Abstract
Objectives
To understand change in work productivity, activity impairment, quality of life (QoL), and disease activity in patients with psoriatic arthritis (PsA) receiving anti-tumor necrosis factor (anti-TNF) treatment.
Method
One hundred twenty patients with PsA receiving anti-TNF therapy were recruited to this noninterventional, observational study. Work disability was assessed via the Work Productivity and Activity Impairment (WPAI) questionnaire and disease activity was calculated via the 28-joint Disease Activity Score using C-reactive protein (DAS28-CRP) and Disease Activity Index for Psoriatic Arthritis with 28 joints (DAPSA28) score. Patient-reported outcomes (PROs), from visual analog scores and Health Assessment Questionnaire-Disability Index scores, were evaluated to understand the clinical effectiveness at baseline and every 3 months until the month-9 final visit. The American College of Rheumatology (ACR)20/50/70 response criteria were assessed at month 9.
Results
A total of 120 patients (females, n = 73) were enrolled in the study. Mean (SD) age and disease duration were 41.6 ± 11.1 years and 6.9 ± 6.5 years, respectively. The most commonly used TNFα inhibitor was adalimumab (42.4%), followed by etanercept (25.8%). All WPAI questionnaire parameters were reduced at the follow-up visits compared with baseline (p < 0.001 for all). PROs and disease activity indicators (DAS28-CRP and DAPSA28) significantly improved during the course of anti-TNF treatments (p < 0.001 for all). Additionally, ACR20/50/70 responses were determined as 86.8%, 63.7%, and 41.8% of patients at the month-9 visit.
Conclusions
The real-world data in PsA patients receiving anti-TNF treatment showed improvement in WPAI, QoL, and disease activity over 9 months of treatment.
Trial registration
NCT02028169
Key Points • Psoriatic arthritis (PsA), with debilitating effects on quality of life, occurs mostly in young adults and has negative impacts on employment status and work productivity. • Early PsA diagnosis and treat-to-target treatment strategies aim to reduce pain and joint damage, as well as improve work productivity. • Real-world data on the impact of treatment with anti-tumor necrosis factor (anti-TNF) agents on work productivity in PsA in the literature is scarce. • Our study of real-world data in patients with PsA receiving anti-TNF treatment showed improvement in work productivity, as well as in clinical and patient-reported outcomes. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory and heterogeneous disease characterized by psoriasis and arthritis [1,2,3]. It causes inflammation at peripheral and axial joints, tendons, entheses, and dactyls and restricts daily activities due to pain and loss of function [1,2,3,4,5]. Moreover, PsA, which mostly appears in younger adults, decreases quality of life (QoL) [6,7,8]. In addition, PsA negatively affects employment status and work productivity [9].
Results from previous studies show the importance of an early diagnosis of PsA and target-to-treat strategies to reduce pain and joint damage, as well as improve work productivity [4, 6]. Work disability is a major patient outcome for chronic rheumatic diseases, such as PsA [6, 10, 11]. The Work Productivity and Activity Impairment (WPAI) questionnaire is a validated measurement tool of work productivity that results in the following four work-related outcomes: absenteeism (time missed from work), presenteeism (impairment at work), productivity loss (absenteeism plus presenteeism), and overall activity impairment [2, 12].
The primary goals of PsA treatment are to reduce pain and maintain regular joint function and QoL [7, 13]. Additionally, low disease activity and clinical remission are expected from treatment. Several measurement instruments are used to assess disease activity in PsA, such as the 28-joint Disease Activity Score (DAS28) and the Disease Activity Index for PsA (DAPSA) [4, 14]. While DAPSA has been validated with 66 swollen and 68 tender joint counts, DAPSA with 28-joint counts has also been proposed as the modified DAPSA28 score because many databases and registries collect only 28-joint counts, and DAPSA28 is a correlated and validated tool that is sensitive to change [15, 16]. Moreover, the American College of Rheumatology 20% (ACR20) response is a significant parameter for the evaluation of disease activity in clinical trials. This criterion is assessed with the 20%, 50%, and 70% of improvements in tender and swollen joints [2].
A number of biologic and targeted synthetic drugs are used for the efficient treatment of patients with PsA [3]. Most patients need treatment with disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate [7]. However, nearly 40% of patients receive treatment with approved biologic disease-modifying antirheumatic drugs (bDMARDs), including tumor necrosis factor (TNF) inhibitors (adalimumab, etanercept, infliximab, golimumab, and certolizumab pegol), interleukin antagonists (ustekinumab and secukinumab) and the selective costimulation modulator abatacept [3, 7, 17]. Anti-TNF agents are the first class of bDMARDs used for the treatment of PsA, with clinical trials showing that they alter the disease process and improve work productivity and QoL of patients with PsA [3, 9]. These agents also have resulted in clinical remission by suppressing disease activity [3, 18].
This postmarketing, noninterventional, observational study aims to understand the change in work productivity and activity impairment, QoL, and disease activity in a real-world setting in patients with PsA receiving anti-TNF treatment over a 9-month period.
Materials and methods
This was an open-label, multicenter, noninterventional, prospective, and observational cohort study in Turkey to investigate the work disability and household productivity of patients with PsA who were on anti-TNF treatment. Diagnosis of PsA was based on the decision of the treating rheumatologist. This study was approved by the Clinical Research Ethics Committee of Hacettepe University on October 31, 2013, with an approval number: 2013/13–03 (KA-130083) and have been conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. One hundred twenty adults (aged ≥ 18 years) and who were actively working (full-time or part-time) or active as homemakers and students and who met the CASPAR criteria with previously initiated (at any time) anti-TNF therapy (adalimumab, etanercept, golimumab, infliximab, or certolizumab) were enrolled after receipt of their written informed consent [19]. In accordance with the one of the main aims of the study, to understand the change in work productivity and activity impairment, active patients were selected. Patients with a history of allergic reaction to the anti-TNF agents, unable to arrive at follow-up visits on time, and who were enrolled in an interventional clinical trial within 30 days were excluded. Physician- and patient-reported outcome measures were collected at baseline and throughout the 9-month period at 3-month intervals. The study was registered at ClinicalTrials.gov (identifier: NCT02028169).
The primary endpoint of this study was to determine the alteration in work disability. This parameter (work productivity) was assessed via the WPAI questionnaire, measured at baseline and at months 3, 6, and 9 [20]. The WPAI is a six-item questionnaire that investigates the disability of patients at or out of work for the past 7 days. The results are expressed as percentages (%) for four outcomes: work time missed (absenteeism), impairment at work (presenteeism), work productivity loss, and general activity impairment. Only patients employed and self-employed at baseline were assessed for WPAI scores. The WPAI is a validated psychometrically and commonly preferred instrument for measuring health-associated work productivity [21].
As secondary outcomes, disease activity scores; tender and swollen joint counts (TJCs and SJCs, respectively); and patient-reported outcomes (PROs), such as the Health Assessment Questionnaire-Disability Index (HAQ-DI) with 20 questions related to daily life activities and the visual analog scale (VAS) for pain (nocturnal back and total back), fatigue, and global assessment of disease activity (PtGA, patient; physician), were calculated. Additionally, clinical parameters of inflammation, such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), were measured and basic demographic data were collected at baseline. Enthesitis and dactylitis parameters also were assessed for PsA inflammation. Enthesitis was measured using the Maastricht Ankylosing Spondylitis Enthesitis Score in 13 body sites: the bilateral first and seventh costochondral joints, the anterior and posterior superior iliac spine, iliac crests, the fifth lumbar spinous process, and the proximal insertion of the Achilles tendon. These sites were analyzed for the presence of tenderness (1 = tender, 0 = not tender; the sum of the sites score range 0–13) [22]. Dactylitis was assessed in each of the 20 digits of the hands and feet and evaluated as to whether they were swollen through a severity score of 0–3 (where 0 = no swelling or pain and 3 = severe swelling and pain) [23]. For peripheral joint disease activity in patients with PsA, the DAS28-CRP was calculated using 28 TJCs and SJCs, respectively, plus CRP values and PtGA. The expected DAS28 score should be ≤ 2.6 (indicating remission) and > 2.6 to ≤ 3.2 (indicating low disease activity) [14]. DAPSA scores were calculated by summing the 28 TJCs and SJCs, CRP, patient disease activity, and pain assessment values using the following formula:
[15].
The cutoff values of disease activities were evaluated as remission (≤ 4), low (> 4 to ≤ 14), moderate (> 14 to ≤ 28), and high (> 28) [24]. Each assessment including questionnaires administered was done at baseline, 3rd, 6th, and 9th month visits.
Data analysis
Analyses of demographic characteristics, medication history, clinical outcomes, and work disability of patients were conducted using descriptive analysis methods. Categorical variables (e.g., sex, employment status, prevalence of anti-TNF type, concomitant medications, work disability outcomes) were summarized using frequency counts and percentages. Continuous variables (e.g., age, clinical measurements, disease activity responsive measurements) were summarized using counts, means, and standard deviation of means. Statistical comparisons between groups as treatment-responsive data for VAS-associated outcomes, DAS28 and DAPSA28 scores, enthesitis and dactylitis scores, ESR and CRP levels, TJCs and SJCs, and HAQ-DI scores, as well as ACR20/50/ACR70 responses, at months 3 and 6 and at the end of the study (month 9) compared with baseline were evaluated using the Friedman test. The Friedman test was used to compare work disability parameters according to WPAI scores between baseline and follow-up visits. When there was significant difference, pairwise comparisons were made using Wilcoxon test. All analyses were performed using IBM SPSS Statistics for Windows, version 21.0. Armonk, NY: IBM Corp.
Results
Demographic data of patients
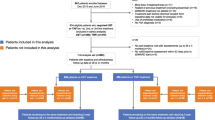
A total of 120 patients (females, n = 73 [60.8%]) with PsA were enrolled in the study. The mean age was 41.6 ± 11.1 years and the mean disease duration since the initial PsA diagnosis was 6.9 ± 6.5 years (Table 1). Approximately half of the patients were homemakers (51%), followed by employers who worked outside the home (35.8%). Among the registered patients, the most commonly used TNFα inhibitor was adalimumab (42.4%), followed by etanercept (25.8%). The detailed baseline characteristics of the patients with PsA are shown in Table 1. Among study patients, 104 (86.7%) completed 3 months, 99 (82.5%) completed 6 months, and 92 (76.7%) completed 9 months (Fig. 1).
Primary endpoint: work productivity in response to therapy
The WPAI questionnaire showed the improvement in work disability of patients with PsA with anti-TNF treatment. The absenteeism values decreased with treatment from 20.8 ± 22.6 (n = 54) to 5.5 ± 11.1 (n = 3 3) at the final follow-up compared with baseline (p < 0.001). The mean presenteeism was 54.7 ± 22.6 (n = 55) at baseline, which decreased significantly to 18.2 ± 18.6 (n = 35) at the month-9 follow-up visit (p < 0.001). Moreover, work productivity loss showed improvement. The mean work productivity score was 43.6 ± 21.5 (n = 54) at baseline and decreased significantly at month 9 to 14.9 ± 13.1 (n = 33) (p < 0.001). The overall activity impairment decreased from 55.0 ± 21.5 (n = 56) to 16.3 ± 18.2 (n = 37) at the final follow-up visit (p < 0.001), indicating the main improvement of anti-TNF treatment. Moreover, compared with baseline, each follow-up assessment showed significant improvement in all four components of the WPAI questionnaire (p < 0.001) (Fig. 2).
Mean Work Productivity and Activity Impairment scores, PtGA, and VASs. PtGA, patient’s global assessment; VAS, visual analog scale. ap values are the result of Friedman’s test; however, they are also valid for pairwise comparisons: baseline versus month-3, baseline versus month-6, baseline versus month-9 comparisons for all parameters
Secondary endpoints: disease activity in response to therapy
The disease activity was assessed globally by patients and physicians using the VAS tool. The PtGA was 6.73 ± 1.63 (n = 120) at baseline and then decreased to an average of 3.93 ± 2.76 (n = 103) at the following visit. Similar results were obtained from physician VAS scores (Fig. 2). All PROs and disease activity indicators significantly changed in response to anti-TNF treatments (p < 0.001, for all follow-up visits). The data from the global assessment of pain were obtained using the VAS tool and indicated a decrease in pain and fatigue at the 3-, 6-, and 9-month visits compared with baseline (Fig. 2). Furthermore, CRP and ESR values, as well as TJCs and SJCs, decreased sharply at the month-3 follow-up compared with baseline and continued at other visits. Similarly, enthesitis and dactylitis scores were improved (p < 0.001), decreasing from 1.75 ± 2.37 to 0.45 ± 1.50 and 0.47 ± 1.01 to 0.03 ± 0.24 at the final visit compared with baseline, respectively (Table 2). Disease activity was evaluated using DAS28-CRP and DAPSA28 scores, as well as ACR response. DAS28-CRP values decreased from 4.17 ± 1.07 to 2.78 ± 1.23 at month 3, 2.07 ± 0.75 at month 6, and 2.17 ± 0.97 at month 9, indicating a reduction in disease activity. Remission and low disease activity were determined in 72.0% and 13.3% of patients at the final visit, respectively (Table 3). Moreover, according to the DAPSA28 classification, the high disease activity observed at baseline decreased from 53.23 ± 51.55 to 22.92 ± 22.11 at month 3, 13.43 ± 10.64 at month 6, and 16.49 ± 17.26 at month 9. Remission and low disease activity were determined in 18.67% and 45.33% of patients at the final visit, respectively (Table 3). In addition to these results, ACR20/50/70 responses were improved, to 86.8%, 63.7%, and 41.8% of patients, at the month-9 final visit (Table 3).
Discussion
PsA is a progressive inflammatory disease that causes loss of function and health-related QoL and negatively affects work productivity [3]. Recent clinical trials have planned to determine the effect of anti-TNF treatment on work disability in rheumatic diseases—especially comparing different classes of drug treatments [6]. Numerous studies showed that remission or low disease activity were the main accomplishable results in patients with PsA treated with anti-TNF [3, 18].
Significant improvements in symptoms of PsA were revealed with anti-TNF treatments in several studies. Moreover, clinical trials and observational studies conducted over 10 years showed the effectiveness of anti-TNF agents in all PsA-related outcomes, mainly in peripheral joint involvement [3]. For instance, infliximab significantly decreased pain and function and improved QoL, assessed using HAQ-DI, in patients with PsA with 24 weeks of treatment [25]. Similarly, another anti-TNF agent, adalimumab, also had better impacts on pain and QoL in patients with PsA [26, 27]. For example, in the ACCLAIM study, a statistically significant improvement of − 0.44 ± 0.53 points in the mean HAQ-DI score was observed at the 12th week compared with baseline [27]. Additionally, work disability decreased in response to adalimumab treatments in employed patients at the end of the 12-week period [26, 27]. Similar results were obtained in the GO-REVEAL trial [28]. The function, health-related QoL, and work productivity results revealed improvements in patients with PsA treated with golimumab for 24 weeks. HAQ-DI values and work disability significantly decreased with golimumab treatment [28]. Furthermore, another anti-TNF agent, certolizumab pegol, improved HAQ-DI scores and pain and fatigue results of patients with PsA over a 24-week period in the RAPID-PsA trial [29]. However, real-world data showing the impact of anti-TNF, especially on work productivity in patients with PsA, in the literature is scarce. Our results indicate reduced pain and fatigue in the 9-month period and significant improvement in work productivity and functional impairment in a cohort of patients with PsA receiving anti-TNF treatment in the real-world setting.
Several trials and observational studies conducted in recent years confirmed the efficiency of infliximab on the reduction of the SJCs and TJCs, with an ACR50 response ranging between 40 and 53% [30, 31]. The SJCs and TJCs were reduced to 58.0% and 54.1%, respectively, in the IMPACT-2 trial [31]. Similar results were obtained with etanercept therapy. ACR20/50/70 responses were 73%, 50%, and 13% with a 12-week treatment period [32]. Golimumab use also improved the ACR20 response significantly. In addition, there was no significant difference in the ACR20 response of patients using methotrexate as a concomitant medication, suggesting the efficiency of golimumab with or without concomitant methotrexate [33]. In the RAPID-PsA trial, certolizumab pegol provided improvements in the ACR response of patients with PsA. At the end of the 4-week period, an ACR20 response was achieved by 56.3% of patients, as well as ACR50 and ACR70 responses being achieved by 40.0% and 23.7% of patients, respectively [34]. Similarly, our results also suggested an improvement in the ACR response.
Dactylitis and enthesitis were previously defined as efficiency criteria for anti-TNF agents [3]. The improvement of dactylitis was shown in the ACCLAIM study with adalimumab, in the PRESTA study with etanercept, in the IMPACT-1 and 2 trials with infliximab, in the GO-REVEAL trial with golimumab, and in the RAPID-PsA trial with certolizumab [27, 30, 31, 33,34,35]. In addition, enthesitis was improved in 81.3% of patients with etanercept therapy in the PRESTA study [35]. Similarly, in the RAPID-PsA trial, enthesitis scores significantly decreased by − 1.8 with certolizumab compared with patients receiving placebo [34]. In our study, dactylitis and enthesitis results support the literature, as scores decreased significantly after 9 months of treatment compared with baseline.
We determined disease activity using DAS28-CRP and DAPSA28 scores and obtained significant remission and low disease activity levels. Remission and low disease activity values reached 72.0% and 13.3%, respectively, after 9 months, indicating the improvement in joints and pain. DAPSA is a measure of articular disease in PsA; for this reason, we used it for the calculation of the disease activity of patients with PsA [4]. We used DAPSA28 score with 28-joint counts instead of 66/68 joint counts in this study. Since DAPSA28 is also apprehended as an evaluation criterion with correlational and construct validity and sensitivity to change, herewith we propose to use it to calculate a modified DAPSA score, especially in patients with low disease activity [15, 16]. In this study, DAPSA28 scores showed an improvement in patients with PsA. These results also show improved low disease activity status.
There is high percentage of female patients in this cohort. However, the rates are similar to the registry results from Turkey where around 64% of the patients were female [36]. The same study also showed worse outcomes of female patients compared to the male patients at the same objective disease activity. Another literature showed that female psoriasis and psoriatic arthritis patients were 1.47 times more likely to seek care [37]. As a result, closer follow-up requirements of women compared with men may be seen given the higher unmet need in women. This could explain why women are more frequently recruited. This is also supported by a recent inception cohort study from the same registry which included fewer women than the established group (57% versus 66.8%) [38].
It is important to highlight certain methodologic limitations of the study. Given the observational design of the study, the inability to control concomitant treatment modalities limited the assessment of the exact response to anti-TNF agents and head-to-head comparison with other treatment modalities which could better be assessed in a randomized trial. Another limitation is the high dropout rate; the most common reason was “lost to follow-up” and those patients were not replaced because this was a noninterventional observational study. Because all patients with PsA who were to receive anti-TNF treatment were enrolled, there could be a patient selection bias. Patients who continued with anti-TNF treatment were more likely to achieve an adequate treatment response compared with those who discontinued treatment, which may also bias the results. The inclusion of patients who were on an anti-TNF agent at any time instead of the initiation of the treatment is another limitation of the study.
The data from our real-world study showed improvement in work productivity, clinical outcomes, and PROs in patients with PsA receiving anti-TNF treatment. Work disability, functional impairment, QoL, and clinical outcomes improved significantly in response to therapy despite the initial high disease activity. Our results may also suggest that work disability may improve upon reaching remission or low disease activity after efficient treatment of PsA. However, prospective studies should be conducted to determine the effects of anti-TNF agents on work productivity loss in patients with PsA.
Data availability
Data are available upon reasonable request.
Code availability
Not applicable.
References
Ritchlin CT, Colbert RA, Gladman DD (2017) Psoriatic arthritis. N Engl J Med 376(10):957–970. https://doi.org/10.1056/NEJMra1505557
Tucker LJ, Coates LC, Helliwell PS (2019) Assessing disease activity in psoriatic arthritis: a literature review. Rheumatol Ther 6(1):23–32. https://doi.org/10.1007/s40744-018-0132-4
Lubrano E, Scriffignano S, Perrotta FM (2019) TNF-alpha inhibitors for the six treatment targets of psoriatic arthritis. Expert Rev Clin Immunol 15(12):1303–1312. https://doi.org/10.1080/1744666x.2020.1685382
Pukšić S, Bolton-King P, Sexton J, Michelsen B, Kvien TK, Berner Hammer H (2018) DAPSA and ultrasound show different perspectives of psoriatic arthritis disease activity: results from a 12-month longitudinal observational study in patients starting treatment with biological disease-modifying antirheumatic drugs. RMD Open 4(2):e000765. https://doi.org/10.1136/rmdopen-2018-000765
Bagel J, Schwartzman S (2018) Enthesitis and dactylitis in psoriatic disease: a guide for dermatologists. Am J Clin Dermatol 19(6):839–852. https://doi.org/10.1007/s40257-018-0377-2
Tillett W, Shaddick G, Jobling A, Askari A, Cooper A, Creamer P, Clunie G, Helliwell PS, James J, Kay L, Korendowych E, Lane S, Packham J, Shaban R, Thomas ML, Williamson L, McHugh N (2017) Effect of anti-TNF and conventional synthetic disease-modifying anti-rheumatic drug treatment on work disability and clinical outcome in a multicentre observational cohort study of psoriatic arthritis. Rheumatology (Oxford) 56(4):603–612. https://doi.org/10.1093/rheumatology/kew433
Palsson O, Love TJ, Gunnarsdottir AI, Gunnarsson PS, Runarsdottir EE, Krogh NS, Gudbjornsson B (2019) Patients with psoriatic arthritis who are not eligible for randomised controlled trials for TNF inhibitors have treatment response and drug survival similar to those who are eligible. RMD Open 5(2):e000984. https://doi.org/10.1136/rmdopen-2019-000984
Tsuruta N, Narisawa Y, Imafuku S, Ito K, Yamaguchi K, Miyagi T, Takahashi K, Fukamatsu H, Morizane S, Koketsu H, Yamaguchi M, Hino R, Nakamura M, Ohyama B, Ohata C, Kuwashiro M, Sato T, Saito K, Kaneko S, Yonekura K, Hayashi H, Yanase T, Morimoto K, Sugita K, Yanagihara S, Kikuchi S, Mitoma C, Nakahara T, Furue M, Okazaki F (2019) Cross-sectional multicenter observational study of psoriatic arthritis in Japanese patients: relationship between skin and joint symptoms and results of treatment with tumor necrosis factor-α inhibitors. J Dermatol 46(3):193–198. https://doi.org/10.1111/1346-8138.14745
van der Heijde D, Braun J, Rudwaleit M, Purcaru O, Kavanaugh AF (2018) Improvements in workplace and household productivity with certolizumab pegol treatment in axial spondyloarthritis: results to week 96 of a phase III study. RMD Open 4(1):e000659. https://doi.org/10.1136/rmdopen-2018-000659
Black CM (2012) Sickness absence and musculoskeletal disorders. Rheumatology (Oxford) 51(2):204–205. https://doi.org/10.1093/rheumatology/ker323
Short P, Jones AC, Walker D, Kavanaugh A, Moots RJ (2012) Working at arthritis. Rheumatology (Oxford) 51(2):201–203. https://doi.org/10.1093/rheumatology/ker415
Beaton DE, Dyer S, Boonen A, Verstappen SM, Escorpizo R, Lacaille DV, Bosworth A, Gignac MA, Leong A, Purcaru O, Leggett S, Hofstetter C, Peterson IF, Tang K, Fautrel B, Bombardier C, Tugwell PS (2016) OMERACT filter evidence supporting the measurement of at-work productivity loss as an outcome measure in rheumatology research. J Rheumatol 43(1):214–222. https://doi.org/10.3899/jrheum.141077
Gossec L, Baraliakos X, Kerschbaumer A, de Wit M, McInnes I, Dougados M, Primdahl J, McGonagle DG, Aletaha D, Balanescu A, Balint PV, Bertheussen H, Boehncke WH, Burmester GR, Canete JD, Damjanov NS, Kragstrup TW, Kvien TK, Landewé RBM, Lories RJU, Marzo-Ortega H, Poddubnyy D, Rodrigues Manica SA, Schett G, Veale DJ, Van den Bosch FE, van der Heijde D, Smolen JS (2020) EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 79(6):700–712. https://doi.org/10.1136/annrheumdis-2020-217159
Fransen J, van Riel PL (2009) The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am 35(4):745-757 vii-viii. https://doi.org/10.1016/j.rdc.2009.10.001
Michelsen B, Sexton J, Smolen JS, Aletaha D, Krogh NS, van der Heijde D, Kvien TK, Hetland ML (2018) Can disease activity in patients with psoriatic arthritis be adequately assessed by a modified Disease Activity index for PSoriatic Arthritis (DAPSA) based on 28 joints? Ann Rheum Dis 77(12):1736–1741. https://doi.org/10.1136/annrheumdis-2018-213463
Kalyoncu U BO, Oksuz MF, Kimyon G, Erden A, Yavuz S, Cetin G, Kucuksahin O, Kilic L, Omma A, Ozisler C, Solmaz D, Onat AM, Kisacik B, Ersozlu Bakirli D, Cinar M, Tufan A, Yildiz F, Mercan R, Kasifoglu T, Yilmazer B, Yilmaz S, Aksu K, Erten S, Sayarlioglu M, Dalkilic E, Akar S, Acikel C, Aydin Tufan M, Balkarli A, Kasapoglu-Gunal E, Senel S, Kobak S, Duruoz MT, Dogru A, Tarhan EF, Can M, Akyol L, Pehlevan S, Erbasan F, Arslan F, Kucuk A, Gonullu E, Kabasakal Y, Sahin M, Atakan N, Aydin SZ (2016) Dapsa-28 may be used instead of Dapsa-66/68 in psoriatic arthritis [abstract]. Arthritis Rheumatol 68(10):3689–3690. https://acrabstracts.org/abstract/dapsa-28-may-be-used-instead-of-dapsa-6668-in-psoriatic-arthritis/
Lee MP, Lii J, Jin Y, Desai RJ, Solomon DH, Merola JF, Kim SC (2018) Patterns of systemic treatment for psoriatic arthritis in the US: 2004–2015. Arthritis Care Res (Hoboken) 70(5):791–796. https://doi.org/10.1002/acr.23337
Perrotta FM, Marchesoni A, Lubrano E (2016) Minimal disease activity and remission in psoriatic arthritis patients treated with anti-TNF-α drugs. J Rheumatol 43(2):350–355. https://doi.org/10.3899/jrheum.150805
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 54(8):2665–2673. https://doi.org/10.1002/art.21972
Reilly MC, Zbrozek AS, Dukes EM (1993) The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 4(5):353–365. https://doi.org/10.2165/00019053-199304050-00006
Klaudius I, Krueger K, Remstedt S, Thiele A (2018) SAT0187 Golimumab improves work productivity and activity impairment in patients with rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PSA): 1-year results from a non- interventional trial in Germany. Ann Rheum Dis 77(Suppl 2):954–955. https://doi.org/10.1136/annrheumdis-2018-eular.2925
Mease P (2020) Enthesitis in psoriatic arthritis (Part 3): clinical assessment and management. Rheumatology (Oxford) 59(Supplement_1):i21–i28. https://doi.org/10.1093/rheumatology/keaa042
Kavanaugh A, Mease P (2012) Treatment of psoriatic arthritis with tumor necrosis factor inhibitors: longer-term outcomes including enthesitis and dactylitis with golimumab treatment in the Longterm Extension of a Randomized, Placebo-controlled Study (GO-REVEAL). J Rheumatol Suppl 89:90–93. https://doi.org/10.3899/jrheum.120254
Schoels MM, Aletaha D, Alasti F, Smolen JS (2016) Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 75(5):811–818. https://doi.org/10.1136/annrheumdis-2015-207507
Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, Zhou B, Dooley LT, Kavanaugh A (2005) Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 64(8):1150–1157. https://doi.org/10.1136/ard.2004.032268
Mease PJ, Ory P, Sharp JT, Ritchlin CT, Van den Bosch F, Wellborne F, Birbara C, Thomson GT, Perdok RJ, Medich J, Wong RL, Gladman DD (2009) Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the Adalimumab Effectiveness in Psoriatic Arthritis Trial (ADEPT). Ann Rheum Dis 68(5):702–709. https://doi.org/10.1136/ard.2008.092767
Gladman DD, Sampalis JS, Illouz O, Guérette B (2010) Responses to adalimumab in patients with active psoriatic arthritis who have not adequately responded to prior therapy: effectiveness and safety results from an open-label study. J Rheumatol 37(9):1898–1906. https://doi.org/10.3899/jrheum.100069
Kavanaugh A, McInnes IB, Krueger GG, Gladman D, Beutler A, Gathany T, Mack M, Tandon N, Han C, Mease P (2013) Patient-reported outcomes and the association with clinical response in patients with active psoriatic arthritis treated with golimumab: findings through 2 years of a phase III, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken) 65(10):1666–1673. https://doi.org/10.1002/acr.22044
Gladman D, Fleischmann R, Coteur G, Woltering F, Mease PJ (2014) Effect of certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res (Hoboken) 66(7):1085–1092. https://doi.org/10.1002/acr.22256
Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, Manger B, Wassenberg S, Weier R, Wallace DJ, Weisman MH, Kalden JR, Smolen J (2005) Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 52(4):1227–1236. https://doi.org/10.1002/art.20967
Kavanaugh A, Krueger GG, Beutler A, Guzzo C, Zhou B, Dooley LT, Mease PJ, Gladman DD, de Vlam K, Geusens PP, Birbara C, Halter DG, Antoni C (2007) Infliximab maintains a high degree of clinical response in patients with active psoriatic arthritis through 1 year of treatment: results from the IMPACT 2 trial. Ann Rheum Dis 66(4):498–505. https://doi.org/10.1136/ard.2006.058339
Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ (2000) Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 356(9227):385–390. https://doi.org/10.1016/s0140-6736(00)02530-7
Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M, Visvanathan S, Beutler A (2009) Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 60(4):976–986. https://doi.org/10.1002/art.24403
Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, van der Heijde D (2014) Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA). Ann Rheum Dis 73(1):48–55. https://doi.org/10.1136/annrheumdis-2013-203696
Damjanov N, Karpati S, Kemeny L, Bakos N, Bobic B, Majdan M, Tlustochowicz W, Vitek P, Dokoupilova E, Aldinc E, Szumski A (2018) Efficacy and safety of etanercept in psoriasis and psoriatic arthritis in the PRESTA study: analysis in patients from Central and Eastern Europe. J Dermatolog Treat 29(1):8–12. https://doi.org/10.1080/09546634.2017.1329509
Kalyoncu U, Bayindir Ö, Ferhat Öksüz M, Doğru A, Kimyon G, Tarhan EF, Erden A, Yavuz Ş, Can M, Çetin GY, Kılıç L, Küçükşahin O, Omma A, Ozisler C, Solmaz D, Bozkirli ED, Akyol L, Pehlevan SM, Gunal EK, Arslan F, Yılmazer B, Atakan N, Aydın SZ (2017) The Psoriatic Arthritis Registry of Turkey: results of a multicentre registry on 1081 patients. Rheumatology (Oxford) 56(2):279–286. https://doi.org/10.1093/rheumatology/kew375
Bhutani T, Wong JW, Bebo BF, Armstrong AW (2013) Access to health care in patients with psoriasis and psoriatic arthritis: data from National Psoriasis Foundation survey panels. JAMA Dermatol 149(6):717–721. https://doi.org/10.1001/jamadermatol.2013.133
Ayan G, Aydin SZ, Kimyon G, Ozisler C, Tinazzi I, Dogru A, Omma A, Kilic L, Yılmaz S, Kucuksahin O, Gönüllü E, Yıldız F, Can M, Balkarlı A, Solmaz D, Dalkılıc E, Bayindir O, Yıldırım Çetin G, Ergulu Esmen S, Ersozlu ED, Duruoz MT, Akyol L, Kucuk A, Bes C, Cınar M, Erden A, Mercan R, Bakirci S, Kasifoglu T, Yazısız V, Kalyoncu U (2021) PsART-ID inception cohort: clinical characteristics, treatment choices and outcomes of patients with psoriatic arthritis. Rheumatology (Oxford) 60(4):1755–1762. https://doi.org/10.1093/rheumatology/keaa663
Acknowledgements
Servet Yolbas, MD, (Inonu University—Turgut Ozal School of Medicine, Department of Internal Medicine, Division of Rheumatology, Malatya—Turkey) and Gokhan Temiz, MD, (Atasehir Memorial Hospital—Nephrology Clinic, Istanbul—Turkey) served as subinvestigators in this study. Murat Ozdemir and Monitor CRO provided medical writing, editing, and reviewing services support in the development of this manuscript, which was funded by AbbVie.
Funding
AbbVie sponsored the study; contributed to the design; and participated in the collection, analysis, and interpretation of data; and in writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship.
Author information
Authors and Affiliations
Contributions
All authors contributed to conduction and writing of this study and have approved the final version.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Clinical Research Ethics Committee of Hacettepe University on October 31, 2013, with an approval number: 2013/13–03 (KA-130083).
Consent to participate
Written informed consent was received before enrolment from all participants.
Consent to publication
Not applicable.
Conflict of interest
Omer Karadag: AbbVie (research grant, consulting fee), Pfizer (research grant, consulting fee), Roche (research grant, consulting fee), Novartis (research grant), Abdi İbrahim (consulting fee), Amgen (consulting fee), Farmanova (consulting fee), Janssen (consulting fee), Lilly (consulting fee), UCB (consulting fee). Ediz Dalkilic: AbbVie (speaker fee), MSD (speaker fee), Roche (speaker fee), Pfizer (speaker fee), UCB (speaker fee), Novartis (speaker fee). Gizem Ayan: None. Orhan Kucuksahin: None. Timucin Kasifoglu: AbbVie, Amgen, Roche, MSD, Novartis, Pfizer, and UCB (speaker and consulting fees). Neslihan Yilmaz: AbbVie (speaker fee), Pfizer (speaker fee), UCB (speaker fee), Roche (speaker fee), Janssen (speaker fee). Suleyman Serdar Koca: AbbVie (speaker fee), Pfizer (speaker fee), Roche (speaker fee), UCB (speaker fee), Novartis (speaker fee), Amgen (speaker fee), Pharmactive (speaker fee), MSD (speaker fee). Veli Yazisiz: AbbVie (consulting fee), Pfizer (consulting fee), UCB (consulting fee). Pinar Talu Erten: None. Mehmet Sayarlioglu: Genzyme (speaker fee), MSD (consulting fee), Novartis (research grant). Mehmet Ender Terzioglu: AbbVie, Pfizer, Novartis, Boehringer Ingelheim, Pharmactive, and UCB (speaker/consulting fee and/or research grant). Sukran Erten: Janssen (speaker fee), Roche (speaker fee), Novartis (speaker fee). Umut Kalyoncu: AbbVie, Pfizer (research grant, speaker, and consulting fee), Janssen (research grant, speaker fee), UCB, Novartis (speaker and consulting fee), Lilly (consulting fee).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karadag, O., Dalkilic, E., Ayan, G. et al. Real-world data on change in work productivity, activity impairment, and quality of life in patients with psoriatic arthritis under anti-TNF therapy: a postmarketing, noninterventional, observational study. Clin Rheumatol 41, 85–94 (2022). https://doi.org/10.1007/s10067-021-05893-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05893-3