed by 20 (19.9%) patients with VAS and 25 (9.2%) patients with CTD.
Prevalence of abnormal caloric testing (total/NOS), highlighting a peripheral vestibulopathy, was the highest in the VAS cohort with 84 (42.8%), followed by 46 (20.9%) patients with CTD and 25 (47.3%) patients in the OIMD subgroup. Most abnormal caloric testing results in the VAS and CTD cohorts were due to decreased response or paresis on testing: VAS 55 (47.3%), CTD 45 (20.4%), and OIMD 8 (38.1%). Caloric testing was more likely to yield absent findings in the VAS subgroup (24 [71.1%]) compared to the CTD cohort (1 [3.57%]). cVEMPs were only reported for one study in the CTD cohort [132]. In the VAS subgroup, cVEMPs were abnormal (total/NOS) in 16 (16.6%) and absent in 3 (4.84%). Rotational chair testing was mentioned in 6 of 54 included articles of which five were VAS and one was OIMD [125, 133,134,135,136]. Results for rotational chair testing were too heterogenous to pool. Sensory organization test (SOT) and clinical test of sensory integration and balance (CTSIB) were only reported in one study each [133, 137].
Only CTD and VAS cohorts were able to undergo analysis for vestibular testing due to insufficient reporting and low sample sizes in SID and OIMD subgroups. In the VAS cohort, AHSN (OR = 47.5 [9.7, 232.5]) and spontaneous nystagmus (OR = 19.6 [4.0, 96.6]) were significantly more likely to be present in CwC patients than controls. Positional nystagmus (NOS) was not significantly more notable in either CTD or VAS groups compared to controls. There also is no significant difference in abnormal Dix Hallpike test in CTD CwC patients and controls. However, CTD CwC patients were 12.5 times more likely to have an abnormal (total/NOS) caloric test (OR = 12.5 [3.9, 39.5]). Within oculographic testing, abnormal saccadic movement was OR = 3.7 [1.6, 8.8] times more likely to be present in CwC CTD patients versus controls. cVEMPs (total/NOS) were significantly abnormal in the CwC VAS group versus controls (OR = 6.9 [2.0, 23.6]). Only one study reported oVEMPs; thus, there was insufficient data for pooling [138].
Audiologic symptoms
Hearing loss and tinnitus were the most reported otologic symptoms while headache was also reported in the above AIED cohorts. In the CTD cohort, headaches were reported in 83 (57.5% [49.1–65.6%]) patients, tinnitus in 154 (42.7% [28.0–58.0%]), and hearing loss (total/NOS) in 259 (39.5% [24.7–55.4%]). In the VAS cohort, headaches were present in 74 (60.2% [32.3–84.9]), tinnitus in 272 (46.0% [29.3–63.0%]), and hearing loss in 351 (63.1% [45.0–79.4%]) patients as shown in Table 4. Hearing loss (total/NOS) was proportionally greatest in OIMD 175 (77.3%), followed by VAS 351 (63.1%) and CTD 259 (39.5%) subgroups. Tinnitus was reported in approximately 40% across all subgroups (ranging 38.3–46.0%), indicating it may be a nonspecific symptom. Only one study reported 15 (25.0%) patients with oscillopsia [139].
Hearing loss (total/NOS) is significantly more likely to be present in CwC patients with CTD (OR = 9.1 [6.3–13.1]) or VAS (OR = 10.0 [4.5–21.8]) pathologies. CwC CTD patients were significantly more likely to present with subjective hearing loss (OR = 31.7 [2.4–425.3]). VAS CwC patients are significantly more likely to present with tinnitus (OR = 37.5 [5.2–267.8]) compared to controls.
Cogan syndrome
Though a hallmark otolaryngologic pathology, typical CS is unique in affecting the vestibular system and the cochlear system [140]. In our study, 8 of the 26 VAS cohorts were CS. A sub-analysis of the VAS subgroup was completed to isolate the confounding effects of CS versus other vasculitis pathologies. The presence of any vestibular symptom, vertigo, and imbalance were significantly more prevalent in CS cases. Upon removing CS patients from the VAS cohort, rates of vertigo in the non-CS cohort (29.8%) were comparable to the CTD subgroup (33.3%). Yet, prevalence of any vestibular symptom, dizziness/lightheadedness, and imbalance are still higher in non-CS cases compared to controls, with up to a third of patients endorsing a complaint of vertigo.
While vestibular testing results were limited by small sample size post-stratification, absent caloric response and abnormal results (total/NOS) were significantly higher in the CS cohort (24, 71.1%) versus non-CS (60, 34.7%) and CTD cases (46, 20.9%). The CS subgroup likely skewed and, naturally, predominated audiologic symptoms in the overall VAS cohort. Prevalence of audiologic symptoms for non-CS cases was comparable to CTD subgroup in the absence of CS cases. However, it is still noteworthy that a third of vasculitis (without Cogan syndrome) and a fifth of CTD subjects had peripheral vestibular abnormalities on objective reflexive testing, confirming that the vestibular labyrinth had been affected.
Descriptive analysis of individual cases
A total of 66 articles were included with 90 respective patients or case presentations. Patients were a mean age of 44.0 ± 16.9 (18–85) years old and 62.2% female (n = 56). Of the 90 patients, 17 (18.9%) were identified as white, 3 (3.3%) as black, 4 (4.4%) as “other,” and 66 (73.3%) were not identified by race. Five (5.6%) patients had a history of connective tissue disease, 58 (64.4%) had a history of vasculitis, 13 (14.4%) had a history of systemic inflammatory disorder, and 14 (15.6%) had a history of autoimmune pathology not otherwise specified. Three (3.3%) patients presented with a history of Behcet’s disease, 13 (14.4%) patients with sarcoidosis, 36 (40.0%) patients with Cogan syndrome, 5 (5.6%) patients with Vogt-Koyanagi-Harada (VKH) disease, 19 (11.1%) patients with granulomatosis with polyangiitis, 3 (3.3%) patients with systemic lupus erythematosus (SLE), 1 (1.1%) patient with IgG4 disease, 8 (8.9%) patients with relapsing polychondritis, 1 (1.1%) patient with systemic sclerosis, 1 (1.1%) patient with rheumatoid arthritis, 2 (2.2%) patients with polyarteritis nodosa, 1 (1.1%) patient with antiphospholipid syndrome, and 3 (3.3%) patients with giant cell arteritis.
On clinical presentation, 28 patients (31%) complained of SNHL, 24 (27%) experienced tinnitus, 29 (32%) reported a history of vertigo episodes, 12 (13%) experienced disequilibrium/imbalance, 5 (6%) experienced headache, and 4 patients (4%) experienced otalgia. Upon examination, 34 patients (38%) displayed uveitis or keratitis, 75 (83%) indicated hearing loss (23 unilateral and 52 bilateral), 47 (52%) reported tinnitus, 7 (8%) reported headache, 52 (58%) displayed vertigo, 11 (12%) displayed dizziness, 18 (20%) displayed imbalance, 2 (2%) displayed oscillopsia, 19 (21%) displayed nausea and/or vomiting, 2 (2%) displayed hyperacusis, and 10 (11%) displayed aural fullness. Of the 46 patients who underwent cranial imaging (27 MRI, 23 CT, 5 X-Ray), 21 (45.7%) were described as “abnormal”; most commonly present were hyperintensities on MRI (2 VAS, 1 SID), cerebellar atrophy (1 SID), recent hemorrhage, infarction, or stenosis (3 VAS, 1 CTD), enhancements in the labyrinthine (1 VAS), inner ear (1 VAS, 1 OIMD), or posterior region (1 OIMD) or nonspecific mucosal thickening in mastoid region (2 VAS, 1 SID).
Central nystagmus was present in 3 patients (one positional, one gazed-evoked, one unspecified), peripheral nystagmus was present in 12, and spontaneous nystagmus was present in 15 patients. Audiometry found SNHL in 47 patients (unilateral in 15 and bilateral in 33).
Treatment regimens were carried out over a mean duration of 4.3 ± 3.9 (0.3–15) months. The most frequently utilized treatment was corticosteroids (n = 66), methotrexate (n = 10), azathioprine (n = 10), cyclophosphamide (n = 7), cyclosporine (n = 5), acetylsalicylic acid (n = 3), antihistamine (n = 3), and intravenous immunoglobulin (n = 2). Tetracyclines, vestibular rehabilitation therapy, plasmapheresis, rituximab, and mycophenolate mofetil were each used in one patient.
Out of the 91 patients, treatment response was recorded in 79. Sixty-four (81.0%) patients were classified as “recovered” if resolution occurred of their presenting symptom which included, but is not limited to, hearing loss, dizziness, imbalance, and/or tinnitus. Fifteen (19.0%) were classified as “not recovered.” Within the recovery group, 24 (38.1%) underwent a full recovery, 39 (61.9%) underwent a partial recovery, and 1 (1.5%) was not specified. Post-audiometry treatment was reported for 24 (31.2%) patients, and 8 (10.9%) patients underwent cochlear implantation.
Discussion
Diagnostic criteria or reliable pathognomonic tests for the diagnosis of secondary AIED are not standardized. For this study, we reviewed audiovestibular manifestations in systemic autoimmune or secondary AIED.
Particularly, the discussion of vestibular symptoms and methods of investigating in patients with AIED is scarce, especially given that the ear is an organ system providing a crucial function for patients. Agrawal et al. [141] reported that loss of vestibular function extended beyond just balance function and resulted in decreased performance in vision, speech, dexterity, and emotion while significantly decreasing overall quality of life [141]. This is the first review to collectively pool and quantify the prevalence of vestibular symptoms in the above pathologies. Identification of vestibular testing by subgroup type may highlight which methods are common or preferred in certain disease processes. In our study, presence of any vestibular symptom, vertigo, and imbalance were all most common in the VAS subgroup with almost 2/3 of the patients (63%) having those symptoms and 50% having vertigo. After excluding CS cases (which skewed vestibular presentations of the overall subgroup), non-CS VAS cases had a higher overall vestibular symptom, dizziness, imbalance, nystagmus, and abnormal caloric testing rate compared to the CTD subgroup. Discussions with patients regarding symptoms of dizziness and vertigo should be incorporated by rheumatologists when managing VAS patients, and a multidisciplinary approach should be initiated by timely referral to an otolaryngologist [142]. Latency in referral can lead to fibrosis and irreversible damage to the inner ear [143]. This is further emphasized by this study’s finding that CTD and VAS patients have > 60 times likelihood of experiencing vertigo or imbalance when compared to control patients.
VAS and CTD groups had the highest prevalence of peripheral vestibulopathy as found on vestibular testing (42.8% and 20.9%, respectively). The presence of a spontaneous nystagmus and AHSN is often a marker of uncompensated, asymmetric input of the peripheral vestibular system, but may also reflect alterations in central vestibular end organs. These findings were more prevalent in VAS patients (OR of 19.6 and 47.5, respectively) simply due to compromised arterial flow, secondary to inflammation, to central or peripheral vestibular systems. OIMD patients also showed the highest rate of spontaneous, positional nystagmus, and abnormal caloric testing (32.3%, 29.5%, and 47.3% respectively). Although these are not specific findings, the decreased or absent response on caloric testing in OIMS, CTD, and VAS patients indicate that the peripheral vestibular system may indeed be involved and a major contributing factor toward audiovestibular symptoms [144]. This emphasizes the need for vestibular testing in those patients.
Preferences in vestibular testing methods and reporting may change with time and be dependent on the availability of vestibular testing resources in a particular clinical setting, institution, or country. Figure 6 in the Appendix further highlights this temporal distribution of study publication between included cohort studies and case reports. The first included cohort study in this review was approximately 30 years after the first included case report (1980 versus 1952) [145, 146]. Commercially available equipment has gotten more accessible for vestibular testing since their inception in the early 2000s [147].
Hearing loss, particularly at lower frequencies, has been shown to be more common in patients with CTD and VAS in congruence with the increased odds (OR = 9.1) demonstrated in our study [148, 149]. In patients with ulcerative colitis, sensorineural hearing loss is the most common auditory symptom as reflected in the present study with 77.3% of included patients experiencing symptoms. Fousekis et al. [150] also found that patients with AIED secondary to IBD has minimal to no response to steroid treatment [150]. The propensity of multiple autoimmune disorders occurring in patients makes pinpointing the true cause of secondary AIED difficult. Subjective symptoms such as hearing loss should be investigated upon presentation in patients as hearing loss could represent an early sign of autoimmune disease.
Even when excluding Cogan syndrome cases, a third of patients with VAS and a fifth of patients with CTD have a confirmed vestibular pathology. It is very likely that this is even underrepresented given the heterogeneity of vestibular testing and the fact that most articles may not have conducted or reported a complete panel of vestibular testing, including VNG, rotational chair, video HIT, and VEMPs. While those symptoms could be related to neuropathy or cardiac/hemodynamic involvement or secondary to treatment side effects, identifying a vestibular dysfunction early may help initiate targeted treatment or vestibular rehabilitation sooner [151,152,153].
Though an array of vestibular testing is available, abnormal findings on vestibular testing may not always translate to a loss of function. Abnormal results should always be correlated with clinical symptoms. Standardized vestibular reporting will allow clinicians to properly understand and better relay information about the vestibular system which may help early detection of dysfunction and early referral to vestibular rehabilitation if needed to minimize risk of falls.
The author, country of publication, and audience toward which the work is directed are all factors which influence the clarity of vestibular reporting (Appendix). Only 64% of authors were otolaryngologists or neurologists by training, calling into the question if vestibular symptoms, signs, and testing have been appropriately interpreted. Thus, the importance of defining vestibular terms cannot be overlooked. An organized checklist or system including, but not limited to, standardizing the way vestibular results and terms are reported in academic journals, regardless of specialty, to better pool data can better facilitate understanding among physicians of various specialties. Identifying vestibular dysfunction early can change quality of life and reduce morbidity in these patients.
Limitations and future directions
This review highlights that audiovestibular pathologies are frequent in patients with systemic rheumatologic disorders. While otolaryngologists are the specialists most likely to interface with audiovestibular symptoms, the treatment of these patients is often multidisciplinary, and management should be done in collaboration with a rheumatologist. Increasing visibility of this problem to rheumatologists screening for organ involvement of the systemic diseases they are treating is primordial. While vital organs should continue to be monitored, the inner ear should also be investigated with emphasis on treating audiovestibular manifestations in a timely way to help reduce risks of complete failure. Many studies have linked loss of hearing and vestibular function to detrimental quality of life impact [141, 154].
One of the major limitations of this paper is the heterogeneous reporting of vestibular data (symptomatic and objective data). The word dizziness is a very nonspecific marker that could implicate vestibular and nonvestibular diagnoses, and our results likely underrepresent the subjects with vestibular diagnoses. Furthermore, making sense of the grouped results of vestibular testing is difficult in the absence of a clear understanding of what the finding is. For instance, while positional nystagmus can reflect benign paroxysmal positional vertigo, it can also be a central vestibular finding, and reviewed papers did not always allow for that determination. Our results further emphasize the need for a standardization in reporting vestibular results. This standardization would allow for greater comparison between independent samples and studies, increase reproducibility, and would increase power when studies are pooled. Due to changing standards in vestibular testing, the addition of new technologies, and the availability of resources in individual clinics, it is difficult to compare disease diagnostics overtime. Furthermore, autoimmune conditions are already rare pathologies, and studies often have small sample sizes. In such scenarios, quality of reporting in these studies should be held to a higher standard. Often, a mismatch exists between what the authors sought in their methods and what the articles ultimately report in their results. Thus, there is a need for a published standard of reporting for vestibular results, akin to those in place for audiometric standards (i.e., New Hearing Reporting Standard for clinical trials) [155]. Pooling is difficult if only “processed results” (i.e., standardized mean differences) are provided without access to raw values.
As previously mentioned, this study included a two-pronged approach. The cohort studies added power to our analysis, but the individual stories of the patients and complete clinical picture of vestibular disease process are lost. It is difficult to categorize a patient into a vestibular pathology in the absence of other clinical information. Additionally, it is important to note the varying exclusion criteria for these studies. Although authors largely excluded baseline otologic disease that may not have been due to autoimmune pathology, confounding factors such as another non-autoimmune otologic comorbidity could have been present. Abnormal oculographic findings are normal and non-pathologic in older patients, but, in a cohort design with pooled analysis, this is difficult to ascertain. To build this clinical context, a summary of case reports was utilized.
Conclusion
Vestibular symptoms are often overlooked in the setting of secondary AIED with no clear-cut diagnostic algorithm. Thus, it is likely that vestibular dysfunction is likely underdiagnosed in patients suffering from AIED. Primary care providers, rheumatologists, and otolaryngologists should collaborate to manage patients presenting with potential cochlear and vestibular involvement. Undergoing multiple workups for diagnosis of autoimmune disorders can be a difficult journey for patients; however, with early identification of audiovestibular issues, early intervention may improve quality of life and reduce morbidity. Pairing clinical data with audiometric and vestibular testing can help guide both otolaryngologists and rheumatologists with accurate diagnosis and more precise management. To improve our ability to understand the impact on the vestibular system, vestibular clinicians need to develop a standardized reporting method that can improve communication and reporting across specialties. Early identification can also aid in tracking disease progression and quality of life for patients.
Data availability
Data will be made available per individual request.
References
Moscicki RA (1994) Immune-mediated inner ear disorders. Baillieres Clin Neurol 3(3):547–563
Harris JP, Gopen Q, Keithley E (2009) Autoimmune inner ear disease and other autoimmune diseases with inner ear involvement. James B Snow Jr and P Ashley Wackym, editors. Ballengers Otolaryngol Head Neck Surg 17:305–12
Berrettini S, Ravecca F, Bruschini L, Ursino F, Sellari-Franceschini S (1998) Progressive sensorineural hearing loss: immunologic etiology. Acta Otorhinolaryngol Ital 18(4 Suppl 59):33–41
Vambutas A, Pathak S (2016) AAO: Autoimmune and autoinflammatory (disease) in otology: what is new in immune-mediated hearing loss. Laryngoscope Investig Otolaryngol 1(5):110–115
Ciorba A, Corazzi V, Bianchini C, Aimoni C, Pelucchi S, Skarzynski PH et al (2018) Autoimmune inner ear disease (AIED): a diagnostic challenge. Int J Immunopathol Pharmacol 32:2058738418808680
McCabe BF (1979) Autoimmune sensorineural hearing loss. Ann Otol Rhinol Laryngol 113(7):526–30
Welling DB (1996) Clinical evaluation and treatment of immune-mediated inner ear disease. Ear Nose Throat J 75(5):301–305
Ruckenstein MJ (2004) Autoimmune inner ear disease. Curr Opin Otolaryngol Head Neck Surg 12(5):426–430
Bovo R, Aimoni C, Martini A (2006) Immune-mediated inner ear disease. Acta Otolaryngol 126(10):1012–1021
Agrawal Y, Ward BK, Minor LB (2013) Vestibular dysfunction: prevalence, impact and need for targeted treatment. J Vestib Res 23(3):113–117
Ralli M, D’Aguanno V, Di Stadio A, De Virgilio A, Croce A, Longo L et al (2018) Audiovestibular symptoms in systemic autoimmune diseases. J Immunol Res 2018:5798103
SafaviNaini A, Ghorbani J, MontazerLotfeElahi S, Beigomi M (2017) Otologic manifestations and progression in patients with Wegener’s granulomatosis: a survey in 55 Patients. Iran J Otorhinolaryngol 29(95):327–31
Adler YD, Jovanovic S, Jivanjee A, Krause L, Zouboulis CC (2002) Adamantiades-Behçet’s disease with inner ear involvement. Clin Exp Rheumatol 20(4 Suppl 26):S40–S42
Agari D, Koide R, Kashiyama T, Yoshida H, Naito R, Bandoh M (2007) Neurosarcoidosis: a treatable cause of vestibular dysfunction. Lancet 369(9564):878
Akdoğan Ö, Exilus S, Ward BK, McArthur JC, Della Santina CC, Carey JP (2021) Sudden sensorineural hearing and vestibular loss in a case of relapsing polychondritis. Ann Otol Rhinol Laryngol 130(12):1412–1416
Albrite LCJP, Resnick DM (1961) Cogan's syndrome: case presentations. Arch Otolaryngol 74(5):501–6
Almorza Hidalgo T, García González AJ, Castañeda S, Tomero EG, Pablos Álvarez JL (2021) Cogan syndrome: descriptive analysis and clinical experience of 7 cases diagnosed and treated in two third level hospitals. Reumatol Clin (Engl Ed) 17(6):318–321
Amor-Dorado JC, Llorca J, Garcia-Porrua C, Costa C, Perez-Fernandez N, Gonzalez-Gay MA (2003) Audiovestibular manifestations in giant cell arteritis: a prospective study. Medicine 82(1):13–26
Aydin T, Taspinar O, Guneser M, Keskin Y (2016) Association of Vogt Koyanagi Harada syndrome and seronegative rheumatoid arthritis. Ethiop J Health Sci 26(2):193–196
Bassyouni IH, Emad Y, Rafaat HA, Dabbous AO (2011) Relationship between nailfold capillary abnormalities and vestibular dysfunction in systemic sclerosis. Joint Bone Spine 78(3):266–269
Batuecas-Caletrío A, del Pino-Montes J, Cordero-Civantos C, Calle-Cabanillas MI, Lopez-Escamez JA (2013) Hearing and vestibular disorders in patients with systemic lupus erythematosus. Lupus 22(5):437–442
Bayir O, Çomoğlu S, Ozdek A, Aytaç E, Güven H, Ozdal P et al (2012) Vestibular evoked myogenic potential responses in Behçet’s disease. J Int Adv Otol 8:113–117
Benitez JT, Arsenault MD, Licht JM, Cohen SD, Greenberg RV (1990) Evidence of central vestibulo-auditory dysfunction in atypical Cogan’s syndrome: a case report. Am J Otol 11(2):131–134
Benitez JT, Bojrab DI, Lubbers DE, Arsenault MD (1999) Investigation of endolymphatic hydrops by electrocochleography in patients with Cogan’s syndrome. Ear Nose Throat J 78(12):929–933
Bennett RW, Staker LV (1987) Wegener’s granulomatosis presenting as vertigo. West J Med 146(3):359–361
Berrettini S, Ferri C, Pitaro N, Bruschini P, Latorraca A, Sellari-Franceschini S et al (1994) Audiovestibular involvement in systemic sclerosis. ORL J Otorhinolaryngol Relat Spec 56(4):195–198
Bomholt A, Knudsen JB, Permin H, Tommerup B, Gormsen J (1982) Profound sensorineural hearing loss in polyarteritis nodosa. An atypical case of Cogan's syndrome. Arch Otorhinolaryngol 236(1):53–8
Bowman CA, Linthicum FH Jr, Nelson RA, Mikami K, Quismorio F (1986) Sensorineural hearing loss associated with systemic lupus erythematosus. Otolaryngol Head Neck Surg 94(2):197–204
Bulman CH, Kane RJ, Srivastva VN (1985) The ENT manifestations of systemic vasculitis. J Laryngol Otol 99(9):941–945
Caldarelli DD, Rejowski JE, Corey JP (1986) Sensorineural hearing loss in lupus erythematosus. Am J Otol 7(3):210–213
Casella G, Corbetta D, Zolezzi M, Di Bella C, Villanacci V, Salemme M et al (2015) Symptomatic sensorineural hearing loss in patients with ulcerative colitis. Tech Coloproctol 19(12):729–731
Casoli P, Tumiati B (1995) Cogan’s syndrome: a new possible complication of antiphospholipid antibodies? Clin Rheumatol 14(2):197–198
Casselman JW, Majoor MH, Albers FW (1994) MR of the inner ear in patients with Cogan syndrome. AJNR Am J Neuroradiol 15(1):131–138
Chiarella G, Tognini S, Nacci A, Sieli R, Costante G, Petrolo C et al (2014) Vestibular disorders in euthyroid patients with Hashimoto’s thyroiditis: role of thyroid autoimmunity. Clin Endocrinol (Oxf) 81(4):600–605
Cinamon U, Kronenberg J, Hildesheimer M, Taitelbaum R (1997) Cochlear implantation in patients suffering from Cogan’s syndrome. J Laryngol Otol 111(10):928–930
Cody DT, Sones DA (1971) Relapsing polychondritis: audiovestibular manifestations. Laryngoscope 81(8):1208–1222
Cody DTR, Williams HL (1960) Cogan’s syndrome. Laryngoscope 70(4):447–478
Das S, Bakshi SS, Seepana R (2019) Demystifying autoimmune inner ear disease. Eur Arch Otorhinolaryngol 276(12):3267–3274
Dion J, Costedoat-Chalumeau N, Sène D, Cohen-Bittan J, Leroux G, Dion C et al (2016) Relapsing polychondritis can be characterized by three different clinical phenotypes: analysis of a recent series of 142 patients. Arthritis Rheumatol 68(12):2992–3001
Engberg J, Jepsen O (1962) Hearing impairment and dizziness in sarcoidosis: report of four cases. Dan Med Bull 9:28–32
Erbek S, Erbek SS, Yilmaz S, Yucel E, Ozluoglu LN (2008) Vestibular evoked myogenic potentials in Behcet’s disease. Eur Arch Otorhinolaryngol 265(11):1315–1320
Ertugrul O, Mutlu A, Zindanci I, Cam OH, Ozluoglu L (2019) Audiological and vestibular measurements in Behçet’s disease. Eur Arch Otorhinolaryngol 276(6):1625–1632
Evereklioglu C, Cokkeser Y, Doganay S, Er H, Kizilay A (2001) Audio-vestibular evaluation in patients with Behçet’s syndrome. J Laryngol Otol 115(9):704–708
Aiello FV, U (1973) Atypical Cogan’s syndrome. Acta Med Romana 11:67–70
Feld J, Shupak A, Zisman D (2015) Diffuse systemic sclerosis presenting as Meniere’s disease-like symptoms as part of autoimmune inner ear disease. Isr Med Assoc J 17(4):263–264
Fidler H, Jones NS (1989) Late onset Cogan’s syndrome. J Laryngol Otol 103(5):512–514
Fujimoto S, Kim CH, Green T, Xu H (2019) Otologic manifestations of immunoglobulin G4-related disease. Ear Nose Throat J 98(10):630–631
Fujiwara K, Morita S, Fukuda A, Yanagi H, Hoshino K, Nakamaru Y et al (2021) Usefulness of the video head impulse test for the evaluation of vestibular function in patients with otitis media with antineutrophil cytoplasmic antibody-associated vasculitis. Otol Neurotol 42(4):e483–e488
Galarza-Delgado DA, Villegas Gonzalez MJ, Riega Torres J, Soto-Galindo GA, Mendoza Flores L, Treviño González JL (2018) Early hearing loss detection in rheumatoid arthritis and primary Sjögren syndrome using extended high frequency audiometry. Clin Rheumatol 37(2):367–373
Gaudreau P, Moy J, Lindsay F (2012) An unusual cause of vertigo, tinnitus, and hyperacusis: Vogt-Koyanagi-Harada syndrome. Ear Nose Throat J 91(12):E7-9
Gelfand ML, Kantor T, Gorstein F (1972) Cogan’s syndrome with cardiovascular involvement: aortic insufficiency. Bull N Y Acad Med 48(4):647–660
Gemignani G, Berrettini S, Bruschini P, Sellari-Franceschini S, Fusari P, Piragine F et al (1991) Hearing and vestibular disturbances in Behçet’s syndrome. Ann Otol Rhinol Laryngol 100(6):459–463
Glišić B, Stevic-Carevic S, Ristić G, Dedović J (2018) Cogan’s syndrome – a case series. Vojnosanitetski Pregled 75:1128–33
Gopen Q, Keithley EM, Harris JP (2006) Mechanisms underlying autoimmune inner ear disease. Drug Discov Today Dis Mech 3(1):137–142
Heydari N, Hajiabolhassani F, Fatahi J, Movaseghi S, Jalaie S (2015) Vestibular evoked myogenic potentials in patients with rheumatoid arthritis. Med J Islam Repub Iran 29:216
Hirvonen TP, Aalto H (2013) Recovery of bilateral vestibular loss in Cogan’s syndrome–a case report. Otol Neurotol 34(9):1736–1738
Hooper R, Holden H (1970) Acoustic and vestibular problems in sarcoidosis. Arch Otolaryngol 92(4):386–391
Igarashi Y, Watanabe Y, Aso S (1994) A case of Behçet’s disease with otologic symptoms. ORL J Otorhinolaryngol Relat Spec 56(5):295–298
Ishii T, Watanabe I, Suzuki J (1995) Temporal bone findings in Cogan’s syndrome. Acta Otolaryngol Suppl 519:118–123
Issing WJ, Selover D, Schulz P (1999) Anti-labyrinthine antibodies in a patient with relapsing polychondritis. Eur Arch Otorhinolaryngol 256(4):163–166
Wysocki J, Jurczak P (1997) A case of relapsing polychondritis with E.N.T. symptoms. Med Sci Monit 3(2):225–8
Jenkins HA, Pollak AM, Fisch U (1981) Polyarteritis nodosa as a cause of sudden deafness A human temporal bone study. Am J Otolaryngol 2(2):99–107
Kang KT, Young YH (2008) Sudden sensorineural hearing loss in a patient with primary antiphospholipid syndrome. J Laryngol Otol 122(2):204–206
Karatas E, Onat AM, Durucu C, Baglam T, Kanlikama M, Altunoren O et al (2007) Audiovestibular disturbance in patients with systemic lupus erythematosus. Otolaryngol Head Neck Surg 136(1):82–86
Kato M, Katayama N, Naganawa S, Nakashima T (2014) Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings in a patient with relapsing polychondritis. J Laryngol Otol 128(2):192–194
Kawamura S, Sakamoto T, Kashio A, Kakigi A, Ito K, Suzuki M et al (2010) Cochlear implantation in a patient with atypical Cogan’s syndrome complicated with hypertrophic cranial pachymeningitis. Auris Nasus Larynx 37(6):737–741
Klement V, Hahn A, Hojdarova A, Sejna I (2007) Cogan’s syndrome: a case report. Acta Oto-Laryngol 127(10):1115–7
Kondo Y, Ito S, Ohi Y, Satou H, Hiraoka T, Tsuboi H et al (2009) Atypical Cogan’s syndrome with aortitis. Intern Med 48(12):1093–1097
Kühn D, Hospowsky C, Both M, Hey M, Laudien M (2018) Manifestation of granulomatosis with polyangiitis in head and neck. Clin Exp Rheumatol 36 Suppl 111(2):78–84
Lee SU, Kim JS, Hyon JY, Ha YJ, Kim HJ, Song JJ et al (2019) Pearls & Oy-sters: Cogan syndrome: a potentially grave disorder of audiovestibulopathy with many faces. Neurology 93(1):39–41
Li H, Zhang M, Wang M, Zhang S, Ma S, Wang X (2021) Clinical feature and prognosis of sudden sensorineural hearing loss with rheumatoid arthritis. Otol Neurotol 42(3):e267–e271
Maciaszczyk K, Waszczykowska E, Pajor A, Bartkowiak-Dziankowska B, Durko T (2011) Hearing organ disorders in patients with systemic sclerosis. Rheumatol Int 31(11):1423–1428
Maciaszczyk K, Durko T, Waszczykowska E, Erkiert-Polguj A, Pajor A (2011) Auditory function in patients with systemic lupus erythematosus. Auris Nasus Larynx 38(1):26–32
Maciążek-Chyra B, Szmyrka M, Skoczyńska M, Sokolik R, Lasocka J, Wiland P (2019) Relapsing polychondritis - analysis of symptoms and criteria. Reumatologia 57(1):8–18
McKennan KX, Nielsen SL, Watson C, Wiesner K (1993) Menière’s syndrome: an atypical presentation of giant cell arteritis (temporal arteritis). Laryngoscope 103(10):1103–1107
Minet M, Deggouj N, Gersdorff M (1997) Cochlear implantation in patients with Cogan’s syndrome: a review of four cases. Eur Arch Otorhinolaryngol 254(9–10):459–462
Munakomi S, Lui F. Caloric Reflex Test (2023) In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557481/
Murata J, Horii A, Tamura M, Mitani K, Mizuki M, Kubo T (2006) Endolymphatic hydrops as a cause of audio-vestibular manifestations in relapsing polychondritis. Acta Otolaryngol 126(5):548–552
O’Reilly BJ, Burrows EH (1995) VIIIth cranial nerve involvement in sarcoidosis. J Laryngol Otol 109(11):1089–1093
Orsoni JG, Zavota L, Pellistri I, Piazza F, Cimino L (2002) Cogan syndrome. Cornea 21(4):356–359
Orsoni JG, Laganà B, Rubino P, Zavota L, Bacciu S, Mora P (2010) Rituximab ameliorated severe hearing loss in Cogan’s syndrome: a case report. Orphanet J Rare Dis 5(1):18
Pelosi S, Chandrasekhar SS (2011) Intratympanic steroid use for hearing salvage in Vogt-Koyanagi-Harada syndrome. Ear Nose Throat J 90(12):574–577
Rarey KE, Bicknell JM, Davis LE (1986) Intralabyrinthine osteogenesis in Cogan’s syndrome. Am J Otolaryngol 7(6):387–390
Roberts RA (2018) Management of recurrent vestibular neuritis in a patient treated for rheumatoid arthritis. Am J Audiol 27(1):19–24
Robinson PK (1954) A case of uveitis with deafness; the Vogt-Koyanagi syndrome. Br Med J 2(4891):792–793
Rowe-Jones JM, Macallan DC, Sorooshian M (1990) Polyarteritis nodosa presenting as bilateral sudden onset cochleo-vestibular failure in a young woman. J Laryngol Otol 104(7):562–564
Ruckenstein MJ, McKown KM, Jacewicz M (2000) Unusual and instructive case of immune-mediated inner ear disease associated with central nervous system vasculitis. Otolaryngol Head Neck Surg 122(1):109–111
Russo FY, Ralli M, De Seta D, Mancini P, Lambiase A, Artico M et al (2018) Autoimmune vertigo: an update on vestibular disorders associated with autoimmune mechanisms. Immunol Res 66(6):675–685
Santos F, Salviz M, Domond H, Nadol JB (2015) Otopathology of vasculitis in granulomatosis with polyangitis. Otol Neurotol 36(10):1657–1662
Saravanan V, Pugmire S, Smith M, Kelly C (2019) Patient-reported involvement of the eighth cranial nerve in giant cell arteritis. Clin Rheumatol 38(12):3655–3660
Scharl M, Frei P, Fried M, Rogler G, Vavricka SR (2011) Association between Cogan’s syndrome and inflammatory bowel disease: a case series. J Crohns Colitis 5(1):64–68
Scherg F, Haag F, Krieger T (2019) Off-label application of intravenous immunoglobulin (IVIG) for treatment of Cogan's syndrome during pregnancy. BMJ Case Rep 12(10):e227917. https://doi.org/10.1136/bcr-2018-227917
Schuknecht HF, Nadol JB Jr (1994) Temporal bone pathology in a case of Cogan’s syndrome. Laryngoscope 104(9):1135–1142
Seccia V, Fortunato S, Cristofani-Mencacci L, Dallan I, Casani AP, Latorre M et al (2016) Focus on audiologic impairment in eosinophilic granulomatosis with polyangiitis. Laryngoscope 126(12):2792–2797
Sekhon RK, Rocha Cabrero F, Deibel JP (2023) Nystagmus Types. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539711/
Seo YJ, Choi JY, Kim SH, Kim TJ (2012) Cochlear implantation in a bilateral sensorineural hearing loss patient with relapsing polychondritis. Rheumatol Int 32(2):479–482
Shah UK, White JA, Gooey JE, Hybels RL (1997) Otolaryngologic manifestations of sarcoidosis: presentation and diagnosis. Laryngoscope 107(1):67–75
Sil A, Chatrath P, Gatland DJ (2006) Deafness in Vogt-Koyanagi-Harada syndrome. J Laryngol Otol 120(5):416–418
Smith JL (1970) Cogan’s syndrome. Laryngoscope 80(1):121–132
Sugimoto K, Miyazawa T, Nishi H, Izu A, Enya T, Okada M et al (2014) Childhood Cogan syndrome with aortitis and anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Pediatr Rheumatol Online J 12:15
Sugiura M, Naganawa S, Teranishi M, Sato E, Kojima S, Nakashima T (2006) Inner ear hemorrhage in systemic lupus erythematosus. Laryngoscope 116(5):826–828
Szmulewicz DJ, Waterston JA (2012) Two patients with audiovestibular sarcoidosis. J Clin Neurosci 19(1):158–161
Takagi D, Nakamaru Y, Maguchi S, Furuta Y, Fukuda S (2002) Otologic manifestations of Wegener’s granulomatosis. Laryngoscope 112(9):1684–1690
Ten Dam L, van de Beek D, Brouwer MC (2022) Clinical characteristics and outcome of hydrocephalus in neurosarcoidosis: a retrospective cohort study and review of the literature. J Neurol 269(5):2727–2733
Tharwat S, Mohamed SZ, Nassar MK (2021) Challenges of Egyptian patients with systemic lupus erythematosus during the COVID-19 pandemic. Reumatologia 59(4):237–243
Tirelli G, Tomietto P, Quatela E, Perrino F, Nicastro L, Cattin L et al (2015) Sudden hearing loss and Crohn disease: when Cogan syndrome must be suspected. Am J Otolaryngol 36(4):590–597
Ulusoy B, Limon M, Yılmaz S, Çolpan B, Aygün AA, Körez MK et al (2022) Effects of primary Sjögren’s syndrome on hearing and vestibular systems. J Laryngol Otol 136(12):1254–1258
Van Doornum S, McColl G, Walter M, Jennens I, Bhathal P, Wicks IP (2001) Prolonged prodrome, systemic vasculitis, and deafness in Cogan’s syndrome. Ann Rheum Dis 60(1):69
von Brevern M, Lempert T, Bronstein AM, Kocen R (1997) Selective vestibular damage in neurosarcoidosis. Ann Neurol 42(1):117–120
Vos FI, Merkus P, van Nieuwkerk EB, Hensen EF (2016) Rare cause of bilateral sudden deafness. BMJ Case Rep 2016:bcr2016216004. https://doi.org/10.1136/bcr-2016-216004
Vyse T, Luxon LM, Walport MJ (1994) Audiovestibular manifestations of the antiphospholipid syndrome. J Laryngol Otol 108(1):57–59
Wagner W, Fehrmann A (2006) Association of retinal vasculitis (Eales’ disease) and Meniere-like vestibulocochlear symptoms. Eur Arch Otorhinolaryngol 263(2):100–104
Weber RS, Jenkins HA, Coker NJ (1984) Sensorineural hearing loss associated with ulcerative colitis. A case report. Arch Otolaryngol 110(12):810–812
White AS, Taylor RL, McNeill C, Garsia R, Welgampola MS (2014) Behçet’s disease presenting as a peripheral vestibulopathy. J Clin Neurosci 21(6):1060–1063
Yamazaki H, Fujiwara K, Shinohara S, Kikuchi M, Kanazawa Y, Kurihara R et al (2012) Reversible cochlear disorders with normal vestibular functions in three cases with Wegener’s granulomatosis. Auris Nasus Larynx 39(2):236–240
Yildirim N, Arslanoglu A, Aygun N (2008) Otologic and leptomeningeal involvements as presenting features in seronegative Wegener granulomatosis. Am J Otolaryngol 29(2):147–149
Yilmaz S, Erbek S, Erbek SS, Ozgirgin N, Yucel E (2007) Abnormal electronystagmography in rheumatoid arthritis. Auris Nasus Larynx 34(3):307–311
Ying YL, Hirsch BE (2010) Atypical Cogan’s syndrome: a case report. Am J Otolaryngol 31(4):279–282
Howick J, Chalmers I, (James Lind Library), Glasziou P, Greenhalgh T, Heneghan C, Liberati A et al (2011) OCEBM levels of evidence working group. The Oxford 2011 Levels of Evidence. Oxford Centre for Evidence-Based Medicine
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2022) Cochrane handbook for systematic reviews of interventions version 6.3. cochrane. Available from https://www.training.cochrane.org/handbook.
Macdonald NK, Kaski D, Saman Y, Al-Shaikh Sulaiman A, Anwer A, Bamiou DE (2017) Central Positional Nystagmus: A Systematic Literature Review. Front Neurol 8:141.https://doi.org/10.3389/fneur.2017.00141
Strupp M, Thurtell MJ, Shaikh AG, Brandt T, Zee DS, Leigh RJ (2011) Pharmacotherapy of vestibular and ocular motor disorders, including nystagmus. J Neurol 258(7):1207–1222
Özkırış M, Kapusuz Z, Günaydın İ, Kubilay U, Pırtı İ, Saydam L (2014) Does rheumatoid arthritis have an effect on audiovestibular tests? Eur Arch Otorhinolaryngol 271(6):1383–1387
Kimura H, Ohashi N, Aso S, Watanabe Y (1996) Clinical study of the role of melanocytes in the inner ear of patients with Harada’s disease. ORL J Otorhinolaryngol Relat Spec 58(4):233–237
Morita Y, Takahashi K, Izumi S, Kubota Y, Ohshima S, Horii A (2017) Vestibular involvement in patients with otitis media with antineutrophil cytoplasmic antibody-associated vasculitis. Otol Neurotol 38(1):97–101
Noguchi Y, Nishio A, Takase H, Miyanaga M, Takahashi H, Mochizuki M et al (2014) Audiovestibular findings in patients with Vogt-Koyanagi-Harada disease. Acta Otolaryngol 134(4):339–344
Tarnutzer AA, Straumann D, Salman MS (2018) Neuro-ophthalmologic assessment and investigations in children and adults with cerebellar diseases. Handb Clin Neurol 154:305–327
Purves D, Augustine GJ, Fitzpatrick D, et al (2001) Editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates. Available from: https://www.ncbi.nlm.nih.gov/books/NBK10799/
McGrath S, Sohn H, Steele R, Benedetti A (2020) Meta-analysis of the difference of medians. Biom J 62(1):69–98
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54(10):1046–1055
El-Wakd MM, El-Gazzar II, Hosni NA, El-Din Teleb DAG (2015) Otolith function assessment in patients with systemic sclerosis. Egypt Rheumatol 37(3):105–112
Cadoni G, Agostino S, Manganelli C, Scipione S, Turco S, Focosi F et al (2004) Vestibular evaluation in Behcet’s disease. Personal experience. Acta Otorhinolaryngol Ital 24(5):262–266
Choung YH, Cho MJ, Park K, Choi SJ, Shin YR, Lee ES (2006) Audio-vestibular disturbance in patients with Behcet’s disease. Laryngoscope 116(11):1987–1990
Helmchen C, Arbusow V, Jager L, Strupp M, Stocker W, Schulz P (1999) Cogan’s syndrome: clinical significance of antibodies against the inner ear and cornea. Acta Otolaryngol 119(5):528–536
Sugasawa J, Ishikawa S (1986) Vestibulo-ocular reflex abnormality in Behçet’s disease. Jpn J Ophthalmol 30(1):91–99
Amor-Dorado JC, Arias-Nunez MC, Miranda-Filloy JA, Gonzalez-Juanatey C, Llorca J, Gonzalez-Gay MA (2008) Audiovestibular manifestations in patients with limited systemic sclerosis and centromere protein-B (CENP-B) antibodies. Medicine (Baltimore) 87(3):131–141
Bayram A, Dogan M, Koc A, Kalkan M, Akcadag A, Ozcan I (2015) Cervical and ocular vestibular evoked myogenic potentials in Behcet’s disease. Am J Otolaryngol 36(4):503–508
Gluth MB, Baratz KH, Matteson EL, Driscoll CL (2006) Cogan syndrome: a retrospective review of 60 patients throughout a half century. Mayo Clin Proc 81(4):483–488
Salman EJ, Tripathy K (2023) Cogans Syndrome. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK580546/
Agrawal Y, Pineault KG, Semenov YR (2018) Health-related quality of life and economic burden of vestibular loss in older adults. Laryngoscope Investig Otolaryngol 3(1):8–15
Koike H, Nishi R, Ohyama K, Morozumi S, Kawagashira Y, Furukawa S et al (2022) ANCA-associated vasculitic neuropathies: a review. Neurol Ther 11(1):21–38
Girasoli L, Cazzador D, Padoan R, Nardello E, Felicetti M, Zanoletti E et al (2018) Update on vertigo in autoimmune disorders, from diagnosis to treatment. J Immunol Res 2018:5072582
Gonçalves DU, Felipe L, Lima TM (2008) Interpretation and use of caloric testing. Braz J Otorhinolaryngol 74(3):440–446
Hedges TR, Taylor GW (1952) Uveal and vestibuloauditory disease with sarcoid. AMA Arch Ophthalmol 48(1):88–89
Brama I, Fainaru M (1980) Inner ear involvement in Behcet’s disease. Arch Otolaryngol 106(4):215–217
Halmagyi GM, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS (2017) The video head impulse test. Front Neurol 8:258
Yuen E, Fried J, Nguyen SA, Rizk HG, Ward C, Meyer TA (2021) Hearing loss in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus 30(6):937–945
Rahne T, Plontke S, Keyßer G (2020) Vasculitis and the ear: a literature review. Curr Opin Rheumatol 32(1):47–52
Fousekis FS, Saridi M, Albani E, Daniel F, Katsanos KH, Kastanioudakis IG et al (2018) Ear involvement in inflammatory bowel disease: a review of the literature. J Clin Med Res 10(8):609–614
Sakano H, Harris JP (2018) Emerging options in immune-mediated hearing loss. Laryngoscope Investig Otolaryngol 4(1):102–108
Hall CD, Herdman SJ, Whitney SL, Cass SP, Clendaniel RA, Fife TD et al (2016) Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American Physical Therapy Association Neurology Section. J Neurol Phys Ther 40(2):124–155
Hall CD, Herdman SJ, Whitney SL, Anson ER, Carender WJ, Hoppes CW et al (2022) Vestibular rehabilitation for peripheral vestibular hypofunction: an updated clinical practice guideline from the Academy of Neurologic Physical Therapy of the American Physical Therapy Association. J Neurol Phys Ther 46(2):118–177
Mira E (2008) Improving the quality of life in patients with vestibular disorders: the role of medical treatments and physical rehabilitation. Int J Clin Pract 62(1):109–114
Gurgel RK, Jackler RK, Dobie RA, Popelka GR (2012) A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg 147(5):803–807
Acknowledgements
The authors would like to acknowledge Emily Brennen, Andrea Li, MD, and Neil Mehta, MD.
Author information
Authors and Affiliations
Contributions
Sunny Shah: conception/design of study, acquisition, analysis, and interpretation of data, intellectual contribution, and drafting of the manuscript.
Shreya Chidarala: conception/design of study, acquisition, analysis, and interpretation of data, intellectual contribution, and drafting of the manuscript.
Seth Jeong: conception/design of study, acquisition, analysis, and interpretation of data, intellectual contribution, and drafting of the manuscript.
Kathy Zhang: conception/design of study, acquisition, analysis, and interpretation of data, intellectual contribution, and drafting of the manuscript.
Shaun A. Nguyen: acquisition, analysis, and interpretation of data, intellectual contribution, and drafting of the manuscript.
Rachel Wilkinson: analysis, intellectual contribution, and drafting of the manuscript.
Celine Ward: interpretation of data, intellectual contribution, and drafting of the manuscript.
Habib Rizk: conception/design of study, acquisition, analysis, and interpretation of data, intellectual contribution, and drafting of the manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Disclaimer
All co-authors take full responsibility for the integrity and accuracy of all aspects of the work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shah, S., Chidarala, S., Jeong, S. et al. Secondary autoimmune immune ear disease (AIED): a systematic review and meta-analysis on vestibular manifestations of systemic autoimmune and inflammatory disorders. Clin Rheumatol 42, 2747–2759 (2023). https://doi.org/10.1007/s10067-023-06674-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06674-w