Abstract
Introduction/objectives
This study was to investigate the role of pelvic incidence (PI) in the development of Andersson lesions (ALs) in ankylosing spondylitis (AS) patients with kyphosis and to evaluate the difference in sagittal spinopelvic parameters between inflammatory ALs and mechanical ALs.
Method
A total of 135 AS patients with kyphosis were reviewed. The patients were classified into AL group and non-AL group based on the presence or absence of ALs. Additionally, AS patients with ALs were also classified as either inflammatory or mechanical lesions depending on the radiological features of the lesions. The sagittal spinopelvic parameters of all these AS patients were measured and compared. Logistic regression analysis was performed to determine the powerful variables for predicting ALs in AS patients.
Results
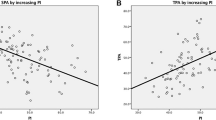
ALs were detected in 34 patients (25.2%) of the total 135 AS patients. The mean PI of the AL group was 40.0°, which was significantly lower than that (48.3°) of the non-AL group (P < 0.001). No statistically significant difference was observed in PI (P = 0.350) between the inflammatory lesion group and the mechanical lesion group. Logistic regression analysis showed that only PI was a statistically significant risk factor for ALs (P < 0.001) and was negatively correlated with ALs (odds ratio = 0.76).
Conclusions
These data suggest that low PI is closely associated with ALs in AS patients with kyphosis and that it might be a possible risk factor for the development of ALs. Moreover, both inflammatory and mechanical ALs patients had similarly low PI.
Key Points • Low PI was closely associated with ALs in AS patients with kyphosis and might be a possible risk factor for development of ALs. • Either inflammatory or mechanical ALs patients had similar low PI. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Andersson lesions (ALs) are a type of destructive vertebral or discovertebral lesion that occurs as a late complication in ankylosing spondylitis (AS) [1], and these lesions were first described by Andersson in 1937 [2]. The reported prevalence of ALs in AS patients ranges dramatically from 1% to over 28% [1,2,3]. Progressive localized thoracolumbar pain or sharp localized pain after minor trauma is the main complaint in the patients with ALs and is often accompanied by evolutional spinal kyphotic deformity [1, 4]. Neurologic symptoms, such as radiculopathy or spinal cord compression, are also occasionally observed [5]. Since the initial study of Andersson, these lesions of the spine in AS have been termed spondylodiscitis, vertebral lesions, destructive vertebral lesions, discitis, spinal pseudarthrosis, or (stress) fractures [1,2,3]. Two main theories, including inflammatory and traumatic/mechanical lesions, have been proposed to explain the cause and pathology of these lesions [2]. However, the exact etiology of ALs and the factors that are important for the development of ALs remain unclear [1, 2, 6].
In recent years, the sagittal morphology of the spine and pelvis has become one of the topics of most focus in spine research. Pelvic incidence (PI) is an individual, static, and position-independent anatomic sacropelvic parameter [7,8,9] that was first introduced by Legaye et al. [10]. PI is a critical measurement in the evaluation of overall spinopelvic balance, and it is defined as the angle between the line perpendicular to the sacral plate at its midpoint and the line connecting this point to the axis of the femoral heads [10]. PI equals the sum of the sacral slope (SS) and pelvic tilt (PT) (PI = SS + PT). Numerous studies have shown that abnormal PI is a risk factor for sagittal imbalance of the spine, such as spondylolisthesis, and these studies have found that PI might be related to certain spinal pathologies [11,12,13].
Previous studies have demonstrated that AS patients have abnormal spinopelvic balance and pelvic morphology, but the correlation between sagittal spinopelvic parameters and the development of spinal disorders in patients with AS has not yet been confirmed [14,15,16,17]. To the best of our knowledge, however, no published studies have analyzed the potential effects of abnormal PI on ALs in AS patients. Hence, the objectives of the present study were to investigate the role of PI in the development of ALs in AS patients with kyphosis and to evaluate the difference in sagittal spinopelvic parameters between inflammatory ALs and mechanical ALs.
Materials and methods
Participants
We performed a retrospective review of 256 consecutive AS patients with kyphosis who underwent spinal surgery at our center between January 2011 and December 2016. All AS patients met the most recent modified New York criteria [18]. The inclusion criteria were (1) kyphotic deformity with global kyphosis > 40° [19], (2) no scoliosis or a coronal curve < 10°, and (3) no hip flexion contractures. The exclusion criteria were (1) previous spine surgery, (2) previous hip or lower extremity surgery, (3) preoperative infection of the spine, and (4) preoperative acute spinal fractures. Therefore, this study included 135 patients (121 males and 14 females) with a mean age of 37.7 years old (range from 19 to 65). The present study was approved by the Clinical Research Ethics Committee of our institution.
All these 135 patients with AS underwent spinal surgery mainly for kyphotic deformity. All of them were examined by X-ray and CT; however, only part of them underwent MRI. The patients were classified into two groups based on the presence or absence of ALs (AL group and non-AL group) according to the features of X-ray, CT, and MRI. Additionally, AS patients with ALs were also classified into an inflammatory lesion group or a mechanical lesion group, depending on the previously reported radiological characteristics of the lesions [1, 2]. The criteria for the diagnosis of inflammatory ALs are narrow disc space, abnormal radiodensity of the adjacent vertebrae, and erosional end plate but without severely destructed vertebral bodies on X-ray or CT images. On MRI, low signal intensity of the vertebral bodies adjacent to intervertebral disc was seen on the T1-weighted images and high signal intensity of the corresponding vertebras was noticeable on the T2-weighted images. The criteria for the diagnosis of mechanical ALs are irregular vertebral or discovertebral osteolysis, destruction of intervertebral disc with surrounding reactive sclerosis and hypertrophy, and pseudarthrosis at the lesion on X-ray or CT images. On the T1-weighted images, low signal intensity of the mechanical lesion was noticeable. T2-weighted images may reveal irregular mixture signal intensity in the central destructive zone and reduced signal intensity in the surrounding area.
Measures
Full-length spine radiographs that included the whole spine and pelvis of AS patients who were standing in a neutral and unsupported position were taken preoperatively. The following radiographical parameters were assessed: the PI, the angle between the line perpendicular to the sacral plate at its midpoint and the line connecting this point to the center of the femoral heads; PT, the angle between the line drawn from the midpoint of the sacral plate to the center of the femoral heads and the vertical axis; SS, the angle between the line along the sacral plate and the horizontal line; thoracic kyphosis (TK), the angle between the upper end plate of T4 and the lower end plate of T12; thoracolumbar kyphosis (TLK), the angle between the upper end plate of T11 and the lower end plate of L2; lumbar lordosis (LL), the angle between the upper end plate of L1 and the upper end plate of S1; sagittal vertical axis (SVA), the distance between the C7 plumb line (C7PL) and the posterosuperior corner of S1; global kyphosis (GK), the angle between the superior endplate of the maximally tilted upper end vertebra and the inferior endplate of the maximally tilted lower end vertebra; and T1 pelvic angle (TPA), the angle between the line from the femoral heads to the center of the T1 vertebral body and the line from the femoral heads to the center of the superior sacral end plate [20]. For GK, TK, TLK, and LL, the angle was negative if the curve was lordotic and was positive if the curve was kyphotic. Surgimap (New York, NY, USA) was used to measure all the parameters. All measurements were performed twice independently by 3 spine surgeons with an interval of 2 weeks between measurements.
Statistical analysis
The statistical analyses were performed using IBM SSPS (version 24, IBM Corp.). Continuous variables are presented as the means ± standard deviations. The two groups were compared with Student’s t test and the chi-square test. Stepwise multiple logistic regression was used to determine the powerful variables for predicting ALs in AS patients. In the logistic regression analysis, multicollinearity was assessed as negative based on a variance inflation factor < 10 and a coefficient tolerance > 0.1. P < 0.05 was considered statistically significant.
Results
Andersson lesions were detected in 34 patients (25.2%) of the total 135 AS patients with kyphosis. Inflammatory lesions were identified in 22 segments in 15 patients, and mechanical lesions were found in 19 segments in 19 patients (Table 1 and Figs. 1 and 2). The most common levels were L1–2 in inflammatory lesions and T12-L1 in mechanical lesions.
Preoperative lateral X-ray of a 45-year-old male patient with a mechanical Andersson lesion at T12-L1 showed kyphotic deformity (a). The value of PI was 40.2°. Preoperative CT showed severe destruction of intervertebral disc with surrounding reactive sclerosis, irregular osteolysis, and pseudarthrosis at T12-L1 (b). Postoperative lateral X-ray showed the kyphosis was corrected by posterior instrumentation and fusion (c)
Preoperative lateral X-ray of a 34-year-old female patient with inflammatory Andersson lesions at L1–2 and L2–3 showed kyphotic deformity (a). The value of PI was 41.8°. Preoperative CT showed narrow disc space, abnormal radiodensity of the adjacent vertebrae, and erosional end plate but without severe destruction at the same levels (b). Postoperative lateral X-ray showed the kyphosis was corrected by posterior L3 pedicle subtraction osteotomy
The comparison of sagittal spinopelvic parameters between the AL and non-AL groups is presented in Table 2. The mean PI of the AL group was 40.0°, which was significantly lower than that (48.3°) of the non-AL group (P < 0.001). Similarly, the PT (34.6° vs. 39.8°, P = 0.034), SVA (137.4 mm vs. 165.9 mm, P = 0.037), and TPA (40.8° vs. 48.1°, P = 0.007) of the AL group were significantly lower than those of the non-AL group. The TLK (39.6° vs. 33.5°, P = 0.026) of the AL group was significantly higher than that of the non-AL group. No significant differences were observed between the two groups in terms of SS (P = 0.119), TK (P = 0.124), LL (P = 0.545), and GK (P = 0.188).
Table 3 shows the sagittal spinopelvic parameters of AS patients with ALs. The PI (P = 0.350), PT (P = 0.345), SS (P = 0.513), TK (P = 0.809), TLK (P = 0.950), LL (P = 0.080), and GK (P = 0.148) did not show any statistically significant differences between the two groups (inflammatory lesion group and mechanical lesion group). The mean PI was 40.8 ± 5.4° in the inflammatory lesion group and 39.4 ± 3.3° in the mechanical lesion group. However, the SVA (P = 0.009) and TPA (P = 0.011) were significantly higher in the inflammatory lesion group than in the mechanical lesion group.
According to the comparisons between the AL group and the non-AL group, the PI, PT, SVA, TPA, and TLK were all considered for multivariate analysis. Multivariate logistic regression analysis showed that only PI was a statistically significant risk factor for ALs (P < 0.001) and was negatively correlated with ALs (odds ratio = 0.76; 95% CI, 0.66–0.87) (Table 4).
Discussion
ALs represent a well-known complication in the late stage of AS, and they are most commonly observed in middle-aged patients (63–86%) with long-standing AS [21]. AL can cause back pain, spinal pseudarthrosis, and progressive kyphosis deformities, which may lead to sagittal imbalance [4]. The reported prevalence of ALs in the literature varies greatly, ranging from 1% to over 28% [1,2,3]. In our study, 25.2% of AS patients had Andersson lesions, among which 11.1% were inflammatory lesions and 14.1% were mechanical lesions. However, Park et al. [2] investigated 622 patients with AS and found that the prevalence of ALs was 5.3%, in which 3.9% were inflammatory lesions and 1.4% were mechanical lesions. The difference between these two studies might be mainly due to the different AS populations included. The patients in our study were advanced cases with kyphosis, who only presented one subset of AS. Thus, the prevalence of ALs in our patients was possibly higher than that in the whole AS population.
Until now, the exact etiology of ALs has been unknown. Several possibilities for the cause and pathology of ALs have been postulated in the literature, including infection, inflammation, trauma, and mechanical stress [1, 22]. However, most authors suggested either an inflammatory or a traumatic/mechanical etiology when describing ALs in studies [1,2,3,4]. Bron et al. [1] summarized the possible etiologies into three different groups: (1) localized lesions that always had an inflammatory origin; (2) extensive lesions without fracture of posterior elements, which had a combination of inflammatory and mechanical origins; and (3) extensive lesions with fracture of posterior elements that had a mechanical origin. Nikolaisen et al. [23] proposed a primary inflammatory origin for ALs according to the early immunohistopathological findings in a severe symptomatic spondylodiscitis patient with AS. Fang et al. [24] assumed that trauma without an inflammatory element was the primary cause of extensive lesions based on the histological observations in a study of 35 patients with AS. Recently, Qiao et al. [6] also reported a traumatic origin rather than an inflammatory origin for AS-related pseudarthrosis by analyzing the radiological and histological findings of 18 patients with extensive Andersson lesions. The differential opinions among these authors may be explained by the heterogeneous group of lesions and the lack of proper and generally accepted diagnostic criteria [1].
PI, one of the spinopelvic parameters, has the advantage of position independence and remains unchanged regardless of the motions or postures of the spine or pelvis [9]. It has been well recognized that PI regulates the sagittal alignment of the spine, pelvis, and hips [7, 13] and determines the ability of pelvic compensation for sagittal imbalance [25]. In the literature, a large number of investigations have demonstrated that either low PI or high PI is associated with spinal or hip pathology [8, 11,12,13, 26,27,28]. It is understandable that the differential roles of PI in the development of these diseases have different mechanisms due to their own characteristics of pathogenesis. To our knowledge, this is the first study to investigate the role of PI in the development of ALs. Our data showed that AS patients with ALs had a lower PI (40.0° vs. 48.3°, P < 0.001) than those without ALs, while there was no difference in PI between the inflammatory lesion and mechanical lesion groups (40.8° vs. 39.4°, P = 0.350). The present study also found that a lower PI value might be a risk factor for the development of ALs in AS patients (odds ratio = 0.76, P < 0.001).
With regard to the relationship between a low PI and ALs, we suggested there might be two possible explanations. One explanation is the role of PI in the regulation of other spinal parameters, such as LL. The low PI has been associated with flattening, hence decreasing, of the lumbar lordosis which is frequently found in AS [7, 25]. The decrease of lumbar lordosis in AS is generally associated with co-occurrence of thoracic or thoracolumbar kyphosis, which may normally slide the plumb line anteriorly [14, 15]. In that case, we would expect an increase of loading on anterior side of motion segment. This abnormal mechanical stress may be related to lesions in AS. The other explanation is that the compensatory capacity of patients with a low PI for severe kyphosis is less than that of patients with a high PI [15]. To maintain sagittal balance and an upright posture, AS patients with thoracolumbar kyphosis need to initiate several compensatory mechanisms, such as retroversion of the pelvis, extension of the hips, and flexion of the ankles and knees [25]. Among these compensatory mechanisms, pelvic retroversion is the major accommodation of kyphotic deformities in AS patients [15, 19, 29]. The SS, demonstrating the orientation of the sacral plate, decreases and the PT increases when the pelvis retroverts. According to the relationship among the PI, PT, and SS, PI = SS + PT, the pelvic incidence angle, which is the patient’s native anatomic parameter, determines the orientation of the sacral plate and the ability of pelvic retroversion [19, 25, 30]. Therefore, AS patients with either a high PI or low PI can compensate for the same degree of spinal kyphosis through pelvic retroversion, as long as the deformity is not severe enough to exceed the maximum compensation of pelvis (Fig. 3). When the kyphotic deformity progresses beyond the maximum compensation for pelvic retroversion, AS patients with a low PI are not able to compensate; however, AS patients with a high PI have good capacity for compensation of the sagittal imbalance by producing a large posterior tilt of the pelvis under the circumstances of similar deformities (Fig. 4). As a result, more stress may be applied to the spines of AS patients with a low PI than to those of patients with a high PI, especially to the thoracolumbar junction, thus resulting in the development of ALs.
When the kyphotic deformity progresses beyond the maximum compensation of pelvic retroversion, AS patients with a low PI are not able to compensate, which might induce more stress on the spine. However AS patients with a high PI have greater capacity of compensation for the sagittal imbalance under the circumstances of similar deformities
It was reported that the incidence of pseudarthrosis in kyphotic AS patients was higher than that in AS patients without kyphosis [4]. In this study, we also found that TLK of the AL group was significantly higher compared with that of the non-AL group, which indicated the kyphotic deformity might be associated with ALs. However, multivariate logistic regression analysis showed that TLK was not the risk factor for ALs. In addition, there were no significant differences in TK and GK between the AL group and the non-AL group. Therefore, the causal association between the location and severity of kyphosis and AL development was not still confirmed. The relationship among PI, kyphosis, and ALs was possibly more complex, which might involve more other factors, such as full body sagittal alignment and the range of movement of hips or knees. More further researches are needed to analyze this relationship in the future.
Previous studies have mainly focused on imaging appearances according to the possible etiologies of ALs [1, 2, 22]. In our study, the mechanical ALs were mostly observed at T12-L1 and the inflammatory lesions were mostly at L1–2. The segments most frequently affected by ALs were those of the thoracolumbar junction, which was consistent with findings from previous studies [1, 4]. The stresses at the thoracolumbar junction are radically increased compared with other levels in AS patients with kyphosis [2, 4]. Since all ALs patients had no previous histories of trauma in the present study, the mechanical stress theory for these lesions might be supported. However, the inflammatory lesions usually affect multiple levels, while the mechanical lesions often involve only one single level [2], which were also confirmed by our study. The levels and distribution of lesions, as well as the radiological characteristics of lesions, were different between the mechanical and inflammatory groups, which indicated that the mechanical and inflammatory lesions might have different origins of etiology. For the cause of inflammatory lesions, the inflammatory process combined with its mechanical effects on the spine has become widely accepted [1].
To the best of our knowledge, however, there has been minimal investigation into the spinopelvic parameters of differential lesions in the patients with ALs. The present study also analyzed the spinopelvic parameters in different groups of ALs. The spinopelvic parameters in the inflammatory lesion group, including the PI, PT, SS, TK, TLK, LL, and GK, were similar to those in the mechanical lesion group. The result of a low PI in both the inflammatory and mechanical lesion groups might imply that biomechanical factors possibly play a role in the progression of both subtypes of ALs. Furthermore, the SVA and TPA, which indicate the severity of sagittal imbalance, were significantly higher in the inflammatory lesion group than in the mechanical lesion group. Similarly, the SVA and TPA in the non-AL group were significantly larger than those in the AL group. The specific reasons for this difference are still unclear; perhaps, the symptoms of ALs, especially those of mechanical lesions, are more obvious, prompting patients to come to the clinic before experiencing obvious or severe deformity.
There are some limitations to our study that require consideration. First, the present study was essentially a retrospective review. Therefore, a prospective study with a long follow-up may be needed to further confirm the role of PI in the development of ALs. Second, it was intended as a preliminary radiographic study focusing on spinopelvic parameters. However, many other factors, such as body weight, bone mineral density, or peripheral joint involvement, have been identified to be associated with ALs [3], and these other factors were not included in this investigation. Furthermore, the number of patients with ALs was relatively small, and only AS patients with kyphosis deformities were included, who did not present the whole AS population. As such, selection bias existed in our study. In the further investigation, more subsets of AS patients should be included.
In summary, this study demonstrated that a low PI was closely associated with ALs in AS patients with kyphotic deformity and that a low PI might be a possible risk factor for the development of ALs. Furthermore, both inflammatory and mechanical AL patients had a similar low PI, which implied that biomechanical factors possibly played a role in the progression of both subtypes of ALs. The relationship between PI and ALs had not been previously published and deserved attention in terms of the operative design of AS patients and in future investigations.
References
Bron JL, de Vries MK, Snieders MN, van der Horst-Bruinsma IE, van Royen BJ (2009) Discovertebral (Andersson) lesions of the spine in ankylosing spondylitis revisited. Clin Rheumatol 28(8):883–892. https://doi.org/10.1007/s10067-009-1151-x
Park YS, Kim JH, Ryu JA, Kim TH (2011) The Andersson lesion in ankylosing spondylitis: distinguishing between the inflammatory and traumatic subtypes. J Bone Joint Surg Br Vol 93(7):961–966. https://doi.org/10.1302/0301-620X.93B7.26337
Shaik I, Bhojraj SY, Prasad G, Nagad PB, Patel PM, Kashikar AD, Kumar N (2018) Management of Andersson lesion in ankylosing spondylitis using the posterior-only approach: a case series of 18 patients. Asian Spine J 12(6):1017–1027. https://doi.org/10.31616/asj.2018.12.6.1017
Kim KT, Lee SH, Suk KS, Lee JH, Im YJ (2007) Spinal pseudarthrosis in advanced ankylosing spondylitis with sagittal plane deformity: clinical characteristics and outcome analysis. Spine 32(15):1641–1647. https://doi.org/10.1097/BRS.0b013e318074c3ce
Jobanputra P, Kirkham B, Duke O, Crockard A, Panayi GS (1988) Discovertebral destruction in ankylosing spondylitis complicated by spinal cord compression. Ann Rheum Dis 47(4):344–347
Qiao M, Qian BP, Qiu Y, Mao SH, Wang YH (2019) Radiologic and pathological investigation of Pseudarthrosis in ankylosing spondylitis: distinguishing between inflammatory and traumatic etiology. J Rheumatol 46(3):259–265. https://doi.org/10.3899/jrheum.171249
Boulay C, Tardieu C, Hecquet J, Benaim C, Mouilleseaux B, Marty C, Prat-Pradal D, Legaye J, Duval-Beaupere G, Pelissier J (2006) Sagittal alignment of spine and pelvis regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J 15(4):415–422. https://doi.org/10.1007/s00586-005-0984-5
Carender CN, Morris WZ, Poe-Kochert C, Thompson GH, Son-Hing JP, Liu RW (2016) Low pelvic incidence is associated with proximal junctional kyphosis in patients treated with growing rods. Spine 41(9):792–797. https://doi.org/10.1097/BRS.0000000000001352
Weinberg DS, Morris WZ, Gebhart JJ, Liu RW (2016) Pelvic incidence: an anatomic investigation of 880 cadaveric specimens. Eur Spine J 25(11):3589–3595. https://doi.org/10.1007/s00586-015-4317-z
Legaye J, Duval-Beaupere G, Hecquet J, Marty C (1998) Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. Eur Spine J 7(2):99–103
Hanson DS, Bridwell KH, Rhee JM, Lenke LG (2002) Correlation of pelvic incidence with low- and high-grade isthmic spondylolisthesis. Spine 27(18):2026–2029
Toy JO, Tinley JC, Eubanks JD, Qureshi SA, Ahn NU (2012) Correlation of sacropelvic geometry with disc degeneration in spondylolytic cadaver specimens. Spine 37(1):E10–E15. https://doi.org/10.1097/BRS.0b013e3182257bb0
Bae JS, Jang JS, Lee SH, Kim JU (2012) Radiological analysis of lumbar degenerative kyphosis in relation to pelvic incidence. Spine J 12(11):1045–1051. https://doi.org/10.1016/j.spinee.2012.10.011
Lee JS, Suh KT, Kim JI, Goh TS (2014) Analysis of sagittal balance of ankylosing spondylitis using spinopelvic parameters. J Spinal Disord Tech 27(3):E94–E98. https://doi.org/10.1097/BSD.0b013e31829186c1
Debarge R, Demey G, Roussouly P (2010) Radiological analysis of ankylosing spondylitis patients with severe kyphosis before and after pedicle subtraction osteotomy. Eur Spine J 19(1):65–70. https://doi.org/10.1007/s00586-009-1158-7
Shin JK, Lee JS, Goh TS, Son SM (2014) Correlation between clinical outcome and spinopelvic parameters in ankylosing spondylitis. Eur Spine J 23(1):242–247. https://doi.org/10.1007/s00586-013-2929-8
Wang T, Zhao Y, Zheng G, Wang Y, Wang C, Wang Z, Wang Y (2018) Can pelvic tilt be restored by spinal osteotomy in ankylosing spondylitis patients with thoracolumbar kyphosis? A minimum follow-up of 2 years. J Orthop Surg Res 13(1):172. https://doi.org/10.1186/s13018-018-0874-2
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368
Qian BP, Jiang J, Qiu Y, Wang B, Yu Y, Zhu ZZ (2014) The presence of a negative sacral slope in patients with ankylosing spondylitis with severe thoracolumbar kyphosis. J Bone Joint Surg Am 96(22):e188. https://doi.org/10.2106/JBJS.M.01070
Protopsaltis T, Schwab F, Bronsard N, Smith JS, Klineberg E, Mundis G, Ryan DJ, Hostin R, Hart R, Burton D, Ames C, Shaffrey C, Bess S, Errico T, Lafage V, International Spine Study G (2014) TheT1 pelvic angle, a novel radiographic measure of global sagittal deformity, accounts for both spinal inclination and pelvic tilt and correlates with health-related quality of life. J Bone Joint Surg Am 96(19):1631–1640. https://doi.org/10.2106/JBJS.M.01459
Barman A, Sinha MK, Rao PB (2016) Discovertebral (Andersson) lesion of the ankylosing spondylitis, a cause of autonomic dysreflexia in spinal cord injury. Spinal Cord Ser Cases 2:16008. https://doi.org/10.1038/scsandc.2016.8
Dave BR, Ram H, Krishnan A (2011) Andersson lesion: are we misdiagnosing it? A retrospective study of clinico-radiological features and outcome of short segment fixation. Eur Spine J 20(9):1503–1509. https://doi.org/10.1007/s00586-011-1836-0
Nikolaisen C, Nossent H (2005) Early histology in ankylosing spondylitis related spondylodiscitis supports its inflammatory origin. Scand J Rheumatol 34(5):396–398. https://doi.org/10.1080/03009740510026625
Fang D, Leong JC, Ho EK, Chan FL, Chow SP (1988) Spinal pseudarthrosis in ankylosing spondylitis. Clinicopathological correlation and the results of anterior spinal fusion. J Bone Joint Surg Br Vol 70(3):443–447
Roussouly P, Pinheiro-Franco JL (2011) Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J 20(Suppl 5):609–618. https://doi.org/10.1007/s00586-011-1928-x
Gebhart JJ, Weinberg DS, Bohl MS, Liu RW (2016) Relationship between pelvic incidence and osteoarthritis of the hip. Bone Joint Res 5(2):66–72. https://doi.org/10.1302/2046-3758.52.2000552
Gebhart JJ, Bohl MS, Weinberg DS, Cooperman DR, Liu RW (2015) Pelvic incidence and acetabular version in slipped capital femoral epiphysis. J Pediatr Orthop 35(6):565–570. https://doi.org/10.1097/BPO.0000000000000342
Cho KJ, Suk SI, Park SR, Kim JH, Kang SB, Kim HS, Oh SJ (2010) Risk factors of sagittal decompensation after long posterior instrumentation and fusion for degenerative lumbar scoliosis. Spine 35(17):1595–1601. https://doi.org/10.1097/BRS.0b013e3181bdad89
Le Huec JC, Aunoble S, Philippe L, Nicolas P (2011) Pelvic parameters: origin and significance. Eur Spine J 20(Suppl 5):564–571. https://doi.org/10.1007/s00586-011-1940-1
Labelle H, Roussouly P, Berthonnaud E, Transfeldt E, O'Brien M, Chopin D, Hresko T, Dimnet J (2004) Spondylolisthesis, pelvic incidence, and spinopelvic balance: a correlation study. Spine 29(18):2049–2054
Acknowledgments
The authors thank all patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Ethical approval
The study was approved by the Ethics Committee of Chinese PLA General Hospital. All study procedures were performed according to the ethical principles of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, Dy., Zheng, Gq., Wang, Th. et al. Low pelvic incidence is associated with Andersson lesions in ankylosing spondylitis patients with kyphosis. Clin Rheumatol 39, 1505–1512 (2020). https://doi.org/10.1007/s10067-019-04907-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-019-04907-5