Abstract
Objective
SB5 showed comparable efficacy and safety profile in respect to adalimumab originator (ADA) in randomized clinical trials of rheumatoid arthritis (RA) and psoriasis. We aimed to describe the efficacy and safety of SB5 after switching from ADA in RA, axial spondyloarthritis (axSpA), psoriatic arthritis (PsA) and juvenile idiopathic arthritis (JIA) patients.
Method
Adult RA, PsA, axSpA, JIA patients treated with ADA for at least 6 months, switched to SB5 in stable clinical conditions, were eligible. Data on safety, activity indexes and patient-reported outcomes were collected at baseline, 3 and 6 months after switching.
Results
Eighty-two patients (19 RA, 28 PsA, 32 axSpA and 3 JIA; 45 females, mean age 54 ± 14 years, disease duration 13 ± 7 years, ADA duration 6 ± 3 years) were enrolled. RA patients showed stable conditions, while PsA patients showed an increase in both HAQ, DAS28(CRP) and DAPSA and axSpA patients an increase in VAS pain, VAS patient disease activity and ASDAS, both at 3 months. There were changes in the concomitant medications profile, with regression of activity indexes increases at 6 months. Adverse events were reported by 33.7% patients at 3 months and 16.6% patients at 6 months, mostly disease flares and infectious events. Two patients stopped SB5.
Conclusions
Despite temporary changes in the concomitant medication profile for mild disease flares, our real-life data replicate the safety profile of switching from ADA to SB5 in RA, with additional data for its applicability in PsA and axSpA patients, further supporting switching to biosimilars in treating inflammatory rheumatic conditions.
Key Points • Switching from adalimumab originator to SB5 is feasible in real life rheumatic inflammatory joint diseases. • Mild disease flares can present after switching from originator adalimumab to SB5, in particular in axial spondyloarthritis and psoriatic arthritis. • Changes in concomitant medications profile allows the control of minor disease flares presenting after switching from adalimumab originator to SB5. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatic musculoskeletal diseases (RMDs) are a heterogeneous group of conditions sharing common features of inflammation, reduced functionality and joint damage. Rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondiloarthritis (axSpA), with juvenile idiopathic arthritis (JIA) for the younger age onset, share similar pathogenetic features and, therefore, also cathegories of available therapeutic options [1,2,3,4,5]. This is, in particular, the case of tumour necrosis factors inhibitors (TNFi), members of the biological disease modifying anti-rheumatic drugs (bDMARDS) that are useful to control disease activity and reduce damage progression, both when used as a monotherapy or combined with other conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs), most frequently methotrexate (MTX) [6,7,8,9,10,11,12,13].
In the last years, in order to cope with the elevated costs deriving from sophisticated production processes and extensive randomized clinical trials, biosimilar drugs have emerged [14].
Adalimumab (Humira® [ADA]) is a TNF-alfa inhibiting fully human and high-affinity monoclonal immunoglobulin G1 antibody, successfully employed in the treatment of different inflammatory RMDs. One of its biosimilars, SB5 (Imraldi®), is currently approved in Europe for the majority of its originator’s indications [15]. In 2017, SB5 similarity to the reference ADA was previously assessed by premarketing registration studies. A randomized, double-blind trial was conducted on 542 patients with moderate-to-severe active RA despite MTX therapy, testing ADA versus SB5 for 24 weeks, followed by a second phase where patients treated with ADA were randomized 1:1 to continue ADA or switching to SB5 (ADA/SB5) [16, 17]. After 52 weeks, American College of Rheumatology Criteria response criteria as well as health assessment questionnaire (HAQ), disease activity score (DAS28ESR), physician global assessment (PhyGA), patient’s global assessment (PtGA), patient visual analogic scale for pain (VAS pain), CDAI and SDAI (Simplified/clinical disease activity index) were comparable between the two non-switched populations and the ADA/SB5 group. The SB5 tolerability and safety were also similar to those of the reference ADA, with similar severity and frequency of adverse events (AEs) in the ADA/SB5 switching group [16, 17].
Recently, a Bayesian network meta-analysis including eight randomized controlled trials compared the efficacy of SB5 + MTX and ADA + MTX with placebo + MTX in active RA. Results showed that both ADA and SB5 were more effective than placebo and that SB5 presented a similar efficacy and safety as the reference ADA [18].
A small cohort of real-life PsA patients was recently published, showing signs of axial disease flare in 3/12 patients after switching from ADA to SB5, leading to change in concomitant medication in 2 cases and back-switch to ADA originator in the third [19]. With the exclusion of this report, no other data on ADA/SB5 switching are available for non-RA patients affected by other inflammatory RMDs. Therefore, we aimed at describing the efficacy and the safety of switching from reference ADA to SB5 in a cohort of clinically stable inflammatory RMDs, including also PsA, axSpA and JIA patients.
Methods
A retro-prospective observational study was performed on a cohort of RA, JIA, axSpA and PsA patients treated with SB5 at the Rheumatology Division, University Hospital of Careggi, from October 2018 to November 2019. At the time of switching, patients with an age ≥ 18 years, fulfilling the diagnosis/classification of RMDs according to international criteria and in clinically stable conditions, treated for at least 6 months with ADA and then switched to SB5 for medical and non-medical decision, were enrolled. Patients with missing baseline data or no follow-up visit available were excluded.
Data were collected at the time of switching from ADA to SB5 (baseline) and again after 3 and 6 months, including blood tests results for inflammatory markers [erythrocyte sedimentation rate (ESR) and C-reactive proteins levels (CRP)], clinical data for all diseases [tender joints count (TJC), swollen joints count (SJC), PhyGA, PtGA, VAS pain, patient fatigue VAS (VAS fatigue), patient global health VAS (GH) and HAQ] as well as disease-specific scores [DAS28(ESR) and DAS28(CRP) used for RA and PsA, CDAI and SDAI for RA, BASDAI both for PsA and axSpA, Disease Activity in Psoriatic Arthritis (DAPSA) for PsA and Ankylosing Spondylitis disease activity score (ASDAS) for axSpA], safety data [in terms of local injection site or systemic AEs, both serious and non-serious] and data on concomitant treatments [SB5 posology, csDMARDs, corticosteroids (CCS), non-steroidal anti-inflammatory (NSAIDs)]. The study obtained approval from local IRB and patients signed informed consent for study participation.
For each continuous variable mean and standard deviation are reported, while for categorical variables absolute frequencies and percentage for each category. To evaluate the association between categorical variables chi-square test or Fisher’s exact test (when appropriate) was used. In order to assess the variation over time (baseline vs the other time points) for each scale, a GEE (generalized estimating equation model) linear model was used. The significant level was set at 5% (p < 0.05).
Results
During the observation period, a total of 115 patients were switched from reference ADA to SB5. Overall, 82 patients were eligible for the study (45 females − 54.8%, mean age 54 ± 14 years, mean disease duration 13 ± 7 years), with missing baseline data as the main reason for exclusion. Treatment with ADA represented the first-line bDMARD for the majority of the patients (55 patients, 67.1%), with a mean treatment duration of 6 ± 3 years at baseline. Enrolled patients included 19 RA (18 females, 94.7%), 32 axSpA (10 females, 31.3%), 28 PsA (15 females, 53.5%) and 3 JIA (2 females, 66.6%) patients. The distribution of first, second and third line bDMARD was comparable between the 3 major RMD groups, with similar disease duration and reference ADA treatment duration. Baseline characteristics in terms concomitant medications, laboratory markers and patient-reported outcomes are reported in Table 2 for the whole population, while Tables 3, 4 and 5 present disease-specific data and activity indexes for RA, axSpA and PsA, respectively. Considering the paucity of enrolled JIA patients, no disease specific within group analysis for this subpopulation was performed.
Efficacy
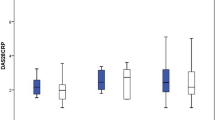
Among the overall study population, a significant increase in PtGA and a trend towards statistically significant increase in TJC and VAS pain at 3 months were found, while all scores were not statistically different from baseline at 6 months (Table 1).
In RA patients, a statistically significant increase in TJC at 3 months was detected, which however returned to original values at 6 months. Moreover, a statistically significant decrease in PhyGA, HAQ and DAS28ESR was also observed at 6 months. Possibly, this could be associated with changes in concomitant medication. In fact, there was a reduction of csDMARD prescription and an increase in number of patients taking NSAIDs, with an OR 6.8 (95% CI 1.03–45.17, p = 0.046) for RA patients to take NSAIDs after 6 months (see Table 2).
In axSpA, PtGA, VAS pain and ASDAS were significantly increased at 3 months, with return to original values at 6 months. Conversely, TJC was significantly increased score both at 3 and 6 months, being paralleled by a non-statistically significant increase in number of patients with csDMARD as concomitant medication (Table 3).
In PsA, PtGA, VAS pain, HAQ and DAPSA were significantly increased at 3 months, paralleled by an increase in NSAIDs prescription. At 6 months, all parameters were not different from baseline (see Table 4).
Safety
Safety data analysis showed 27 (34.2%) and 13 (17.1%) patients with at least one AE at 3 and 6 months, respectively. These were mostly infectious AEs and joint disease relapses (see Table 5). None of the 3 RMDs showed a higher prevalence of AEs, both in terms of number or specific type (Supplement Table 1 and Supplement Table 2). No serious AE was recorded. Two cases of bDMARD treatment withdrawn were seen: SB5 was discontinued in a patient with axSpA for an AE requiring further investigations and in a JIA patient who lost disease control and back-switched to ADA.
Conclusions
We described similar profiles of control of disease activity, everyday life disability and safety after switching from reference ADA to SB5 in real-life patients with RA, PsA and axSpA, with mild features of disease flare not leading to bDMARD treatment interruption.
Our study population was composed of different RMDs, in particular PsA and axSpA, with smaller cohorts of RA and JIA patients. Overall, some features of subjective joint disease relapse were observed at 3 months, despite not being paralleled by other scores of clinical activity or by laboratory parameter variations. In fact, acute phase reactants mean levels were always in the normal ranges during the study course, as the only objective measure available in the overall population. Moreover, overall 6 months scores were not different from the baseline values: this could be related to an increase in the temporary NSAIDs prescription or a slight shortening of the interval between each SB5 administration (still above the regular interval of 14 days). These two changes could have supported the return to the pre-switching disease status overall.
Concerning RA patients, TJC was the only score to be statistically significantly increased at 3 months, when we also registered a slight increase in the mean dosage of steroid and in the number of NSAIDs users. This allowed all disease activity scores and patient reported outcome measures to significantly decrease at 6 months and return to values similar to baseline. Moreover, mean average DAS28 (ESR) was further reduced from mild disease activity to remission; similarly, a minimally clinically important decline in HAQ disability index was also recorded [20]. Despite no changes in the concomitant medications are generally allowed during registration studies, our results resemble the positive effect of SB5 on RA seen in randomized clinical trials [16,17,18], with no RA patient interrupting the treatment in the first 6 months.
PsA patients showed a significant increase in patient-reported outcomes (PtGA, VAS pain and HAQ), in the DAPSA and also presented with a slight non-statistically significant increase in NSAIDs or CCS intake at 3 months. As seen for RA patients, these changes in the concomitant medications allowed the control of disease activity, with 6 months values of all disease activity and disability measures being not significantly different from baseline also in PsA patient. In addition, despite changes at 3 months, mean HAQ remained in the mild disability range and DAPSA in the mild disease activity interval during the 6 months of observation. In comparison to Di Cesare et al., we observed a similar extent of patients with joint disease flare (n = 4, 14.4%) but no significant change in the BASDAI was reported and no patient interrupted the treatment with SB5 [19]. Moreover, we also presented data of clinician-reported outcomes, peripheral and overall disease activities, proving further data on real-life switching from ADA to SB5 in PsA.
The axSpA subpopulation, along with the other two groups, showed a statistically significant increase in PtGA, VAS pain and ASDAS at 3 months, with progressive increase in the prescription of csDMARD as concomitant medication during the study observation. The four increased assessments were not different when comparing baseline and 6 months values and the disease activity evaluation remained in the same range for the axSpA population throughout the study. In particular, BASDAI values remained in the inactive disease interval and ASDAS values in the mild disease activity ranges. Despite these reassuring results, a significant increase in the TJC and a clinically meaningful increase in HAQ were noted both at 3 and 6 months versus baseline, despite mean value being below 1 joint. Currently no study of ADA/SB5 switching in axSpA has been performed and, therefore, we cannot compare our results with previous reports, in particular in terms of disease flares and joint counts.
When analysing the whole study population, the number of patients reporting at least 1 AE was in line with the current literature data, both in terms of prevalence of both local and systemic AEs, and type of systemic AEs [16, 17]. Despite this, notes of disease reactivation requiring changes in the concomitant medication profile were noted, requiring large-scale studies as a confirmation [21]. During the study, only two patients discontinued therapy: a JIA patient, in which insufficient disease control caused a back-switch to reference ADA, and an axSpA patient in which the suspicion of an AE determined a temporary treatment, later reintroduced.
To the best of our knowledge, this is the first study on patients switching from ADA to SB5 on a multi-RMDs evaluation, providing the first exploratory data on axSpA and further real-life data on PsA and RA, using both disease-specific activity indexes and patient-reported outcomes. However, our study has some limitation. First of all, we did not perform any sample size calculation. The aim of our study was to present an explorative description of the first 6 months of real-life treatment after switching from originator adalimumab to SB5, and no formal hypothesis has been formulated. Our preliminary descriptive data will therefore need corroboration by the large-scale international real-life initiative recently started, which includes also patients with axSpA and PsA switched from ADA to SB5 [21]. Moreover, the paucity of our JIA population made it impossible to consider it for a separate statistical analysis. Finally, our observation for 6 months does not allow an estimation of the persistence on treatment and the long-term outcome, in particular of disease flares.
In conclusion, despite the fact that minor adjustment may be needed in concomitant medications, our study confirms the data in the literature for RA and preliminarily support the medical switch from ADA originator to SB5 in AS and PsA patients, with initial data also for JIA patient. Ongoing large-scale initiatives will provide confirmatory data in the future, in particular regarding the overall safety and efficacy profiles, as well as detailed information on the entity and the management of subjective and objective disease flares after switching from reference ADA to SB5.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376(9746):1094–1108. https://doi.org/10.1016/S0140-6736(10)60826-4
Ritchlin CT, Colbert RA, Gladman DD (2017) Psoriatic arthritis. N Engl J Med 376(10):957–970. https://doi.org/10.1056/NEJMra1505557
Taurog JD, Chhabra A, Colbert RA (2016) Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med 374(26):2563–2574. https://doi.org/10.1056/NEJMra1406182
Prakken B, Albani S, Martini A (2011) Juvenile idiopathic arthritis. Lancet Lond Engl 377(9783):2138–2149. https://doi.org/10.1016/S0140-6736(11)60244-4
Ravelli A, Martini A (2007) Juvenile idiopathic arthritis. Lancet Lond Engl 369(9563):767–778. https://doi.org/10.1016/S0140-6736(07)60363-8
Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poór G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R, de Wit M, van der Heijde D (2016) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 76(6):960–977. https://doi.org/10.1136/annrheumdis-2016-210715
Mian A, Ibrahim F, Scott DL (2019) A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol 3(1):42. https://doi.org/10.1186/s41927-019-0090-7
Kerschbaumer A, Sepriano A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, McInnes IB, Bijlsma JWJ, Burmester GR, de Wit M, Falzon L, Landewé R (2019) Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis https://doi.org/10.1136/annrheumdis-2019-216656, annrheumdis-2019-216656
Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, Emery P, Landewé R, Oliver S, Aletaha D, Betteridge N, Braun J, Burmester G, Cañete JD, Damjanov N, FitzGerald O, Haglund E, Helliwell P, Kvien TK, Lories R, Luger T, Maccarone M, Marzo-Ortega H, McGonagle D, McInnes IB, Olivieri I, Pavelka K, Schett G, Sieper J, van den Bosch F, Veale DJ, Wollenhaupt J, Zink A, van der Heijde D (2016) European league against rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 75(3):499–510. https://doi.org/10.1136/annrheumdis-2015-208337
Ringold S, Weiss PF, Beukelman T, DeWitt E, Ilowite NT, Kimura Y, Laxer RM, Lovell DJ, Nigrovic PA, Robinson AB, Vehe RK, American Collge of Rheumatology (2013) 2013 update of the 2011 American College of Rheumatology Recommendations for the treatment of juvenile idiopathic arthritis. Arthritis Rheum 65(10):2499–2512. https://doi.org/10.1002/art.38092
van der Heijde D, Ramiro S, Landewé R, Baraliakos X, van den Bosch F, Sepriano A, Regel A, Ciurea A, Dagfinrud H, Dougados M, van Gaalen F, Géher P, van der Horst-Bruinsma I, Inman RD, Jongkees M, Kiltz U, Kvien TK, Machado PM, Marzo-Ortega H, Molto A, Navarro-Compàn V, Ozgocmen S, Pimentel-Santos FM, Reveille J, Rudwaleit M, Sieper J, Sampaio-Barros P, Wiek D, Braun J (2017) 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 76(6):978–991. https://doi.org/10.1136/annrheumdis-2016-210770
Maksymowych WP, Gladman D, Rahman P, Boonen A, Bykerk V, Choquette D, Dimond S, Fortin P, Karsh J, Klinkhoff AV, Mosher D, Mulholland K, Olszynski WP, Russell AS, Savage L, Shanner L, Shojania K, Starr M, Thomson G, Zummer M, Inman R, Canadian Rheumatology Association/ Spondyloarthritis Research Consortium of Canada (2007) The Canadian rheumatology association/ Spondyloarthritis research consortium of Canada treatment recommendations for the management of spondyloarthritis: a national multidisciplinary stakeholder project. J Rheumatol 34(11):2273–2284
Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, Inman RD, Majithia V, Haroon N, Maksymowych WP, Joyce J, Clark BM, Colbert RA, Figgie MP, Hallegua DS, Prete PE, Rosenbaum JT, Stebulis JA, van den Bosch F, Yu DTY, Miller AS, Reveille JD, Caplan L (2016) American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 recommendations for the treatment of Ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Rheumatol Hoboken NJ 68(2):282–298. https://doi.org/10.1002/art.39298
Ruiz S (2017) Biosimilars in the EU. Biosimilar Drug Prod Dev 395–411
European Medicines Agency (2018). Imraldi. https://www.ema.europa.eu/en/medicines/human/EPAR/imraldi. Accessed 24 February 2020
Weinblatt ME, Baranauskaite A, Dokoupilova E, Zielinska A, Jaworski J, Racewicz A, Pileckyte M, Jedrychowicz-Rosiak K, Baek I, Ghil J (2018) Switching from reference adalimumab to SB5 (adalimumab biosimilar) in patients with rheumatoid arthritis: fifty-two-week phase III randomized study results. Arthritis Rheumatol Hoboken NJ 70(6):832–840. https://doi.org/10.1002/art.40444
Weinblatt ME, Baranauskaite A, Niebrzydowski J, Dokoupilova E, Zielinska A, Jaworski J, Racewicz A, Pileckyte M, Jedrychowicz-Rosiak K, Cheong SY, Ghil J, Sokolovic S, Mekic M, Prodanovic N, Gajic B, Karaselimovic-Dzambasovic E, Pojskic B, Toncheva A, Dimitar P, Rodina L, Geneva-Popova M, Staykov I, Stoilov R, Podrazilova L, Mosterova Z, Simkova G, Kopackova J, Stejfova Z, Vencovsky J, Urbanova Z, Janska L, Galatíkova D, Stropuviene S, Sniuoliene I, Sitek-Ziolkowska K, Rell-Bakalarska M, Kolasa R, Daniluk S, Sliwowska B, Bartosik-Twardowska M, Brzezicki J, Konieczny M, Jeka S, Choe J, Bae S, Kang Y, Prystupa L, Vyacheslav Z, Gasanov I, Yatsyshyn R, Rekalov D, Iaremenko O, Stanislavchuk M, Tseluyko V (2018) Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol Hoboken NJ 70(1):40–48. https://doi.org/10.1002/art.40336
Bae SC, Lee YH (2018) Comparative efficacy and safety of biosimilar adalimumab and originator adalimumab in combination with methotrexate in patients with active rheumatoid arthritis: a Bayesian network meta-analysis of randomized controlled trials. Clin Rheumatol 37(5):1199–1205
Di Cesare A, Tronconi G, Fastame TM et al (2020) SB5 adalimumab biosimilar in the treatment of psoriasis and psoriatic arthritis. Dermatol Ther. https://doi.org/10.1111/dth.13435
Wolfe F, Michaud K, Strand V (2005) Expanding the definition of clinical differences: from minimally clinically important differences to really important differences. Analyses in 8931 patients with rheumatoid arthritis. J Rheumatol 32(4):583–589
Anonymous (2019). A Real-World study of Imraldi ® use (PROPER). https://clinicaltrials.gov/ct2/show/NCT04089514. Accessed 05 May 2020
Acknowledgements
We thank the patients who participated to the study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by CB, RB, FN, LC, LT. The first draft of the manuscript was written by CB and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
CB received honoraria from Actelion and Eli-Lilly; RB, FN, LC, LT, FB, GF: none. MMC reports receipt of grant/research support and/or speaker’s bureau attendance from Actelion, Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Bayer - MSD, Biogen, Eli Lilly.
Ethical approval
Obtained from Comitato Etico Area Vasta Toscana Centro.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Bruni, C., Bitti, R., Nacci, F. et al. Efficacy and safety of switching from reference adalimumab to SB5 in a real-life cohort of inflammatory rheumatic joint diseases. Clin Rheumatol 40, 85–91 (2021). https://doi.org/10.1007/s10067-020-05199-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05199-w