Abstract
Background
Sarcoidosis is a chronic inflammatory disease characterized by non-caseating granuloma which etiology is unknown yet. Adipokines are different proteins synthesized by adipose tissue that have an influence on angiogenesis, hemostasis, lipid metabolism, and immune system regulation. Adipokines may play a role in the pathogenesis of sarcoidosis.
Objectives
To evaluate the serum adipokine levels in patients with sarcoidosis and to determine a possible correlation with clinical and laboratory signs of disease.
Methods
Forty-four biopsy-proven sarcoidosis patients followed at a single center and age- and sex-matched 41 healthy volunteers were included in the study. Demographic, clinical, laboratory, and radiological data were recorded and body mass index (BMI) was calculated in all patients. Routine laboratory tests (blood glucose, liver, and kidney function test) were measured. Serum adiponectin and leptin levels were measured by ELISA method.
Results
Among 44sarcoidosis patients, 13 (29.5%) were male and 31 (70.5%) were female. Twenty-one (47.7%) patients had erythema nodosum, three (6.8%) had uveitis, 40 (90.9%) had arthralgia, 32 (72.7%) had arthritis, 15 (34.1%) had enthesitis. Laboratory evaluation showed increased serum ACE level in 24 (54.5%) patients, increased serum calcium level in 11 (25%) patients, increased serum D3 level in 5 (11.4%) patients, and increased ESR and CRP levels in 22 (50%) and 23 (52.3%) patients, respectively. Compared with the control group, serum adiponectin levels were significantly higher in patients with sarcoidosis(p = 0.007). Serum adiponectin level was associated with arthralgia and ankle joint swelling (p = 0.007, p = 0.006 respectively). Serum leptin levels were similar in sarcoidosis patients and controls (p = 0.327). There was no relationship between serum leptin level and disease features (p > 0.05).
Conclusions
In this study, high serum adiponectin level was detected in patients with sarcoidosis while serum leptin level was similar in the sarcoidosis and control group. Adiponectin, an anti-inflammatory protein, may play a role in the pathogenesis of sarcoidosis. Studies are needed to shed light on this topic.
Key Points • Sarcoidosis is a chronic granulomatous disease characterized by granuloma formation • High serum adiponectin level was found in sarcoidosis patients • Serum adiponectin level was associated with some clinical features such as arthralgia and arthritis • High adiponectin levels in sarcoidosis patients may mitigate the inflammatory response, resulting in a mild form of the disease and/or spontaneous remission |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adipose tissue is not only a storage organ but also plays an important role in many physiological and pathological processes by producing and releasing a variety of proinflammatory mediators. Different proteins (adipokines) synthesized by adipose tissue have effects on angiogenesis, hemostasis, lipid metabolism, appetite regulation, and immune system regulation [1]. The adipose tissue also contains macrophages that synthesized many proinflammatory cytokines. A low-grade systemic inflammation demonstrated by elevated levels of C-reactive protein (CRP) and interleukine-6 (IL-6) has been reported in obese patients when compared to healthy subjects [2]. This relationship between adipose tissue and systemic inflammation can be explained by the production of inflammatory cytokines from adipose tissue which reflects the potential contribution of this organ to the inflammatory responses. It shows that the adipose tissue is an important production area of circulating proinflammatory cytokines [3]. Macrophages that are produced from the adipose tissue contribute to nearly 30% of circulating IL-6. Adiponectin and leptin are the most well-known and investigated proteins synthesized by the adipose tissue [4]. While leptin plays a role in appetite regulation, adiponectin is associated with insulin sensitivity. The effect of adipokines on immunity and inflammation is well-established. It is generally accepted that leptin exhibits a proinflammatory effect, while adiponectin acts primarily as an anti-inflammatory molecule. It has been reported that leptin receptors are expressed on B cells and T cells and leptin has direct effects on lymphocytes. Leptin can stimulate T cell proliferation, initiate a Th1 response, affect T cell activation, and increase phagocytic activity by activating macrophages and monocytes [5]. In animal models, leptin deficiency has been shown to be associated with immunosuppression. Adiponectin interferes with macrophage function by inhibiting IL-6 or TNFα production and phagocytosis, decreasing T cell function, and promoting the release of IL-10 and IL-1 receptor antagonists and exerts various anti-inflammatory activities [6]. As a result, by acting on both hormones, adipose tissue may affect or alter the inflammatory response. There are many studies investigating the role of adipokines in various rheumatic diseases [7]. Sarcoidosis is a chronic inflammatory disease of unknown etiology characterized by non-caseating granuloma formation [8]. The possible role of adipokines in the pathogenesis of sarcoidosis is not known yet. They may have an important role in the development of granuloma formation by stimulating Th1 immune response and activation of various inflammatory cells. In this context, adipokines may also be considered as determinants of disease activity or may be associated with different clinical phenotypes in patients with sarcoidosis.
The aim of this study was to determine serum adipokines levels in patients with sarcoidosis and to determine possible relationship with clinical and laboratory findings.
Material and method
Forty-four patients with biopsy-proven sarcoidosis followed in a single center and 41 sex- and age-matched healthy volunteers were included in the study. The patients and controls with confounding factors (such as cardiovascular disease, metabolic diseases, or any chronic diseases), which may lead to higher adipokine levels in the sera, were excluded from the study. Tissue samples collected with endobronchial ultrasound (EBUS), mediastinoscopy, and skin and axillary lymphadenopathies biopsies were used for histopathological verification of sarcoidosis and diagnosis was made upon identification of non-caseating granulomas by the pathologist. Other possible causes of granulomatous diseases (tuberculosis, bacterial, and fungal infections) were excluded. Laboratory examinations were made for all sarcoidosis patients; routine biochemistry, acute phase reactants (ESR, CRP), serum angiotensin-converting enzyme (ACE), serum calcium, and serum 25-hydroxy- vitamin D3 levels were checked. Lung X-ray and thorax CT scan were made to stage sarcoidosis. Demographic, clinical, laboratory, and radiological data of all patients were recorded and body mass index (BMI) was calculated. Routine laboratory tests (blood glucose, liver function test (LFT) and glomerular filtration rate (GFR) were evaluated. Serum adiponectin and leptin levels were evaluated by microELISA method.
Statistical analysis
All statistical analyses were made by using SPSS version 9.0 (Chicago, II, USA). Prevalence was calculated for each group and comparisons for categorical variables were made with a chi-square test. Continuous variables were compared with Student’s 푡 test. For all statistical tests, a 푃 value of < 0.05 was considered to be statistically significant.
Results
Among 44sarcoidosis patients 13 (29.5%) were male and 31 (70.5%) were female, the mean age was 46.3 years, the mean disease duration was 3.7 years. The system and organ involvement evaluation revealed erythema nodosum in 21 patients (47.7%), uveitis in three patients (6.8%), neurosarcoidosis in one patient (2.3%), arthralgia in 40 (90.9%), and arthritis in 32 patients (72.7%). In patients with arthritis, 28 had ankle joint swelling (87.5%), three had knee joint, and one had wrist joint involvement. None of the patients had cardiac involvement. Thorax CT revealed sarcoidosis stage one in 14 patients (31.8%), stage two in 22 patients (50%), stage three in 4 patients (9.1%), and stage 4 in 4 patients (9.1%) (Table 1). Histopathological verification of sarcoidosis was done with endobronchial ultrasound and mediastinoscopy and skin and axillary LAP biopsy revealing non-caseating granuloma. Laboratory tests revealed elevated serum ACE in 24 (54.5%) patients, elevated serum calcium in 11 (25%) patients, and elevated serum 25-hydroxy-vitamin D3 in 5 (11.5%) patients. The laboratory tests revealed elevated erythrocyte sedimentation rate (normal < 20 mm/h) in 22 (50%) patients and elevated C-reactive protein (normal < 5 mg/dL) in 23 (52.3%) patients. As for the patient’s treatment approaches, 17 patients had short-term treatment with low-dose corticosteroids, 5 patients used hydroxychloroquine (HQ), 1 patient used azathioprine (AZT), 1 patient used methotrexate (MTX), 2 patients used colchicum-dispert (CD) and 18 patients used non-steroidal anti-inflammatory drugs (NSAIDs).
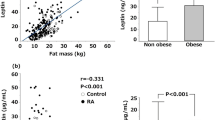
Of the 41 healthy volunteers enrolled in the study, 14 were males and 27 were females; mean age was 45.8 years. Compared with the control group serum adiponectin levels were significantly higher in patients with sarcoidosis (9.12 micg/ml vs. 24.92 micg/ml, p = 0.007). On the other side, serum leptin levels were similar in sarcoidosis patients and healthy controls (8.96 ng/ml vs. 8.18 ng/ml, p = 0.327). When compared with clinical features of sarcoidosis, serum adiponectin level was associated with arthralgia and ankle swelling (p = 0.007, p = 0.006 respectively). There was no relationship between serum adiponectin and leptin levels with various disease features such as erythema nodosum, uveitis, arthritis, CRP, and ESR (p > 0.05) (Table 2). When we compared serum adipokines levels between sarcoidosis patients receiving DMARDs and those receiving only NSAIDs, we found no differences between the two groups (p = 0.006). Most of our patients were female (70.5%) and only 13 (29.5%) patients were male. When we compared the serum adipokines levels of adipokines regarding patient’s gender, we have found that female patients have increased adipokines levels. Among these female patients, we have no information about their reproductive status (menopause), and we cannot compare them in this regard. Also, we cannot compare the patients and control group regarding age parameter because both groups have similar age distribution.
Discussion
In this study, we have found a high serum level of adiponectin in patients with sarcoidosis, while serum leptin level was normal. Also, the association between serum adiponectin and some clinical features of sarcoidosis was detected. There are contradictory reports in the literature about the role of adipokines in pathogenesis of various inflammatory rheumatic diseases. Previous studies have reported high levels of adiponectin in systemic autoimmune diseases, even in patients with a significant inflammatory response [9]. It has been shown to stimulate IL-6 and prostaglandin E2 production by synovial fibroblasts in patients with rheumatoid arthritis (RA) [10]. In addition, serum adiponectin levels were associated with severity of RA and evaluated as a marker of joint destruction [11]. Toussirot et al. showed higher serum IL-6 and adiponectin levels in primary Sjögren’s syndrome patients compared to healthy controls (p = 0.05 and 0.04, respectively) [12]. Xue et al. showed that the level of adiponectin was significantly lower in patients with psoriatic arthritis (PsA) than those in healthy controls and with psoriasis [13]. In contrast, the concentrations of leptin were higher in the erosive PsA patients when compared with psoriasis and non-erosive PsA patients. Yurt et al. investigated the plasma regulatory proteins in pulmonary tuberculosis and sarcoidosis and the possible relationship between these parameters and loss of body weight [14]. The authors did not find any significant difference in adiponectin levels between the tuberculosis and control group. However, the adiponectin levels were significantly higher in sarcoidosis group compared with the control group. Adiponectin may take a role in the regulation of food consumption in sarcoidosis. Our results were in accordance with the results of this study regarding adiponectin levels in sarcoidosis patients. Leptin serum levels have been evaluated in various inflammatory rheumatic diseases. Otero et al. reported increased circulating and synovial leptin levels in RA patients compared with osteoarthritis and control group [15]. They also found higher adiponectin levels in RA patients than the control group. The increased levels of adiponectin in RA patients suggest a compensatory mechanism under catabolic or anabolic imbalance. The authors hypothesized that increased adiponectin levels antagonize the anorexigenic and pro-inflammatory effects of leptin. They suggested that these two adipokines may act as opposing metabolic counterparts. Our study results support this hypothesis; we have found higher serum adiponectin levels, while serum leptin was similar to the control group. So the opinion that adiponectin antagonizes pro-inflammatory effect of leptin was consistent with our study result. Also, we have suggested that this data may be one of the reasons for the benign nature and self-limitation of sarcoidosis. As adiponectin is known to exhibit potent anti-inflammatory properties, high adiponectin levels in sarcoidosis patients may mitigate the inflammatory response, resulting in the mild form of disease and/or spontaneous remission. In another study, the serum leptin levels were positively associated with CRP level in RA patients, suggesting that it may act as pro-inflammatory cytokines in this disease [16]. However, the serum adiponectin level was elevated too, but it was negatively associated with CRP level [17]. There are contradictory data about the serum adipokines in SLE patients [18, 19]. While increased leptin levels were reported in most studies, some authors have reported decreased/normal serum adiponectin levels [20, 21]. Based on the results observed in SLE patients, the authors speculated on the possible effect of leptin on inflammation, some clinical symptoms, or weight changes during the course of the disease [22]. However, no association was found between leptin levels and clinical or biological disease activity indicators. As a result, the role of adiponectin in inflammation seems conflicting, although it is generally considered as an anti-inflammatory agent. In contrast, some studies have proved that adiponectin levels are high in the inflammatory diseases.
In conclusion, we have found high serum adiponectin level in sarcoidosis patients, while serum leptin level was similar to the control group. Adiponectin, an anti-inflammatory protein, may play a role in the pathogenesis or may be associated with some clinical featured of sarcoidosis. While there are a lot of studies about the relationship between adiponectin and systemic autoimmune diseases, not one is about the association of adiponectin and sarcoidosis except our study. More prospective multicenter studies are needed to shed light on this issue.
References
Fantuzzi G (2005) Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 115(5):911–920
Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW (1999) C-reactive protein in healthy subjects: association with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 19:972–978
Lago F, Dieguez C, Gomez-Reino J, Gualillo O (2007) Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 3(12):716–724
Carrión M, Frommer KW, Pérez-García S, Müller-Ladner U, Gomariz RP, Neumann E (2019) The adipokine network in rheumatic joint diseases. Int J Mol Sci 22:17–20
Fantuzzi G (2008) Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol 121:326–330
Toussirot E, Streit G, Wendling D (2007) The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem 14:1095–1100
Toussirot E, Gaugler B, Bouhaddi M, Nguyen NU, Saas P, Dumoulin G (2010) Elevated adiponectin serum levels in women with systemic autoimmune diseases. Mediat Inflamm 2010:938408
Baughman RP, Lower EE, du Bois RM (2003) Sarcoidosis. Lancet 361:1111–1118
Chung CP, Long AG, Solus JF (2009) Adipocytokines in systemic lupus erythematosus: relationship to inflammation, insulin resistance and coronary atherosclerosis. Lupus 18(9):799–806
Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM (2007) Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-κB pathway. J Immunol 179:5483–5492
Kusunoki N, Kitahara K, Kojima F (2010) Adiponectin stimulates prostaglandin E production in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum 62:1641–1649
Toussirot É, Binda D, Gueugnon C, Dumoulin G (2012) Adiponectin in autoimmune diseases. Curr Med Chem 19(32):5474–5480
Xue Y, Jiang L, Cheng Q, Chen H, Yu Y (2012) Adipokines in psoriatic arthritis patients: the correlations with osteoclast precursors and bone erosions. PLoS One 7(10):e46740
Yurt S, Erman H, Korkmaz G, Kosar AF, Uysal P, Gelisgen R et al (2013) The role of feed regulating peptides on weight loss in patients with pulmonary tuberculosis. Clin Biochem 46:40–44
Otero M, Lago R, Gomez R (2006) Towards a proinflammatory and immunomodulatory emerging role of leptin. Rheumatology 45:944–950
Yoshino T, Kusunoki N, Tanaka N, Kaneko K, Kusunoki Y (2011) Elevated serum levels of resistin, leptin, and adiponectin are associated with CRP and also other clinical conditions in rheumatoid arthritis. Intern Med 50:269–275
Ebina K, Fukuhara A, Ando W (2009) Serum adiponectin concentrations correlate with severity of rheumatoid arthritis evaluated by extent of joint destruction. Clin Rheumatol 28:445–451
Garcia-Gonzalez A, Gonzalez-Lopez L, Valera-Gonzalez IC (2002) Serum leptin levels in women with systemic lupus erythematosus. Rheumatol Int 22:138–141
Sada KE, Yamasaki Y, Maruyama M (2006) Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol 33:1545–1552
Vadacca M, Margiotta D, Rigon A (2009) Adipokines and systemic lupus erythematosus: relationship with metabolic syndrome and cardiovascular disease risk factors. J Rheumatol 36:295–297
Wislowska M, Rok M, Stepien MK, Kuklo-Kowalska A (2008) Serum leptin in systemic lupus erythematosus. Rheumatol Int 28:467–473
De Sanctis JB, Zabaleta M, Bianco NE, Garmendia JV, Rivas L (2009) Serum adipokine levels in patients with systemic lupus erythematosus. Autoimmunity 42:272–274
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kobak, S., Semiz, H., Akyildiz, M. et al. Serum adipokine levels in patients with sarcoidosis. Clin Rheumatol 39, 2121–2125 (2020). https://doi.org/10.1007/s10067-020-04980-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-04980-1