Abstract
Interstitial lung disease (ILD) accounts for the major cause of morbidity and mortality in rheumatoid arthritis (RA). However, little is known of the pathogenesis, diagnosis and treatment of RA-associated ILD. In this review, we describe our present understanding and ongoing research in RA-ILD. Its aetiology does appear to associate with anti-cyclic citrullinated peptide antibodies, MUC5B mutation and smoking. Another focus of this article is on recent diagnostic methods in RA-ILD. Compared with other methods, high-resolution computed tomography (HRCT) imaging is a main method for the evaluation of ILD in RA patients. Pulmonary function is better suited for assessing progression. An important topic relates to therapeutic intervention. Disease-modifying anti-rheumatic drugs (DMARDs) in RA lack strong evidence in the onset or worsening of ILD. The available literature support that methotrexate, leflunomide, abatacept and rituximab play beneficial roles in the prevention and treatment of RA-ILD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by symmetric polyarthritis that leads to progressive bone destruction and eventual joint deformity if left untreated. Although arthritis is the most common manifestation of RA, extra-articular manifestations (EAMs) also occur in the RA population [1,2,3]. Among those with multiple EAM, interstitial lung disease (ILD) might be the most frequent EAM and worsens the disease prognosis [4]. Given that ILD may already be irreversible and contributes to the excess morbidity and mortality of patients with RA, it is now recommended that all RA patients should be checked for ILD. Early detection of ILD is very important for the management of RA patients.
In this review, we focus on discussing the epidemiology and pathogenesis of RA-ILD, establishing the diagnosis of ILD in RA, evaluating disease activity and prognosis and the available therapeutic approaches by arranging and analysing recent studies. This analysis will provide a better decision for clinicians in the management of RA-ILD patients and will trigger some pivotal questions for future research in RA-ILD.
The epidemiology of RA-ILD
It is reported that the prevalence of ILD is 7.7–67% among RA patients [5,6,7,8]. The morbidity of RA-ILD varies depending on detection methods and the selected patients in each publication. There are several studies about the prevalence of ILD in the early phases of RA [9,10,11,12]. In the study of Habib.HM et al. [9], they recruited 40 RA patients not more than 2-year disease duration. Ten percent early RA patients were found to be clinically involved by ILD. Other studies proved that anti-cyclic citrullinated peptide antibodies (ACPA) in serum were associated with lung involvement in early RA patients [11, 12]. ILD may precede the development of articular manifestations. Hyldgaard et al. found that 14% of patients diagnosed with RA-ILD 1 to 5 years before the RA diagnosis [13].
The pathogenesis of RA-ILD
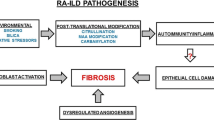
At present, the exact aetiology of RA-associated ILD is not known. However, some factors, such as ACPA, genes mutations, smoking and other factors, have been reported to be involved in the pathogenesis of RA-ILD (Fig. 1). Surprisingly, some overlaps of pathogenic mechanisms between RA-ILD and idiopathic pulmonary fibrosis (IPF) have been found.
The pathogenesis of RA-ILD. Anti-cyclic citrullinated peptide antibodies (ACPA), MUC5B promoter variant, certain human leukocyte antigen (HLA) alleles, older age, male sex, smoking and other serum biomarkers have been reported to be involved in the pathogenesis of RA-ILD. IL interleukin, MMP-7 matrix metalloproteinase-7, PARC pulmonary and activation-regulated chemokine, SP-D surfactant protein-D
ACPA
Citrullination converts arginine to citrulline by an epigenetic post-translational modification. This process can trigger an immune response that causes the production of ACPA [14]. Recent studies found that citrullination is not only involved in the development of joint damage in RA but is also found in the bronchoalveolar lavage fluid of RA-ILD and IPF subjects [15]. Based on these observations, two potential mechanisms that explain the pathogenesis of RA-ILD were proposed by Paulin et al. [16]. It has been hypothesised that an immune response against citrullinated peptides taking place at the joints subsequently shifts to the lungs, resulting in interstitial lung inflammation, most likely in a nonspecific interstitial pneumonia (NSIP) pattern. It also has been proposed that individuals with usual interstitial pneumonia (UIP) pattern and a genetic susceptibility to RA trigger local inflammation in the lung. This process could lead to an immune response against citrullinated peptides in the lung, initiating an inflammatory process that secondarily affects the joints. There are also reports that support this hypothesis, as ILD can precede joint damage in some subsets of RA-ILD patients [17]. Correia et al. [18] reported that the higher ACPA titres are also associated with increasing prevalence of ILD. In their study, two thousand thirty patients were included. The increasing risk of ILD was associated with higher ACPA titres (10.3%, 8.82%, 4.92% and 1.90% in the high, moderate, low and negative titre groups, respectively, p < 0.001). However, some studies reported no association between ACPA and rheumatic lung disease [19,20,21]. Fortunately, a recent meta-analysis revealed that serum ACPA positivity was highly associated with RA-related pulmonary disease, particularly with ILD and IPF, although no association was found in the Asian population subgroup [22]. Therefore, ACPA play a crucial role in the occurrence and development of ILD in RA patients.
MUC5B mutation
MUC5B, an evolutionarily conserved gene, is responsible to encode mucin glycoproteins that the principal macromolecules in airway mucus [23]. The promoter polymorphism (rs35705950) of MUC5B was reported to be associated with familial interstitial pneumonia and IPF. In a recent study by Juge et al. [24], a gain-of-function promoter variant in MUC5B (rs35705950), the strongest risk factor for the development of IPF, could also account for the risk of ILD among patients with RA, specifically involving an UIP pattern on imaging. The MUC5B promoter variant rs35705950 was observed in at least 50% of IPF and could contribute to approximately 30 to 35% of the genetic risk of the development of IPF [25,26,27]. Similar to observations of MUC5B expression in the lungs of IPF patients, Juge and colleagues showed that a MUC5B promoter variant was associated with a higher risk of ILD in RA patients, even when taking other risk factors into account, such as smoking, age, and sex. In the combined analysis, the relationship between MUC5B promoter variation and the increased risk of RA-ILD appears to be specific to the UIP pattern, as this promoter variant seem to be a generalized risk factor for UIP disease and was not simply restricted to IPF and RA-ILD. This promoter variant was not associated with an increased risk of the development of RA, as well as interstitial pneumonia among patients with systemic sclerosis or autoimmune myositis. The increased risk of the MUC5B promoter variant was found in multiple ethnic groups, but not in Asian patients, probably because of the small sample sizes and the rarity of the risk allele. However, the exact mechanism that accounts for development of ILD with the MUC5B promoter variant is unknown [24, 28]. To boot, the promoter polymorphism (rs35705950) of MUC5B was proved to be significantly associated with improved survival [29]. The presence of the MUC5B promoter variant are also significantly linked with radiographical indices of undiagnosed pulmonary fibrosis and could be a genomic marker for early identification of undiagnosed pulmonary fibrosis [30].Therefore, MUC5B genotyping might guide management by providing more information on prognosis and pathogenesis of RA-ILD in the future.
Other factors
Other factors, such as certain human leukocyte antigen (HLA) alleles, older age, male sex, smoking and serum biomarkers, are associated with an increased risk of developing RA-ILD. Current studies suggest that several HLA variants, including HLA-B54, HLA-DQB1*0601, HLA-B40 and HLA-DR4, have been associated with the development of ILD in patients with RA [31,32,33,34]. The risk of developing ILD was higher in RA patients who were older or in RA patients with male sex [4]. Cigarette smoking could trigger an immune reaction that produces serum autoantibodies against multiple citrullinated proteins in the lung, which in turn leads to inflammation and epithelial cell injury, ultimately resulting in ILD [35, 36]. There is also evidence supporting the relationship between autoantibodies, cytokines and the immune environment and the pathogenesis of RA-ILD. Some research suggests the elevated levels of interleukin (IL)-33 and IL-18 in RA-ILD patients lead to the development of ILD in patients with RA. Since IL-33 might mediate immune responses by the expression of T helper-2 cytokines and IL-18 is a member of the IL-1 cytokine superfamily that regulates immune responses [37]. According a study by Doyle et al., increased levels of matrix metalloproteinase-7 (MMP-7), pulmonary and activation-regulated chemokine (PARC) and surfactant protein-D (SP-D) are strongly associated with the presence of clinically evident and subclinical RA-ILD [38].
However, the exact pathogenesis of RA-ILD is still elusive; therefore, further studies are needed to explain the mechanisms driving ILD in patients with RA.
Clinical and laboratory features of RA-ILD
Clinical features
The predominant clinical symptoms of RA-ILD include exertional dyspnoea and chronic dry cough. Other clinical complaints of ILD are fatigue and generalized weakness. Clinical serum biomarkers such as elevated titres of rheumatoid factor (RF) and ACPA have also been detected in RA-ILD patients [39, 40]. Based on Hyldgaard’s study, ILD significantly increased mortality in RA patients compared with a large matched cohort of RA without ILD. The hazard rate ratios for death were 2 to 10 times increased for RA-ILD compared with non-ILD RA [13].
However, the early diagnosis of RA-ILD is challenging and requires interdisciplinary discussions and knowledge. Cough and exertional dyspnoea of RA-ILD are easily overlooked because they are unobvious or limited by joint dysfunction or generalized fatigue from systemic inflammation. Therefore, we need to combine symptoms (dry cough and dyspnoea), clinical risk factors (autoantibodies, sex, age and smoking status) and other methods to diagnose the presence of ILD among RA patients (Table 1).
HRCT
Currently, high-resolution computed tomography (HRCT) imaging has become a main method in the evaluation of ILD among RA patients. Compared with chest radiographs, HRCT can detect more subtle structural abnormalities in the lung tissue at earlier disease stages [8]. HRCT also replaces lung biopsy because the imaging patterns correlate well with the histopathology [41, 42].
The predominant HRCT pattern in RA-ILD is UIP, with NSIP as the second most common. Other types of ILD include organizing pneumonia or bronchiolitis obliterans, acute interstitial pneumonia with diffuse alveolar damage, desquamate interstitial pneumonia and lymphocytic interstitial pneumonia, though these occur less frequently [43, 44]. The UIP pattern on HRCT is characterized by heterogenous honeycombing at the bases and periphery of the lungs, peripheral basilar predominant reticular abnormalities and architectural distortions. The radiographic hallmarks for NSIP are bilateral ground-glass opacification and minimal to no architectural distortion or honeycombing [45,46,47]. NSIP is the most common radiographic pattern in other connective tissue disease (CTD)–associated ILD, while UIP is the most common pattern in RA-ILD [2, 48]. The UIP pattern seen in RA patients is very similar to that observed in IPF patients and predicts worse prognosis when compared with a non-UIP pattern of RA-ILD [42, 49]. At present, the identification of ILD predominantly relies on HRCT detection; discordance between radiographic and histologic findings sometimes occurs on NSIP, as they mimic each other very closely.
Ultrasound
Recently, ultrasound of the lung was introduced as a safe and easily available tool for detecting ILD, as well as IPF, bronchiolitis or pneumothorax [50,51,52,53,54]. Sonographic characters, such as multiple B lines, irregularities of the pleural line or pleural nodules, might be signs of lung interstitial disease [55]. In Moazedi-Fuerst’s study, they recruited 64 rheumatoid arthritis patients and 40 healthy volunteers and then screened their pleura and the pulmonary parenchyma via sonography. In their cohort of RA patients, 28 showed pleural nodules or B-line phenomena. In these patients, CT scans confirmed the signs of incipient interstitial lung disease [56]. Therefore, sonography can be regarded as a non-invasive and radiation-free monitor for incipient ILD among patients with RA.
PFT
Although several studies have shown that pulmonary function testing (PFT) is helpful for the early detection of ILD, it may be better suited for assessing progression. PFT in RA-ILD patients often reveals restrictive ventilatory defects with decreases in gas exchange, and such abnormalities may be detected even in the absence of any clinical symptoms [57]. Compared with routine parameters such as forced vital capacity (FVC) and total lung capacity (TLC), diffusing capacity for carbon monoxide (DLCO) is the most sensitive parameter for assessing progression, clinical disease severity and morbidity from ILD [58, 59]. A reduced FVC and DLCO may predict the progression of ILD. Dawson et al. indicated that a DLCO of less than 54% of the predicted value is a highly specific predictor of disease progression [60].
Velcro sound detector (VECTOR)
Recently, Manfredi and co-workers described a velcro sound detector to detect ILD among RA patients [61]. They enrolled 137 RA patients who had recently undergone HRCT. The detection of lung sounds was applied in all patients in four pulmonary fields bilaterally with a commercial electronic stethoscope (ES), and these sounds were saved to files and were analysed using a suitably developed algorithm (VECTOR). All HRCT images and audio data were blindly evaluated by radiologist and rheumatologist by using VECTOR. In their study, fifty-nine of 137 RA patients showed ILD [43.1%]. VECTOR correctly classified 115/137 patients, showing a diagnostic accuracy of 83.9% and a sensitivity and specificity of 93.2 and 76.9%, respectively. Thus, the velcro sound detector may be a validated tool for the indication of ILD in RA patients.
Bronchoalveolar lavage and lung biopsy
Bronchoalveolar lavage (BAL) is not routinely used as a diagnostic tool in RA-ILD. The main value of BAL is to rule out infections and malignancy. While lung biopsy is the gold standard for confirming ILD subtype, it is not routinely performed in RA-ILD patients. There is also no evidence to guide the decision of when to perform a lung biopsy. Currently, it is recommended that lung biopsy should be considered in patients when HRCT cannot discriminate an accurate pattern, especially in cases with a possible UIP pattern [41, 62]. Since CT imaging to diagnose RA-ILD can be informative to a certain extent.
The management of patients with RA-ILD
At present, there is no guideline or strong evidence from randomized controlled trials (RCTs) to manage RA-ILD patients. Here, we discuss the management of RA-ILD based on recent studies.
Disease activity
At present, the disease activity of RA-ILD depends on PFT and HRCT. Given RA is a systemic inflammation disease, the active joint involvement possible reflects inflammation in the lung. Recently, in the study of Sparks JA et al. [63], the DAS28 of RA patients was associated with increased ILD risks. Although Paulin et al. found that there was no correlation between lung function test and HRCT scores with DAS28 [64], some limitations like small sample and flaws of statistic method existed in their study. More researches are expected to confirm the conclusion in the future.
The timing for RA-ILD treatment
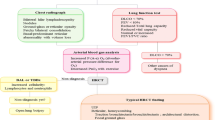
Currently, an approach was recommended to guide clinicians to manage and monitor patients with RA-ILD. In fact, this approach, adapted from Travis et al., in which disease was categorized by behaviour, is used for idiopathic interstitial pneumonias [IIPs] in recent guidelines [65]. Lake et al. and co-workers also applied this method to treatments for RA-ILD [66]. According to this approach (Fig. 2), if there is some reversible course, such as with some types of drug-induced ILD, we should remove the course first. If the changes still cannot be reversed, clinicians should initiate treatment. If the disease progresses quickly, clinicians should initiate treatment and slow the progression. If the disease is in a stable state, clinicians should aim to maintain status and monitor the disease closely. Pulmonary function could be used as a predictor for RA-ILD progression. The DLCO is the most sensitive parameter for assessing disease progression. Progressive loss of DLCO in RA-ILD indicates that the clinician should start treatment, irrespective of whether the pattern of ILD is UIP or NSIP. All RA-ILD patients should have their pulmonary function evaluated every 3 or 6 months by a clinician, even in those patients without any respiratory symptoms.
The therapeutic response
PFT has been considered to be a useful method to evaluate therapeutic response in RA-ILD patients. It has been recommended that maintaining the vital capacity or gas transfer within a 5% range over 2 years can be regarded as suspension of the physiological progression of ILD. It has also been suggested that a 10% improvement in either vital capacity or gas transfer over a 2-year period can be said to be of clinical importance [67].
Treatments
The drug therapy for RA includes conventional disease-modifying anti-rheumatic drugs (cDMARDS) and biologic disease-modifying anti-rheumatic drugs (bDMARDs), with a rules-based treat-to-target strategy. However, both cDMARDS and bDMARDs have been reported to be associated with drug-related pulmonary disease, including lung infection, nodules and ILD [68]. Therefore, clinicians need to weigh the risks and benefits when choosing treatment for RA-ILD patients.
Methotrexate
Methotrexate (MTX) is recommended as the first-line treatment in RA patients, as it effectively decreases disease progression, disability and mortality [69]. However, MTX has been reported to be associated with the onset or exacerbation of ILD in RA patients [70]. This may be a reason why physicians are reluctant to use methotrexate in RA patients with lung disease. According to some studies, the incidence of ILD attributed to methotrexate treatment is as high as 7.6%, with 17% mortality, which may occur with any dose or duration of therapy [71, 72]. Currently, more evidence suggests that cases of MTX-induced ILD may be overstated. They maintain that reports of ILD in patients receiving methotrexate possibly included publication bias and lacked blinding. RA patients on methotrexate treatment probably have been evaluated more carefully via pulmonary tests compared with the general RA population for the fear of causing MTX-induced ILD.
To evaluate whether MTX is the cause of ILD, a large, randomized and blinded study would need to be conducted to decrease the risk of bias. To confirm the causation relationship between MTX and ILD, Conway and his colleague performed a meta-analysis of randomized double-blind controlled trials in patients with RA [73]. They included 22 suitable studies for the meta-analysis, in which 8584 patients were divided into two groups based on whether they were receiving MTX or not. Their findings showed that MTX was associated with an increased risk of respiratory infections, but the risk of ILD appeared to be much less frequent than previously reported. Theoretically, given that MTX might not be a causative agent of ILD and could be an effective treatment for the joint manifestations of RA, it may potentially play an important role in the treatment of RA-ILD. Kiely, et al. wanted to address the issue of whether MTX exposure is associated with RA-ILD diagnosis and delays the onset of RA-ILD. They performed a multicentre prospective early RA cohort study recruiting 2701 patients and determined that MTX exposure was associated with a significantly reduced risk of incident RA-ILD (OR 0.48, 95% CI 0.3 to 0.79, p = 0.004); moreover, they found that treatment may delay the onset of ILD in RA patients (OR 0.41, 95% CI 0.23 to 0.75, p = 0.004). This evidence gives us great confidence that MTX may have a beneficial effect in the prevention and treatment of RA-ILD [74].
Leflunomide
Leflunomide (LEF) is another effective treatment for RA patients. While a large observational study reported that 61 of 5054 RA patients developed new or worsened ILD under the treatment of LEF. Pre-existing ILD, smoking, low body weight and use of a loading dose were proved as independent risk factors for LEF-induced ILD [75]. Therefore, they suggest that LEF should not be recommended for RA patients complicated with ILD. However, a recent meta-analysis of LEF and lung disease in RA suggested that LEF-treated patients were not associated with an increase in the risk of respiratory adverse events; instead, they were associated with a decrease in non-infectious respiratory adverse events. The findings of this study support the safety of LEF in RA patients with regard to the risk of ILD [76]. Therefore, it remains difficult to distinguish any causal association between LEF and ILD.
bDMARDs
bDMARDs are a second-line treatment in the management for RA patients and effectively improve clinical symptoms and joint function, whereas drug-induced ILD has been reported for most conventional antitumour necrosis factor (TNF) agents, such as infliximab, etanercept, adalimumab [77], golimumab [78], certolizumab pegol [79] and IL-6 receptor (IL-6R) antagonist tocilizumab [80]. However, most evidence regarding TNF inhibitors–related ILD is from case reports. A systematic literature search highlights that it is very difficult to identify a causal relationship between RA therapy and the onset or worsening ILD.
Abatacept is also used in the treatment of RA by preventing the activation of T-lymphocytes. Some studies suggest that abatacept might be a favourable option for the treatment of RA-ILD patients. A retrospective cohort study showed that abatacept, over other bDMARDs, was associated with a better prognosis in RA-ILD [81]. Fernandez-Diaz and co-workers reported a retrospective study of RA-associated ILD patients treated with abatacept. Sixty-three RA-associated ILD patients underwent abatacept therapy about 12 months. Two thirds of patients remained stable, 36.4% improvement. Eleven of 63 patients had to withdraw mainly due to adverse events. From these studies, abatacept appears to be an effective in RA-associated ILD [82].
Recently, a study by Annalisa Fui et al. [83] assessed the efficacy of rituximab (RTX) therapy in 14 RA-ILD patients for more than 1 year. In this study, 14 RA-ILD patients were treated with rituximab and showed mild improvements in FVC, FEV1, TLC and stabilization DLCO percentages. The same conclusion was found in other study, in which RTX proved to be an acceptable therapy for RA-ILD patients based on 10-year experience [84]. Therefore, rituximab seems to be effective in stabilizing ILD involvement in RA patients. Limited data about RTX induced ILD in RA patients. There was a systematic review about potential RTX associated with ILD in any patients who was undertaken RTX. ILD was a rare complication of RTX therapy. It occurred more frequently in male and older patients. The mean time of onset of ILD from the last RTX infusion to symptom development or relevant abnormal radiological change was 30 days (0–158 days) [85].
Glucocorticoids and other immunosuppressive therapy
Glucocorticoids remain the first-line treatment (prednisone 0.5–1 mg/kg−1), and therapeutic responses have been observed in RA-ILD patients with NSIP and OP patterns [86]. Cyclophosphamide, azathioprine, cyclosporine and mycophenolate, in association with glucocorticoids, have been reported to have beneficial effects on CTD-ILD and may be considered in this setting [67, 87].
No recent RCTs have been performed for the treatment of cyclophosphamide or mycophenolate in RA-ILD patients. However, a series of RCT or even meta-analysis were performed for CTD-ILD, particularly scleroderma-related ILD [88]. Those studies showed that cyclophosphamide or mycophenolate presented beneficial effects on CTD-ILD. Those patients with rapidly progressive fibrotic disease may benefit the most [89]. Given that cyclophosphamide and mycophenolate alter the progression of effects in other types of CTD-ILD, they could potentially play a role in the treatment of RA-ILD.
Other treatments
At present, the anti-fibrotic drug pirfenidone has only been reported to have therapeutic effects in IPF, not in CTD-ILD. IPF and RA-ILD have the same overlaps in their pathogenesis. Therefore, the anti-fibrotic drug pirfenidone might be an effective treatment for RA-ILD. At this point, some work has been performed in animal models [90]. The clinical trial of pirfenidone (NCT02808871) in RA-ILD has begun to recruit patients now [91]. We will be very looking for forward to their results. N-acetylcysteine is a useful treatment in IPF and may be effective in the treatment of RA-ILD [67]. However, these finding need more research for confirmation.
Annual influenza and pneumonia vaccinations have been recommend for RA-ILD patients to reduce their risk of lung infection. Given that smoking increases the risk of not only developing RA but also lung damage, we strongly suggest all RA-ILD patients stop smoking. Lung transplantation should be considered for patients with severe, fibrotic lung disease, especially for young patients without any significant comorbidity [67].
Conclusions
ILD is a common and potentially life-threatening complication for RA patients. Unfortunately, the exact aetiology of RA-ILD is still poorly understood. ACPA, MUC5B mutation and other factors, such as smoking, are reported to be associated with the pathogenesis of RA-ILD. To better manage RA-ILD patients, all RA patients should be evaluated to determine the presence and extent of their lung involvement. We also should monitor the rate of disease progression. Routine examinations include clinical symptoms, lung function and HRCT. Ultrasound of the lung, velcro sound detector and BAL can assist in the diagnosis of RA-ILD. The DLCO is the most sensitive parameter for assessing disease progression. Clinicians should monitor RA-ILD patients closely for disease progression and start treatment when the disease progresses. To date, there have not been any guidelines or RCTs for the treatment of RA-ILD. The causative relationship between DMRADS and drug-induced-ILD lacks strong evidence. Drug-induced ILD is likely just is a rare condition. From recent data, cDMARDS, including MTX and LEF, play beneficial roles in the prevention and treatment of RA-ILD. bDMARDS such as abatacept and rituximab are effective in stabilizing ILD involvement in RA patients.
Although a great deal of research has been conducted and breakthroughs have been achieved in the field of RA, there are still many questions about RA-ILD. What is the relationship between IPF and RA-ILD, since many similar factors are involved in their pathogenesis? How do clinicians help RA patients prevent the onset of ILD? When should clinicians initiate the treatment and choose the right option for different ILD patterns? However, most approaches are based on experts, without evidence from RCTs. To effectively care for these patients, further research is needed to define the pathogenesis and treatment of RA-ILD.
Data availability
Not applicable.
References
Turesson C (2013) Extra-articular rheumatoid arthritis. Curr Opin Rheumatol 25:360–366
Lee HK, Kim DS, Yoo B, Seo JB, Rho JY, Colby TV, Kitaichi M (2005) Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest 127:2019–2027
Suda T (2016) Up-to-date information on rheumatoid arthritis-associated interstitial lung disease. Clin Med Insights Circ Respir Pulm Med 9:155–162
Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, Gabriel SE, Matteson EL (2010) Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 62:1583–1591
Turesson C, O'Fallon WM, Crowson CS, Gabriel SE, Matteson EL (2002) Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheumatol 29:62–67
Bilgici A, Ulusoy H, Kuru O, Çelenk Ç, Ünsal M, Danacı M (2005) Pulmonary involvement in rheumatoid arthritis. Rheumatol Int 25:429–435
Mori S, Cho I, Koga Y, Sugimoto M (2008) Comparison of pulmonary abnormalities on high-resolution computed tomography in patients with early versus longstanding rheumatoid arthritis. J Rheumatol 35:1513–1521
Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR (2001) Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax 56:622–627
Habib HM, Eisa AA, Arafat WR, Marie MA (2011) Pulmonary involvement in early rheumatoid arthritis patients. Clin Rheumatol 30:217–221
Gabbay E, Tarala R, Will R et al (1997) Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med 156:528–535
Roos Ljungberg K, Joshua V, Skogh T et al. (2019) Secretory anti-citrullinated protein antibodies in serum associate with lung involvement in early rheumatoid arthritis. Rheumatology (Oxford)
Robles-Perez A, Luburich P, Rodriguez-Sanchon B, Dorca J, Nolla JM, Molina-Molina M, Narvaez-Garcia J (2016) Preclinical lung disease in early rheumatoid arthritis. Chron Respir Dis 13:75–81
Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Løkke A, Bendstrup E, Ellingsen T (2017) A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis 76:1700–1706
McInnes IB, Schett G (2011) The pathogenesis of Rheumatois arthritis. NEJM 365:2205–2219
Bongartz T, Cantaert T, Atkins SR, Harle P, Myers JL, Turesson C, Ryu JH, Baeten D, Matteson EL (2007) Citrullination in extra-articular manifestations of rheumatoid arthritis. Rheumatology 46:70–75
Paulin F, Doyle TJ, Fletcher EA, Ascherman DP, Rosas IO (2015) Rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis:shared mechanistic and phenotypic traits suggest overlapping disease mechanisms. Rev Investig Clin 67:280–286
Ytterberg AJ, Joshua V, Reynisdottir G et al (2014) Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis 74:1772–1777
Correia CS, Briones MR, Guo R, Ostrowski RA (2019) Elevated anti-cyclic citrullinated peptide antibody titer is associated with increased risk for interstitial lung disease. Clin Rheumatol 38:1201–1206
Jearn LH, Kim TY (2012) Level of anticitrullinated peptide/protein antibody is not associated with lung diseases in rheumatoid arthritis. J Rheumatol 39:1493–1494
Korkmaz C, Us T, Kasifoglu T et al (2006) Anti-cyclic citrullinated peptide [CCP] antibodies in patients with long-standing rheumatoid arthritis and their relationship with extra-articular manifestations. Clin Biochem 39:961–965
Inui N, Enomoto N, Suda T, Kageyama Y, Watanabe H, Chida K (2008) Anti-cyclic citrullinated peptide antibodies in lung diseases associated with rheumatoid arthritis. Clin Biochem 41:1074–1077
Zhu J, Zhou Y, Chen X, Li J (2014) A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol 41:1282–1289
Young HW, Williams OW, Chandra D et al (2007) Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol 37:273–290
Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, Kannengiesser C, Ottaviani S, Oka S, Tohma S, Tsuchiya N, Rojas-Serrano J, González-Pérez MI, Mejía M, Buendía-Roldán I, Falfán-Valencia R, Ambrocio-Ortiz E, Manali E, Papiris SA, Karageorgas T, Boumpas D, Antoniou K, van Moorsel CHM, van der Vis J, de Man YA, Grutters JC, Wang Y, Borie R, Wemeau-Stervinou L, Wallaert B, Flipo RM, Nunes H, Valeyre D, Saidenberg-Kermanac’h N, Boissier MC, Marchand-Adam S, Frazier A, Richette P, Allanore Y, Sibilia J, Dromer C, Richez C, Schaeverbeke T, Lioté H, Thabut G, Nathan N, Amselem S, Soubrier M, Cottin V, Clément A, Deane K, Walts AD, Fingerlin T, Fischer A, Ryu JH, Matteson EL, Niewold TB, Assayag D, Gross A, Wolters P, Schwarz MI, Holers M, Solomon JJ, Doyle T, Rosas IO, Blauwendraat C, Nalls MA, Debray MP, Boileau C, Crestani B, Schwartz DA, Dieudé P (2018) MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 379:2209–2219
Helling BA, Gerber AN, Kadiyala V, Sasse SK, Pedersen BS, Sparks L, Nakano Y, Okamoto T, Evans CM, Yang IV, Schwartz DA (2017) Regulation of MUC5B expression in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 57:91–99
Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA (2011) A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364:1503–1512
Zhang Y, Noth I, Garcia JG et al (2011) A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med 364:1576–1577
Juge PA, Borie R, Kannengiesser C et al (2017) Shared genetic predisposition in rheumatoid arthritis-interstitial lung disease and familial pulmonary fibrosis. Eur Respir J 49:1602314
Peljto AL, Zhang Y, Fingerlin TE, Ma SF, Garcia JGN, Richards TJ, Silveira LJ, Lindell KO, Steele MP, Loyd JE, Gibson KF, Seibold MA, Brown KK, Talbert JL, Markin C, Kossen K, Seiwert SD, Murphy E, Noth I, Schwarz MI, Kaminski N, Schwartz DA (2013) Association between the MUC5B promoter polymorphism and survival in patients with idiopathic pulmonary fibrosis. JAMA 309:2232–2239
Adegunsoye A (2019) MUC5B promoter variant: genomic fingerprint for early identification of undiagnosed pulmonary fibrosis. Thorax 74:1111–1112
Charles PJ, Sweatman MC, Markwick JR, Maini RN (1991) HLA-B40: a marker for susceptibility to lung disease in rheumatoid arthritis. Dis Markers 9:97–101
Scott TE, Wise RA, Hochberg MC, Wigley FM (1987) HLA-DR4 and pulmonary dysfunction in rheumatoid arthritis. Am J Med 82:765–771
Hillarby MC, McMahon MJ, Grennan DM et al (1993) HLA associations in subjects with rheumatoid arthritis and bronchiectasis but not with other pulmonary complications of rheumatoid disease. Br J Rheumatol 32:794–797
Sugiyama Y, Ohno S, Kano S et al (1994) Diffuse panbronchiolitis and rheumatoid arthritis: a possible correlation with HLA-B54. Intern Med 33:612–614
Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, MacDonald S, Travis WD, Stylianou MP, Rosas IO (2008) Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med 168:159–166
Albano SA, Santana-Sahagun E, Weisman MH (2001) Cigarette smoking and rheumatoid arthritis. Semin Arthritis Rheum 31:146–159
Matsuo T, Hashimoto M, Ito I, Kubo T, Uozumi R, Furu M, Ito H, Fujii T, Tanaka M, Terao C, Kono H, Mori M, Hamaguchi M, Yamamoto W, Ohmura K, Morita S, Mimori T (2019) Interleukin-18 is associated with the presence of interstitial lung disease in rheumatoid arthritis: a cross-sectional study. Scand J Rheumatol 48:87–94
Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, Golzarri MF, Traslosheros A, Chu SG, Frits ML, Iannaccone CK, Koontz D, Fuhrman C, Weinblatt ME, el-Chemaly SY, Washko GR, Hunninghake GM, Choi AMK, Dellaripa PF, Oddis CV, Shadick NA, Ascherman DP, Rosas IO (2015) Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med 191:1403–1412
Wang T, Zheng XJ, Liang BM, Liang ZA (2015) Clinical features of rheumatoid arthritis-associated interstitial lung disease. Sci Rep 5:14897
Zhang Y, Li H, Wu N, Dong X, Zheng Y (2017) Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 36:817–823
Assayag D, Elicker BM, Urbania TH, Colby TV, Kang BH, Ryu JH, King TE, Collard HR, Kim DS, Lee JS (2014) Rheumatoid arthritis-associated interstitial lung disease: radiologic identification of usual interstitial pneumonia pattern. Radiology 270:583–588
Solomon JJ, Ryu JH, Tazelaar HD, Myers JL, Tuder R, Cool CD, Curran-Everett D, Fischer A, Swigris JJ, Brown KK (2013) Fibrosing interstitial pneumonia predicts survival in patients with rheumatoid arthritis-associated interstitial lung disease [RA-ILD]. Respir Med 107:1247–1252
Ascherman DP (2010) Interstitial lung disease in rheumatoid arthritis. Curr Rheumatol Rep 12:363–369
Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, van Uden JH, Lee JS, King TE, Collard HR (2010) Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 35:1322–1132
Rajasekaran BA, Shovlin D, Lord P, Kelly CA (2001) Interstitial lung disease in patients with rheumatoid arthritis: a comparison with cryptogenic fibrosing alveolitis. Rheumatology (Oxford) 40:1022–1025
Travis WD, Costabel U, Hansell DM, King te Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, ATS/ERS Committee on Idiopathic Interstitial Pneumonias (2013) An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188:733–748
Katzenstein AL, Mukhopadhyay S, Myers JL (2008) Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum Pathol 39:1562–1581
Doyle TJ, Dellaripa PF (2017) Lung manifestations in the rheumatic diseases. Chest 152:1283–1295
Jacob J, Hirani N, van Moorsel CHM et al. (2019) Predicting outcomes in rheumatoid arthritis related interstitial lung disease. Eur Respir J 53(1)
Caiulo VA, Gargani L, Caiulo S, Fisicaro A, Moramarco F, Latini G, Picano E (2011) Lung ultrasound in bronchiolitis: comparison with chest X-ray. Eur J Pediatr 170:1427–1433
Doveri M, Frassi F, Consensi A, Vesprini E, Gargani L, Tafuri M, Picano E, Della Rossa A, Delle Sedie A, d'Ascanio A, Giacomelli C, Bazzichi L, Bombardieri S (2008) Ultrasound lung comets: new echographic sign of lung interstitial fibrosis in systemic sclerosis. Reumatismo 60:180–184
Mathis G, Gehmacher O (2001) Lung and pleural ultrasound. Praxis (Bern 1994) 90:681–686
Volpicelli G, Elbarbary M, Blaivas M et al (2012) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 38:577–591
Wohlgenannt S, Gehmacher O, Gehmacher U, Kopf A, Mathis G (2001) Sonographic findings in interstitial lung diseases. Ultraschall Med 22:27–31
Sperandeo M, Varriale A, Sperandeo G, Filabozzi P, Piattelli ML, Carnevale V, Decuzzi M, Vendemiale G (2009) Transthoracic ultrasound in the evaluation of pulmonary fibrosis: our experience. Ultrasound Med Biol 35:723–729
Moazedi-Fuerst FC, Kielhauser SM, Scheidl S et al (2014) Ultrasound screening for interstitial lung disease in rheumatoid arthritis. Clin Exp Rheumatol 32:199–203
Solomon JJ, Chung JH, Cosgrove GP, Demoruelle MK, Fernandez-Perez ER, Fischer A, Frankel SK, Hobbs SB, Huie TJ, Ketzer J, Mannina A, Olson AL, Russell G, Tsuchiya Y, Yunt ZX, Zelarney PT, Brown KK, Swigris JJ (2016) Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 47:588–596
Ley B, Collard HR, King TE et al (2011) Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183:431–440
Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL (2017) Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 69:542–549
Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR (2002) Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis 61:517–521
Manfredi A, Cassone G, Cerri S et al (2019) Diagnostic accuracy of a velcro sound detector [VECTOR] for interstitial lung disease in rheumatoid arthritis patients: the InSPIRAtE validation study [INterStitial pneumonia in rheumatoid arthritis with an electronic device]. BMC Pulm Med 19:111
Wu EK, Ambrosini RD, Kottmann RM et al. (2019) Reinterpreting evidence of rheumatoid arthritis-associated interstitial lung disease to understand etiology. Curr Rheumatol Rev
Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, Gill RR, Hatabu H, Nishino M, Murphy DJ, Iannaccone CK, Mahmoud TG, Frits ML, Lu B, Rosas IO, Dellaripa PF, Weinblatt ME, Karlson EW, Shadick NA, Doyle TJ (2019) Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol 71:1472–1482
Paulin F, Mercado JF, Fernández ME, Caro FM, Alberti ML, Fassola LA (2018) Correlation between lung and joint involvement in patients with rheumatoid arthritis and interstitial lung disease: a cross-sectional study. Rev Investig Clin 70:76–81
Travis WD, Costabel U, Hansell DM, King te Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, ATS/ERS Committee on Idiopathic Interstitial Pneumonias (2013) ATS/ERS Committee on idiopathic interstitial pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188:733–748
Lake F, Proudman S (2014) Rheumatoid arthritis and lung disease: from mechanisms to a practical approach. Semin Respir Crit Care Med 35:222–238
Kelly C, Saravanan V (2008) Treatment strategies for a rheumatoid arthritis patient with interstitial lung disease. Expert Opin Pharmacother 9:3221–3230
Picchianti Diamanti A, Markovic M, Argento G, Giovagnoli S, Ricci A, Laganà B, D’Amelio R (2017) Therapeutic management of patients with rheumatoid arthritis and associated interstitial lung disease: case report and literature review. Ther Adv Respir Dis 11:64–72
Smolen JS, Landewe R, Breedveld FC et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509
Hozumi H, Nakamura Y, Johkoh T, Sumikawa H, Colby TV, Kono M, Hashimoto D, Enomoto N, Fujisawa T, Inui N, Suda T, Chida K (2013) Acute exacerbation in rheumatoid arthritis-associated interstitial lung disease: a retrospective case control study. BMJ Open 3:e003132
McKendry RJ, Dale P (1993) Adverse effects of low dose methotrexate therapy in rheumatoid arthritis. J Rheumatol 20:1850–1856
Hendrick DJ, Spickett GP (2005) Drug-induced lung disease. In: Oxford textbook of medicine, vol 2. Oxford University Press, Oxford, pp 1507–1512
Conway R, Low C, Coughlan RJ, O'Donnell MJ, Carey JJ (2014) Methotrexate and lung disease in rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheumatol 66:803–812
Kiely P, Busby AD, Nikiphorou E et al (2019) Is incident rheumatoid arthritis interstitial lung disease associated with methotrexate treatment? Results from a multivariate analysis in the ERAS and ERAN inception cohorts. BMJ Open 9:e028466
Sawada T, Inokuma S, Sato T, Otsuka T, Saeki Y, Takeuchi T, Matsuda T, Takemura T, Sagawa A, on behalf of the Study Committee for Leflunomide-induced Lung Injury, Japan College of Rheumatology (2009) Leflunomide-induced interstitial lung disease:prevalence and risk factors in Japanese patients with rheumatoid arthritis. Rheumatology (Oxford) 48:1069–1072
Conway R, Low C, Coughlan RJ, O’Donnell MJ, Carey JJ (2016) Leflunomide use and risk of lung disease in rheumatoid arthritis: a systematic literature review and metaanalysis of randomized controlled trials. J Rheumatol 43:855–860
Perez-Alvarez R, Perez-de-Lis M, Diaz-Lagares C et al (2011) Interstitial lung disease induced or exacerbated by TNF-targeted therapies: analysis of 122 cases. Semin Arthritis Rheum 41:256Y26
Hadjinicolaou AV, Nisar MK, Bhagat S, Parfrey H, Chilvers ER, Ostor AJK (2011) Non-infectious pulmonary complications of newer biological agents for rheumatic diseases—a systematic literature review. Rheumatology (Oxford) 50:2297–2305
Pearce F, Johnson SR, Courtney P (2012) Interstitial lung disease following certolizumab pegol. Rheumatology (Oxford) 51:578–580
Koike T, Harigai M, Inokuma S, Ishiguro N, Ryu J, Takeuchi T, Takei S, Tanaka Y, Ito K, Yamanaka H (2011) Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis 70:2148–2151
Kurata I, Tsuboi H, Terasaki M, Shimizu M, Toko H, Honda F, Ohyama A, Yagishita M, Osada A, Ebe H, Kawaguchi H, Takahashi H, Hagiwara S, Asashima H, Kondo Y, Matsumoto I, Sumida T (2019) Effect of biological disease-modifying anti-rheumatic drugs on airway and interstitial lung disease in patients with rheumatoid arthritis. Intern Med 58:1703–1712
Fernández-Díaz C, Loricera J, Castañeda S, López-Mejías R, Ojeda-García C, Olivé A, Rodríguez-Muguruza S, Carreira PE, Pérez-Sandoval T, Retuerto M, Cervantes-Pérez EC, Flores-Robles BJ, Hernández-Cruz B, Urruticoechea A, Maíz-Alonso O, Arboleya L, Bonilla G, Hernández-Rodríguez Í, Palma D, Delgado C, Expósito-Molinero R, Ruibal-Escribano A, Álvarez-Rodríguez B, Blanco-Madrigal J, Bernal JA, Vela-Casasempere P, Rodríguez-Gómez M, Fito C, Ortiz-Sanjuán F, Narváez J, Moreno M, López-Corbeto M, Mena-Vázquez N, Aguilera-Cros C, Romero-Yuste S, Ordóñez S, Villa-Blanco I, Gonzélez-Vela MC, Mora-Cuesta V, Palmou-Fontana N, Hernández JL, González-Gay MA, Blanco R (2018) Abatacept in patients with rheumatoid arthritis and interstitial lung disease: a national multicenter study of 63 patients. Semin Arthritis Rheum 48:22–27
Fui A, Bergantini L, Selvi E et al (2019) Rituximab therapy in interstitial lung disease associated with rheumatoid arthritis. Intern Med J 9
Md Yusof MY, Kabia A, Darby M, Lettieri G, Beirne P, Vital EM, Dass S, Emery P (2017) Effect of rituximab on the progression of rheumatoid arthritis-related interstitial lung disease: 10 years’ experience at a single centre. Rheumatology (Oxford) 56:1348–1357
Hadjinicolaou AV, Nisar MK, Parfrey H, Chilvers ER, Ostor AJK (2012) Non-infectious pulmonary toxicity of rituximab: a systematic review. Rheumatology (Oxford) 51:653–662
Tanoue LT (1998) Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med 19:667–685
Chang HK, Park W, Ryu DS (2002) Successful treatment of progressive rheumatoid interstitial lung disease with cyclosporine: a case report. J Korean Med Sci 17:270–273
Wallace B, Vummidi D, Khanna D (2016) Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol 28:236–245
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, Goldin J, Arriola E, Volkmann ER, Kafaja S, Silver R, Steen V, Strange C, Wise R, Wigley F, Mayes M, Riley DJ, Hussain S, Assassi S, Hsu VM, Patel B, Phillips K, Martinez F, Golden J, Connolly MK, Varga J, Dematte J, Hinchcliff ME, Fischer A, Swigris J, Meehan R, Theodore A, Simms R, Volkov S, Schraufnagel DE, Scholand MB, Frech T, Molitor JA, Highland K, Read CA, Fritzler MJ, Kim GHJ, Tseng CH, Elashoff RM, Sclerodema Lung Study II Investigators (2016) Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease [SLS II]: a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 4:708–719
Wu C, Lin H, Zhang X (2019) Inhibitory effects of pirfenidone on fibroblast to myofibroblast transition in rheumatoid arthritis-associated interstitial lung disease via the downregulation of activating transcription factor 3 [ATF3]. Int Immunopharmacol 74:105700
Solomon JJ, Danoff SK, Goldberg HJ et al (2019) Trail network. The design and rationale of the Trail1 trial: a randomized double-blind phase 2 clinical trial of Pirfenidone in rheumatoid arthritis-associated interstitial lung disease. Adv Ther 36:3279–3287
Acknowledgments
The authors appreciate all researchers who contribute to the scientific research about RA-ILD. We also extremely grateful to Junwu Dong who gave a lot of suggestions when we prepared for this work.
Funding
The grants from the Wuhan health and Family Planning Commission research projects WG15A02 and WX18 Q31 supported this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation and editing of this paper, and they all reviewed and approved the manuscript for submission.
Corresponding authors
Ethics declarations
Disclosures
None.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, Y., Wang, W., Yu, Y. et al. Rheumatoid arthritis–associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clin Rheumatol 40, 1211–1220 (2021). https://doi.org/10.1007/s10067-020-05320-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05320-z