Abstract
Background
Patients with rheumatoid arthritis (RA) tend to be more overweight, take less physical exercise, exhibit decreased cardiorespiratory fitness and demonstrate reduced muscle strength compared with age- and sex-matched controls. Impaired cognitive function in RA is an important associated factor, although it has been less well-recognized. The aim of this study was to investigate the effects of a specifically designed exercise programme on body composition, aerobic capacity, muscle strength and cognition in RA.
Methods
Sixty-six patients with RA were randomized to a specifically designed, personalized exercise programme or standard care. Assessments included body composition, fitness, grip strength and cognitive testing, in addition to disease related measures.
Results
Significant improvements in C-reactive protein (p = 0.025), fatigue scores (p = 0.047) and truncal fat (p = 0.004) were observed in the exercise group compared with controls. Median waist circumference was significantly reduced (94.0 to 91.4 cm, p < 0.0001). Improvements were also seen in aerobic capacity (23.2 to 27.6 ml/kg/min, p = 0.002) and in median right (12.0 to 13.0 kg, p = 0.025) and left grip strength (8.0 to 10 kg, p = 0.005). Cognitive function improved in the exercise group, with median Montreal Cognitive Assessment score 25.5 at 0 months compared to 28.0 at 3 months (p = 0.001).
Conclusion
This study demonstrates that exercise has a significant and positive impact on cognitive function in RA. Furthermore, physical activity is safe and effective in chronic inflammatory joint disease and is recommended as a vital component in the holistic management of these patients.
Key Points • A dedicated physical exercise programme is feasible and safe in patients with rheumatoid arthritis (RA). • Physical exercise helps reduce fatigue scores and improves cardiovascular fitness in stable RA patients. • Physical exercise has a positive impact on cognition in patients with RA. • A structured exercise programme should be an integral part of chronic disease management protocols for patients with RA. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic multisystem inflammatory disease affecting approximately 1% of the population [1,2,3]. Co-morbid diseases are common with increased morbidity and mortality due predominantly to cardiovascular and cerebrovascular pathology and infections [4]. Many patients with RA have multiple associated risk factors for vascular disease including obesity, and the co-morbidities linked to the metabolic syndrome. Inflammation appears to play an additional role in accelerated atherosclerosis [5]. Preventable or modifiable risks include avoidance of weight gain, eating a healthy diet and participation in regular aerobic activity. A large proportion of patients with RA are overweight or obese [6].
Aerobic capacity is a strong and independent predictor of cardiovascular disease and overall mortality [7]. Studies in general populations have shown that engaging in regular physical activity can improve cardiovascular health. Despite the known benefits of exercise, RA patients are less likely to engage in such activity compared with their age- and sex-matched counterparts [8,9,10].
Cognitive impairment is a less well-recognized association with RA, despite the fact that it affects up to 30% of these patients versus 8% of healthy controls [11]. Reduced cognitive function adversely affects function in RA and contributes significantly to morbidity and the ability to actively participate in the modifiable aspects of the disease. Although the pathogenesis of cognitive dysfunction in RA has not been fully elucidated, suggested mechanisms include the adverse effects of inflammation on the brain; the associated vascular risk factors; the consequences of pain, fatigue and sleep disturbance; and medications used to treat the joint disease. Cognitive function improves with regular physical activity [12, 13]
One of the aims of this study was to study the effects of exercise and improved cardiovascular fitness on cognition in patients with RA.
Methods
Patients with RA were identified from outpatient clinics and existing clinical databases at a tertiary academic university hospital rheumatology department.
Inclusion criteria were rheumatoid arthritis as per 1987 American College of Rheumatology criteria or diagnosis documented in the medical notes by a consultant rheumatologist, age between 18 and 75 years, on stable medications for at least 3 months, absence of a diagnosis of cognitive impairment and the ability to exercise (walk independently).
Exclusion criteria were as follows: inability to tolerate cardiorespiratory fitness training due to co-existent co-morbidity, cerebrovascular accident or myocardial infarction within the last 3 months, pregnancy, active malignancy, major surgery (including joint surgery) in the previous 6 months and significant active psychiatric disease.
Ethical approval for the study was obtained in advance from the Ethics Committee of St James’s Hospital, Dublin. After explaining the purpose, benefits and potential risks of the study to each patient and obtaining written informed consent, age and gender-matched patients were randomized on a 1:1 case/control ratio to a personalized exercise programme or standard care, using a computer based randomisation programme [14].
All randomized patients were offered two visits with the investigator (MA). During the first visit, a baseline assessment was performed. After the 3-month study period, patients in both groups attended for final review, and all assessments were repeated by the same investigator.
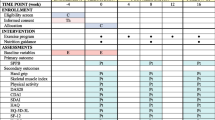
Assessments
Demographic and anthropometric data
Demographic data were collected using a self-administered questionnaire. Standing height was measured to the nearest 0.5 cm. Waist circumference was measured at the approximate midpoint between the lower margin of the last palpable rib and the top of the iliac crest and recorded in centimetres. Weight (to the nearest 0.1 kg) and body composition (body mass index and truncal fat percentage) were recorded using a Tanita Segmental Body Composition Analyser (Tanita BC- 418MA®) [15].
Disease activity and quality of life measurements
Inflammation was evaluated using erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels in venous blood. The Disease Activity Score-28 (DAS28) [16] was used to assess clinical disease activity. Functional capacity was assessed using the health assessment questionnaire (HAQ) [17] disability index. Fatigue was assessed using the Multidimensional Assessment of Fatigue (MAF) scale [18, 19].
Individual CVD risk factors
Each participant had their blood pressure and resting pulse measured using a standard electronic sphygmomanometer after they remained seated for 5 min. Serum lipids and glucose were measured from venous blood samples collected in the fasting state. Smoking status was recorded by patient self-report.
Grip strength and cardiorespiratory fitness
Grip strength was measured in kilogrammes (kg), using a hand-held dynamometer [20], and the best measure out of three for each hand was recorded. Hand dominance was noted. All participants underwent a submaximal treadmill test (modified Bruce protocol [21, 22]) to estimate VO2Max in ml/kg/min, which is the rate of oxygen uptake during maximal exercise. Patients walked on a treadmill according to a multistage protocol, whereby the speed and incline (gradient) on the treadmill was increased every 3 min. The protocol started at stage 1 (0% gradient, speed 1.74 miles per hour), and both speed and incline were increased with increasing stage. For the duration of the test, patients wore a heart rate monitor across their chest for continuous heart rate monitoring. Heart rate was used to guide the intensity of exercise. Prior to the test, patients’ heart rate reserve (the difference between predicted maximum heart rate and resting heart rate) was calculated using the Karvonen formula [23]. This formula calculates exercise heart rate zones at a given percentage training intensity based on age and resting heart rate. The American College of Sports Medicine (ACSM) recommends that to improve aerobic fitness, exercise intensity should be set at 40–85% of heart rate reserve [24]. The test was terminated once the target heart reached 80% or if the patient wished to stop due to exhaustion or pain. Once the test was completed, VO2Max was calculated using formulas described in Advanced Fitness Assessment and Exercise Prescription [24]. Depending on their score and age, patients were categorized into different cardiorespiratory fitness levels (poor, fair and good), as per the classification described in Advanced Fitness Assessment and Exercise Prescription [24]. This was used to guide their exercise prescription.
Cognitive testing
Montreal Cognitive Assessment (MoCA) was used to assess cognition. This is a paper-based test, administered by the investigator and evaluates different cognitive domains, including attention, concentration, executive function, memory, language, visuo-constructional skills, conceptual thinking, calculations and orientation [25]. The total possible score is 30 points, and 1 point is added for individuals with 12 years or fewer of formal education. In the original study, 21 controls had an average score of 27.4, while those with mild cognitive impairment had a score of 22.1 compared to 16.2 in patients with Alzheimer’s. However, MoCA is mostly utilized in distinguishing normal cognition from mild cognitive impairment, with a cut-off value of < 26, giving an area under the curve of 0.921 [25]. We therefore stratified our population into two categories based on this cut-off value.
Other cognitive measurement tools included Picture Memory Test (acquisition, recall, recognition and visual reasoning), Colour trails 1 and 2 and the computer based Sustained Attention Response Time (SART). A detailed summary of these tests, as well as normative values is presented in supplementary Table 1.
Personalized exercise Programme
Patients in the intervention group were enrolled for a 3-month personalized exercise programme, prescribed by the study physiotherapist (CC) and had three sessions with the physiotherapist during the period of study.
On their first visit, they were given an exercise prescription based on their baseline cardiovascular fitness test and strength measurements. They had two follow-up visits to the physiotherapist, at four weekly intervals to assess their progress, and, if needed, the exercise prescription was escalated.
The control group received standard care, which involved advice on benefits of exercise in rheumatoid arthritis and outlining recommendations by ACSM and American Heart Association guidelines for physical activity in older adults (men and women age ≥ 65 years) and adults age 50 to 64 years with clinically significant chronic conditions and/or functional limitations [26].
The type of cardiovascular exercise prescribed (walking, cycling or swimming) depended on the patient’s preferences and perceived ability and on the physiotherapist’s assessment of their ability to attain fitness goals.
The strength training programme consisted of series of exercises for major muscle groups and grip strength. Exercises for the upper body included biceps curls, triceps extensions and shoulder press. Exercises for the lower body included leg squats. Resistance bands and balls were used for grip strength. Patients were asked to write a short daily report (diary) of how much training they had performed at home and to note any side effects such as pain or swelling.
Statistical methods
The primary outcomes for the study were body composition (measured by waist circumference), cardiovascular fitness (measured by VO2Max), muscle strength (measured by grip strength) and cognitive function (measured by MoCA).
Based on existing literature, an expected difference in VO2Max of 5–10% was estimated with an expected standard deviation of values in both groups of 0.1. This was incorporated into a power calculation to estimate a sample size that would allow rejection of the null hypothesis with a probability of 0.95 (α = 0.05). Similar power calculations were carried out for waist circumference, grip strength and MoCA. Sample size was therefore estimated at 60 patients (30 cases, 30 controls). We increased the recruitment target by 10% to allow for an expected drop-out rate, given the nature of the intervention. Data was analysed on an intention-to-treat basis using SPSS version 20 (IBM Corp, Chicago, USA).
Results
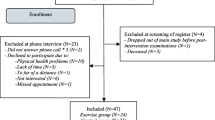
Sixty-six patients were consented and enrolled in the study, 33 were randomized to the intervention (exercise) group and 33 to the control group. Five cases and nine controls did not attend for baseline assessment, and a further four cases and three controls did not return for assessment at 3 months and so were lost to follow-up (Fig. 1).
Study design and patient randomization flowchart. Thirty-three patients were randomized in each group. Five patients did not attend for baseline assessment in the exercise group and nine in the control group. A further four and three patients dropped out during the follow-up period and were not available for 3-month assessments. The final per protocol population was therefore 45 patients. RA, rheumatoid arthritis
Baseline assessments
Fifty-two patients underwent baseline (week 0) assessment. Demographics, disease-related characteristics and medications for exercise and control groups are presented in Table 1. There were no significant differences between the exercise group (n = 28) and controls (n = 24). Over 80% of patients were female in both groups, with similar median age (58.5 versus 63 years, p = 0.108). Median disease duration was 12 years in the exercise group and 9 years in controls. Half of the patients in both groups had received third level education, and only a small proportion of patients were in current employment.
There were no statistically significant differences in baseline medications between the groups.
Most of the patients were on disease-modifying anti-rheumatic drugs (DMARDs; 86% versus 79%, p = 0.716), with smaller numbers on biologic therapy. Duration of therapy was similar in both groups for all medication classes.
median Charlson co-morbidity score was 2 in the exercise group and 3 in the control group. A minority of patients self-reported memory deficits at baseline (11% versus 17%, p = 0.69).
Table 2 presents baseline data of disease activity, quality of life, body composition, cardiovascular fitness and cognition. Baseline CRP was 2.7 versus 3.2 (p = 0.633) and DAS28 was 2.37 versus 2.69 (p = 0.124) in exercise and control groups, respectively.
Global fatigue index was higher in the control group, 23.6 versus 13.1 (p = 0.018) with similar baseline HAQ scores (0.44 versus 0.88, p = 0.162).
Baseline weight, waist circumference, BMI and truncal fat were similar in both groups. About 25% of patients in the exercise group and 30% of controls had a BMI greater than 30 (p = 0.582).
Resting heart rate and blood pressure were similar in both groups. VO2Max values were categorized after assessment as described in methods. About 55% of the exercise group and 50% of controls were within the poor category, while only 35 and 37.5% had good fitness levels, respectively (p = 0.373). We were unable to record accurate VO2Max values for a minority of patients due to inability to walk on the treadmill, and so these patients were excluded from this part of the analysis.
Baseline grip strength was similar in both hands for both groups (p = 0.429).
Cognitive impairment (MoCA < 26) was recorded in 54% of patients in the exercise group and in 58% of controls (p = 0.785).
Assessments after 3-month exercise programme
Forty-five patients completed both baseline and 3-month assessments. The observed effects of our personalized physical exercise programme on disease activity, quality of life, body composition, cardiovascular fitness and cognition in the exercise group (n = 24), in comparison to non-exercise controls (n = 21), are presented in Table 3.
Disease activity and quality of life measurements
A significant improvement in serum CRP level was observed at 3 months for the exercise group (2.8 to 1.9, p = 0.002) compared with controls (3.1 to 3.2, p = 0.5). No difference was recorded in ESR or DAS28. In the exercise group, HAQ improved from 0.5 to 0.25 (p = 0.05) and GFI from 13.2 to 10.9 (p = 0.047), compared to 1.1 to 0.8 (p = 0.026) and 24.8 to 24.8 (p = 0.96), respectively.
Body composition
Patients in the exercise group had a significant reduction in median weight (68.9 to 67.9 kg, p = 0.005), waist circumference (94.0 to 91.4 cm, p = 0.0001), BMI (26.8 kg/m2 to 26.7 kg/m2, p = 0.009) and truncal fat percentage (37.3% to 36.2%, p = 0.004), while there was a non-significant increase in weight (72.6 to 72.7 kg, p = 0.094), no difference in waist circumference (91.4 cm, p = 0.274) and an increase in truncal fat from 37.2 to 37.4%, p = 0.522) at 3 months in the control group.
Cardiovascular fitness and grip strength
There was a reduction in resting heart rate (HR) for both groups, from 72 beats per minute to 68 (p = 0.014) in the exercise group and from 70 to 68 (p = 0.03) in the controls. There were no changes observed in blood pressure or serum cholesterol; however, there was a small improvement in glucose levels in the controls.
VO2Max was significantly improved in the exercise group at 3 months, compared to controls, from a median of 23.2 to 27.6 ml/kg/min, (p = 0.002) compared with 26.1 to 27.6 (p = 0.313) for controls. Right and left handgrip strength was improved in the exercise group compared with controls. Median right handgrip strength improved from 12.0 to 13.0 kg at 3 months (p = 0.025) in the exercise group, while the control group median values were 10.0 and 9.0 kg, respectively (p = 0.905). Similarly, median left handgrip strength increased from 8.0 to 10.0 kg (p = 0.005) compared with a non-significant increase from 8.0 to 9.0 kg at 3 months (p = 0.388). There was no change in dominant handgrip strength for either group.
Cognition
Median MoCA in the exercise group improved from 25.5 to 28.0 at 3 months (p = 0.001) compared with 25.0 to 27.0 (p = 0.214) for controls. There were no significant changes observed in the SART mean reaction time, variation reaction time or in the commission and omission errors at 3 months. Mean variation time for controls was 139.3 (46–289) at 0 months compared to 161.3 (65–396) at 3 months, equating to an increase of 14% (p = 0.004) and signifying a reduction in sustained attention. There was no significant difference in colour trails at 3 months for either group. These data are presented in Table 3.
Discussion
This study has demonstrated significant benefit from a 3-month personalized physical exercise programme in a cohort of RA patients compared with matched disease controls, across a range of measured variables including disease activity, quality of life, body composition, cardiovascular fitness, muscle strength and cognition. Many of these variables correlate directly with mortality, and all are associated with significant morbidity in RA populations. Significant improvements were observed for all primary outcome measurements (waist circumference, VO2Max, grip strength and MoCA), as well as CRP, fatigue (GFI) and HAQ scores.
A significant reduction in CRP level was observed in the exercise group. This is in keeping with previous findings of physical activity reducing systemic inflammation in RA populations [27, 28]. Elevated CRP levels have been associated with increased risk of cardiovascular disease (CVD) in healthy individuals [29], and this may be even more significant in RA populations, where an increased CVD risk already exists [30]. These present data, as well as the increasing evidence suggesting that physical activity reduces inflammation, supports a critical role for physical exercise in the management of RA patients.
There were significant improvements in HAQ disability index in both groups. Regular exercise has been shown to significantly improve symptoms, joint function and psychological well-being [9]. The commonest barrier to exercise reported by patients in the intervention group was fatigue, which was significantly reduced in our exercise group. A systematic review which explored the effectiveness of non-pharmacological interventions for fatigue concluded that both aerobic and resistance exercise reduce fatigue in RA patients [31] and a more recent study from our group showed improvements in fatigue scores in patients following an exercise programme [32].
We observed significant reductions in truncal fat and waist circumference. Positive effects of physical activity on body composition have been shown in other studies. Strasser et al. reported a reduction in body fat and gain in lean body mass after a 6-month combined strength and endurance programme [8]. Similarly, Häkkinen et al. confirmed significant increase in muscle mass in women with RA after a 21-week combined strength and endurance training programme [33]. Excess abdominal visceral fat carries an increased CVD risk in the general population as well as in patients with RA [34]. We measured truncal fat and waist circumference, both of which correlate well with visceral fat [34] and both of which showed significant improvements in our exercise group.
One of the main findings in our study was the consistent impact of physical activity on reducing factors associated with CVD, which is the main cause of mortality among RA patients [30]. This was demonstrated in the form of improved aerobic capacity, which is an independent predictor of CVD risk and mortality [35]. Improved VO2Max levels have been shown to reduce the prevalence and the severity of CVD in RA patients [7]. At the beginning of the study period, most patients fell into the poor fitness level category with significant improvements in VO2Max levels observed after exercise. This is in keeping with existing data on fitness levels and exercise in RA patients. Stavropoulos et al. reported a 10% improvement in VO2Max after 3 months of individualized aerobic and resistance training in RA patients [7]. Similarly, Strasser et al. showed a 10% improvement in cardiorespiratory endurance in RA patients after a six-month combined exercise and strength-training programme [8].
There was a significant improvement in grip strength in both hands for the exercise group. The loss of handgrip strength and function is a major cause of disability in patients with RA [36, 37]. Studies done by Pincus et al. reported that measures of functional status such as grip strength could be used to detect long-term morbidity and mortality in RA [38].
Ours is the only study to date that measures the effects of physical activity on cognition in RA patients. There was a significant improvement in MoCA in the exercise group after 3 months. Patients in the exercise group improved from a median value representing cognitive impairment to a median value which was comfortably within the normal range after our exercise programme. One of the domains of MoCA is executive function, which is crucial for cognitive processes such as working memory, reasoning, task flexibility, planning and problem-solving. There are a number of studies demonstrating positive effects of physical activity on executive function in non-RA populations. A meta-analysis by Smith et al. showed individuals with normal cognitive function randomized to aerobic exercise exhibited improved executive function [12]. Similarly, Smiley-Oyen et al. observed an improvement in speeded tasks (which relies on executive control) after a 10-month aerobic exercise programme involving adults aged 65–79 [39].
Our finding of improved MoCA scores in this RA population is novel and, if replicated, may have implications for the design of future management protocols and clinical trial end points in RA. No medication has been proven to reduce the risk of dementia or age-related cognitive impairment in RA patients. Structured physical exercise appears to be an important non-pharmacological intervention in this domain.
There are some limitations to our study. The number of patients in the study was small with patients lost to follow-up in both groups. Patients enrolled in the study had predominantly stable disease, as a certain degree of mobility and physical conditioning was required to complete the physical fitness assessments. Patients were not matched for pharmacological exposures, and while there were numerical differences between groups (with more patients in the exercise group on biological therapies), observed differences did not reach statistical significance. The study period might not have been long enough for some of the parameters to change significantly, such as lipid profile or blood pressure. Most of the patients who volunteered for the study were motivated and willing to make a lifestyle change, although the personalized nature of our programme did encourage broad participation across all levels of physical capabilities.
These data suggest that the completion of a personalized 3-month exercise programme has considerable benefits for RA patients with stable disease and should inform future design of management and clinical trial protocols.
Abbreviations
- RA:
-
Rheumatoid arthritis
- CVD:
-
Cardiovascular disease
- ACSM:
-
American College of Sports Medicine
- ACPA:
-
Anti-citrullinated protein antibody.
- DMARD:
-
Disease modifying anti-rheumatoid drug
- CRP:
-
c-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- DAS28:
-
Disease activity score
- HAQ:
-
Health assessment questionnaire
- GFI:
-
Global fatigue index
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- VO2Max:
-
Maximal oxygen consumption
- MoCA:
-
Montreal Cognitive Assessment
- SART:
-
Sustained attention reaction time
References
Davis JM 3rd, Matteson EL, American College of R, European League Against R (2012) My treatment approach to rheumatoid arthritis. Mayo Clin Proc 87:659–673
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376:1094–1108
Prete M, Racanelli V, Digiglio L, Vacca A, Dammacco F, Perosa F (2011) Extra-articular manifestations of rheumatoid arthritis: an update. Autoimmun Rev 11:123–131
Nadareishvili Z, Michaud K, Hallenbeck JM, Wolfe F (2008) Cardiovascular, rheumatologic, and pharmacologic predictors of stroke in patients with rheumatoid arthritis: a nested, case-control study. Arthritis Rheum 59:1090–1096
del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44:2737–2745
Katz PP, Yazdany J, Trupin L, Schmajuk G, Margaretten M, Barton J, Criswell LA, Yelin EH (2013) Sex differences in assessment of obesity in rheumatoid arthritis. Arthritis Care Res 65:62–70
Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y (2013) Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis 72:1819–1825
Strasser B, Leeb G, Strehblow C, Schobersberger W, Haber P, Cauza E (2011) The effects of strength and endurance training in patients with rheumatoid arthritis. Clin Rheumatol 30:623–632
Cooney JK, Law RJ, Matschke V et al (2011) Benefits of exercise in rheumatoid arthritis. J Aging Res 2011:681640
Sokka T, Hakkinen A, Kautiainen H et al (2008) Physical inactivity in patients with rheumatoid arthritis: data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum 59:42–50
Appenzeller S, Bertolo MB, Costallat LT (2004) Cognitive impairment in rheumatoid arthritis. Methods Find Exp Clin Pharmacol 26:339–343
Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A (2010) Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 72:239–252
Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC (2011) Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 86:876–884
Urbaniak GC, Plous S (2013) Research Randomizer (Version 4.0)
Nunez C, Gallagher D, Visser M, Pi-Sunyer FX, Wang Z, Heymsfield SB (1997) Bioimpedance analysis: evaluation of leg-to-leg system based on pressure contact footpad electrodes. Med Sci Sports Exerc 29:524–531
Smolen JS, Breedveld FC, Eberl G, Jones I, Leeming M, Wylie GL, Kirkpatrick J (1995) Validity and reliability of the twenty-eight-joint count for the assessment of rheumatoid arthritis activity. Arthritis Rheum 38:38–43
Sokka T, Kautiainen H, Hannonen P, Pincus T (2006) Changes in health assessment questionnaire disability scores over five years in patients with rheumatoid arthritis compared with the general population. Arthritis Rheum 54:3113–3118
Belza BL, Henke CJ, Yelin EH, Epstein WV, Gilliss CL (1993) Correlates of fatigue in older adults with rheumatoid arthritis. Nurs Res 42:93–99
Novaes GS, Perez MO, Beraldo MB, Pinto CR, Gianini RJ (2011) Correlation of fatigue with pain and disability in rheumatoid arthritis and osteoarthritis, respectively. Rev Bras Reumatol 51:451–455
Schmidt RT, Toews JV (1970) Grip strength as measured by the Jamar dynamometer. Arch Phys Med Rehabil 51:321–327
Bruce RA (1971) Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res 3:323–332
Bruce RA, Kusumi F, Hosmer D (1973) Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85:546–562
Karvonen MJ, Kentala E, Mustala O (1957) The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 35:307–315
Heyward V (2010) Advanced fitness assessment and exercise prescription, 6th edn. Human Kinetics
Nasreddine ZS, Phillips NA, Bedirian V et al (2005) The Montreal cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699
Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39:1435–1445
Hakkinen A, Sokka T, Kotaniemi A, Hannonen P (2001) A randomized two-year study of the effects of dynamic strength training on muscle strength, disease activity, functional capacity, and bone mineral density in early rheumatoid arthritis. Arthritis Rheum 44:515–522
van den Ende CH, Breedveld FC, le Cessie S, Dijkmans BA, de Mug AW, Hazes JM (2000) Effect of intensive exercise on patients with active rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 59:615–621
Church TS, Barlow CE, Earnest CP, Kampert JB, Priest EL, Blair SN (2002) Associations between cardiorespiratory fitness and C-reactive protein in men. Arterioscler Thromb Vasc Biol 22:1869–1876
van Breukelen-van der Stoep DF, Klop B, van Zeben D, Hazes JM, Castro CM (2013) Cardiovascular risk in rheumatoid arthritis: how to lower the risk? Atherosclerosis 231:163–172
Neill J, Belan I, Ried K (2006) Effectiveness of non-pharmacological interventions for fatigue in adults with multiple sclerosis, rheumatoid arthritis, or systemic lupus erythematosus: a systematic review. J Adv Nurs 56:617–635
Durcan L, Wilson F, Cunnane G (2014) The effect of exercise on sleep and fatigue in rheumatoid arthritis: a randomized controlled study. J Rheumatol 41:1966–1973
Hakkinen A, Pakarinen A, Hannonen P et al (2005) Effects of prolonged combined strength and endurance training on physical fitness, body composition and serum hormones in women with rheumatoid arthritis and in healthy controls. Clin Exp Rheumatol 23:505–512
Uutela T, Kautiainen H, Jarvenpaa S, Salomaa S, Hakala M, Hakkinen A (2014) Waist circumference based abdominal obesity may be helpful as a marker for unmet needs in patients with RA. Scand J Rheumatol:43, 279–285
Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS Jr, Blair SN (1999) Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282:1547–1553
Fraser A, Vallow J, Preston A, Cooper RG (1999 Please note theat reference sblah and blajh have the same bib details. Please delete the duplicated reference and renumber the affected ref citations) Predicting 'normal' grip strength for rheumatoid arthritis patients. Rheumatology 38:521–528
Dellhag B, Bjelle A (1999) A five-year followup of hand function and activities of daily living in rheumatoid arthritis patients. Arthritis Care Res 12:33–41
Pincus T (2005) Rheumatology function tests: quantitative physical measures to monitor morbidity and predict mortality in patients with rheumatic diseases. Clin Exp Rheumatol 23:S85–S89
Smiley-Oyen AL, Lowry KA, Francois SJ, Kohut ML, Ekkekakis P (2008) Exercise, fitness, and neurocognitive function in older adults: the "selective improvement" and "cardiovascular fitness" hypotheses. Ann Behav Med 36:280–291
Author information
Authors and Affiliations
Contributions
Study design: MA, CL, FW, and GC. Patient recruitment and assessments: MA, CC, TOD. Data analysis and interpretation: MA, CL, and GC. Drafting of manuscript and review of manuscript for intellectual content: MA, CL, and GC. All authors have reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13.7 kb)
Rights and permissions
About this article
Cite this article
Azeez, M., Clancy, C., O’Dwyer, T. et al. Benefits of exercise in patients with rheumatoid arthritis: a randomized controlled trial of a patient-specific exercise programme. Clin Rheumatol 39, 1783–1792 (2020). https://doi.org/10.1007/s10067-020-04937-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-04937-4