Abstract
In rheumatoid arthritis and systemic lupus erythematosus, cardiovascular disease is frequently one of the leading causes of mortality or morbidity. Studies have shown that acute systemic inflammation and chronic systemic vasculitis are associated with endothelial dysfunction and atherosclerotic plaque formation, subsequently leading to cardiovascular disease. This meta-analysis aimed to explore the association of subclinical atherosclerosis and arterial stiffness in primary Sjogren’s syndrome. A comprehensive search of the MEDLINE and Embase databases was performed from date of inception through August 2017. The inclusion criterion was observational studies evaluating the association between primary Sjogren’s syndrome, subclinical atherosclerosis, and arterial stiffness by measuring pulse wave velocity (PWV) and intima–media thickness (IMT). Definitions of PSS and methods to assess PWV and IMT were recorded for each study. Different locations of IMT were evaluated including common carotid, internal carotid, and femoral arteries. The pooled mean difference (MD) of PWV and IMT and 95% confidence interval (CI) were calculated using a random-effect meta-analysis. The between-study heterogeneity of effect size was quantified using the Q statistic and I2. Data were extracted from eight observational studies involving 767 subjects. Pooled result demonstrated a significant increase in PWV in patients who have PSS compared with controls (MD = 1.30 m/s; 95% CI 0.48–2.12; p value = 0.002; I2 = 85%). Patients with PSS also have higher IMT (MD = 0.08 mm; 95% CI 0.04–0.11; p value < 0.01; I2 = 72%). Our study suggests that PSS is associated with arterial stiffness and subclinical atherosclerosis. Further studies need to be conducted to find the correlation of subclinical atherosclerosis in PSS with the cardiovascular event, the pathophysiological changes of arterial stiffness in PSS, and the benefit of statins, because controlling cardiovascular risk factors or disease activity could potentially help avoid progression of atherosclerosis to overt cardiovascular disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sjogren’s syndrome is a common rheumatic disease in the female population, especially in their 50s. It can present as primary Sjogren’s syndrome (PSS) or secondary to other underlying connective tissue diseases, most commonly rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE). Lymphocytic infiltration of lacrimal and salivary glands is the characteristic pathologic finding, causing keratoconjunctivitis sicca and xerostomia, which are the most common manifestations of this disease. However, this lymphocytic infiltration characteristic could also involve extra-glandular organs—these include thyroid, lungs, and the gastrointestinal, vasculitis, renal, central or peripheral nervous system—and includes hematological involvement. Cardiovascular involvement in PSS is less commonly seen. Acute pericarditis and myocarditis were reported as rare complications [1]. The widely known cardiovascular involvement of PSS is congenital heart block associated with anti-SSA and is one of the manifestations of neonatal lupus, yet this is a rare complication in adult patients [2]. In certain rheumatic diseases such as RA and SLE, cardiovascular disease is frequently a major cause of mortality or morbidity because studies have shown that acute systemic inflammation and chronic systemic vasculitis are associated with endothelial dysfunction, and cause atherosclerotic plaque formation, subsequently lead to cardiovascular disease. Necrotizing vasculitis of medium-sized vessels resembling polyarteritis nodosa can occur in patients with Sjogren’s syndrome [3]. However, whether this necrotizing vasculitis causes atherosclerosis remains uncertain. The pathophysiological mechanisms of atherosclerosis in PSS are also yet to be discovered.
Multiple studies reported subclinical atherosclerosis by measuring the pulse wave velocity (PWV) or artery intima–media thickness (IMT) as a surrogate test to evaluate the risk of cardiovascular diseases with mixed results. To explore the association of subclinical atherosclerosis and arterial stiffness in PSS, because it may progress into overt cardiovascular diseases, we perform this systemic review and meta-analysis to compare arterial stiffness parameters in subjects with a diagnosis of PSS to normal subjects.
Materials and methods
This systematic review and meta-analysis was conducted and reported according to the Meta-analysis Of Observational Studies in Epidemiology statement [4] and was registered in PROSPERO (registration number: CRD 42017058316).
Search strategy
Three authors (WCY, AS, SU) independently searched published studies indexed in MEDLINE and EMBASE from date of inception to August 2017. References of all selected studies were also examined. The following main search terms were used: Sjogren’s syndrome, pulse wave velocity, vascular stiffness, arterial stiffness, aortic stiffness, pulse pressure, pulse wave analysis, intima–media thickness. The full search terms used are detailed in the Supplemental Material and Methods.
Inclusion and exclusion criteria
This review included all published observational studies including cross-sectional, prospective cohort, retrospective cohort, and case-control studies that assessed the association of primary Sjogren’s syndrome and arterial stiffness. Reviews, case reports, and abstracts were excluded because their quality of studies could not be evaluated.
We included studies that recruited participants from the general population or used data from medical records from healthcare facilities. Participants were adults with primary Sjogren’s syndrome or healthy individuals and had arterial stiffness measured. We compared outcomes between patients who were diagnosed with primary Sjogren’s syndrome by American College of Rheumatology criteria [5], American-European Consensus criteria [6] for diagnosis of Sjogren’s syndrome, and participants who did not have primary Sjogren’s syndrome. Secondary Sjogren’s syndrome was excluded from our study. The main outcome of this study was aortic stiffness measured by pulse wave velocity (PWV) and intima–media thickness (IMT).
Data extraction
All authors independently reviewed the titles and abstracts of all citations that were identified. After abstracts were reviewed, data comparisons between the three investigators were conducted to ensure completeness and reliability. The inclusion criteria were independently applied to all identified studies. Differing decisions were resolved by consensus.
Full-text versions of potentially relevant papers identified in the initial screening were retrieved. Data concerning study design, the source of information, participant characteristics, Sjogren’s syndrome, and arterial stiffness assessment were independently extracted. We contacted the authors of the original reports to request any unpublished data. If the authors did not reply, we used the available data for our analyses.
Assessment of bias risk
A subjective assessment of the methodological quality of observational studies was evaluated by all three authors using the Newcastle–Ottawa Scale (NOS) [7], which is a quality assessment tool for non-randomized studies. It uses a “star system” based on three major perspectives: the selection of the study groups (0–4 stars, or 0–5 stars for cross-sectional studies), the comparability of the groups by controlling for important and additional relevant factors (0–2 stars), and the ascertainment of outcome of interest or exposure (0–3 stars). A total score of 3 or less was considered poor, 4–6 was considered moderate, and 7–10 was deemed high quality. We excluded studies from our meta-analysis if they had poor quality. Discrepant opinions between authors were resolved by consensus.
Statistical analysis
We performed a meta-analysis of the included studies using Review Manager 5.3 software from The Cochrane Collaboration to generate forest plot and funnel plot and Comprehensive Meta-Analysis 3.3 software from Biostat, Inc. to perform Egger’s regression test. We calculated the pooled mean difference (MD) of PWV and IMT of various anatomical sites. All outcomes were reported as effect estimate and its 95% confidence intervals (CIs) comparing between primary Sjogren’s syndrome and normal groups using a random-effects model. If a study reported multiple anatomical sites, we used outcomes from each site and calculated the pooled effect size with other studies. The mean and standard deviation (SD) of studies that reported median and its respective range or interquartile range (IQR) was calculated using formulas [8,9,10]. We excluded studies from meta-analysis and only presented the result with narrative description (qualitative analysis) when there were not sufficient data available for calculating pooled effect size. The heterogeneity of effect estimates across these studies was quantified using the Q statistic and I2 (p < 0.10 was considered statistically significant). The Q statistic compared the observed between-study dispersion and expected dispersion of the effect size, and was expressed in p value for statistical significance. An I2 is the ratio of true heterogeneity to total observed variation. An I2 of 0 to 40% was considered to exclude heterogeneity, of 30 to 60% was considered to represent moderate heterogeneity, of 50 to 90% was considered to represent substantial heterogeneity, and of 75 to 100% was considered to represent considerable heterogeneity [11]. Publication bias was assessed using funnel plot, Egger’s regression test [12].
Results
Description of included studies
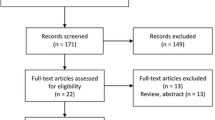
The initial search yielded 113 articles (Fig. 1); 103 articles were excluded based on the title and abstract review. A total of 10 articles underwent full-length review. Two articles were excluded (one article did not study the outcome of interest, and one article had no control group). Data were extracted from eight studies involving a total of 767 participants for qualitative analysis [13,14,15,16,17,18,19,20]. The included studies varied in study location, sample size, and source of data. Among the eight eligible studies that were included in the meta-analysis, two studies [16, 20] reported PWV, five studies [13, 15, 17,18,19] reported IMT, and one study [14] reported both PWV and IMT. The characteristics of the eight extracted studies included in this review were outlined in Table 1.
Meta-analysis results
The pooled result from three studies of PWV in PSS demonstrated a significant increase in PWV in patients who have PSS compared with controls (MD = 1.30 m/s; 95% CI 0.48–2.12; p value = 0.002; I2 = 85%) (Fig. 2). The pooled result from seven studies of IMT also showed that patients with PSS have higher IMT (MD = 0.08 mm; 95% CI 0.04–0.11; p value < 0.01; I2 = 72%) (Fig. 3).
Publication bias
To investigate potential publication bias, we examined the funnel plot of the included studies in the meta-analysis of the PWV and IMT (Figs. 4 and 5). The vertical axis represents study size (standard error) while the horizontal axis represents the difference in means. From these plots, there is an asymmetrical distribution of studies as depicted in the meta-analysis of PWV; no publication bias exists in the meta-analysis of IMT because the studies were symmetrically distributed. However, the Egger’s test results were not statistically significant in both meta-analyses of PWV (p = 0.29) and IMT (p = 0.49).
Discussion
To the best of our knowledge, this is the first meta-analysis to explore the association between PSS, arterial stiffness, and subclinical atherosclerosis by measuring pulse wave velocity and intima–media thickness. Our result showed a significant increased PWV and IMT compare to the control group, which implies that PSS is associated with arterial stiffness and subclinical atherosclerosis. The etiologies of vascular disease in PSS are not well understood. In general, the possible mechanisms of atherosclerosis in autoimmune diseases include side effects of medication, higher traditional cardiac risk factors, the disproportion between endothelial damage and repair, and production of pro-inflammatory cytokines [21]. Patients with PSS are more common in female than in male population with onset of disease in their 50s, which coincides with the menopausal period. Thus, the hormonal protective effect for cardiovascular diseases is diminishing. Secondly, patients with PSS are suffering from fatigue and depression and are associated with low quality of life as the disease progresses; hence a more sedentary lifestyle increases the prevalence of traditional risk factors and an imbalance in lipids. The LDL oxidation in the subendothelial space activates endothelial cells and macrophages to produce chemokines and adhesion molecules as the initial step in endothelial dysfunction [21]. Several studies reported a high level of cytokines and pro-inflammatory markers are likely the cause of atherosclerosis in PSS because they elicit the chemotaxis of dendritic cells, monocytes, and T cells into the intima. These markers include soluble thrombomodulin, anti-endothelial cell antibody, VCAM-1, ICAM-1, and asymmetric dimethylarginine [21]. The intensification of inflammatory process is also initiated by oxidized lipids through the activation of pattern recognition receptors of innate immunity and serves as autoantigens in the humoral and cellular immune responses. This process eventually leads to the infiltration of vascular smooth muscle into the plaque to form a fibrous cap [21].
Similarly, the atherosclerotic process in RA also involves the expression of ICAM and VCAM followed by aggregation of vascular monocytes after activation of the endothelium by innate and adaptive immune systems. The release of cytokines such as IL-1, IL-6, and TNF-a triggers the conversion of these monocytes to foam cells, as well as contributes to the reduction of nitric oxide release by changing the endothelial function. These processes are the harbinger of initial atherosclerotic lesions in RA [22]. The involvement of aforementioned pro-inflammatory cytokines in the pathophysiology of PSS [21] implies the possibility of an identical inflammation cascade in both diseases causing atherosclerosis. To conclude, endothelial dysfunction and flow-mediated dilatation impairment have led to an inflammatory cascade causing vascular fibrosis and smooth muscle proliferation, eventually causing arterial stiffness and atherosclerosis.
Multiple studies found that PWV and IMT are the acceptable non-invasive tests to evaluate subclinical atherosclerosis and arterial stiffness that can reliably predict subsequent cardiovascular disease morbidity and mortality [23,24,25]. There have not been many studies on the cardiovascular or cerebrovascular events in PSS patients and these studies’ results often are inconclusive. Thus we used PWV and IMT as surrogate tests. PWV is defined as the velocity of the arterial pulse moving along the vessel wall, and it is inversely related to the arterial distensibility [26]. It is calculated by measuring the time needed for pulse transit across the distance between two recording sites on the surface of the body. The calculation is based on the following formula: PWV (m/s) = distance (m)/transit time (s) [27]. Alternatively, carotid or femoral intima–media thickness also has been used to evaluate subclinical atherosclerosis by direct measurement of the intima–media thickness under sonography. Unfortunately, there are some limitations on both of the tests. First of all, PWV may not change significantly in early atherosclerotic aorta because it may remain elastic. The recording sites could also affect the accuracy of estimating the arterial stiffness since some arteries are more prone to overt atherosclerosis changes than the other (such as femoral artery being more susceptible to change than radial artery). Also, the PWV does not reflect the entire arterial tree measurement [24]. Finally, the increase of IMT may not be easily detected in the early stage of atherosclerosis.
Although our study reported an increased arterial stiffness and subclinical atherosclerosis, whether this develops into an overt cardiovascular or cerebrovascular disease remains debatable. Chiang et al. [28, 29] reported no increased risk of both diseases after adjusted for age, sex, hypertension, hyperlipidemia, diabetes, chronic kidney disease, and chronic pulmonary obstructive disease; while Kang and Lin also had not found such association [30]. On the contrary, Ramagopalan et al. [31] reported an increased risk of subarachnoid hemorrhage while Zoller et al. [32] reported an increased risk of ischemic stroke. However, there is no greater risk of hemorrhagic stroke in Zoller’s study. Bartoloni et al. [33] is the only study that reported a statistically significant increased risk of myocardial infarction and cerebrovascular disease in patients with PSS. Further study is needed to investigate the contribution of subclinical atherosclerosis causing cardiovascular or cerebrovascular events in PSS patients because the discordance findings probably mean that development of subclinical atherosclerosis is slow. Therefore, it would not have sufficient time to progress into an overt cardiovascular or cerebrovascular disease. The atherosclerotic changes in PSS patients could be due to less aggressive inflammation from lymphocytic infiltration than the more aggressive inflammation from neutrophils infiltration.
Nonetheless, chronic systemic inflammation is a risk factor for atherosclerosis [34]. Other studies on chronic inflammatory diseases such as gout, ankylosing spondylitis (AS), and RA have yielded a comparable result. Krasnokutsky et al. demonstrated a reduction of arterial endothelial function that is independent of other cardiovascular risk factors or comorbidities in patients with gout by measuring the flow-mediated dilation and nitroglycerin-mediated dilation [35]. Biesbroek et al. showed that the aortic arch PWV was increased in their cohort of patients with AS, even after controlling for age, BMI, systolic blood pressure, and heart rate on multivariate regression analysis [36]. Meantime, Rongen et al. revealed that vasodilator function was decreased in RA patients with a flare-up after suspending TNF inhibitor. This finding did not pertain for patients with inactive RA after discontinuing TNF inhibitor and those with the continuation of therapy [22].
Our study was unable to include studies that reported different outcome measurements because of insufficient data to perform an adequate and reliable meta-analysis. Cicek et al. [37] reported a significant decreased of aortic distensibility in patients with PSS. Akyel et al. [13] also reported disruption of flow-mediated dilatation, while Atzeni et al. [14] reported an impairment of coronary flow reserve. Aortic distensibility is inversely correlated to arterial stiffness; flow-mediated dilatation is a marker of endothelial dysfunction that reflects the response of blood vessels to an increase in flow by dilatation; while impairment of coronary flow reserve can accurately predict the presence of severe coronary stenosis. These results also support our study’s finding.
The limitation of our study is the inability to determine whether disease duration, disease severity, antibodies association, or treatment make any difference to the outcome. As we know, the longer the disease duration, or the more severe the disease is, theoretically, the more significant atherosclerotic plaque forms over time and may cause more significant disease. Alternatively, with treatment, it may reduce the inflammation from PSS and thereby minimize the progression of arterial stiffness. Zardi et al. [18] reported a significant correlation between disease duration and increased carotid IMT in PSS. The number of patients with PSS taking steroids, statins, or anti-hypertensive medications might affect the outcome [20], and we will not know how much these medications can help reduce the progression of atherosclerosis from our study. The restoration of arterial vasodilator and endothelial function after treatment with glucocorticoids, disease-modifying anti-rheumatic drugs (DMARDs), or biologic agents such as rituximab is inconclusive since the included studies in our meta-analysis either did not include subjects treated with these medications [17,18,19] or did not report subjects being treated [13]. Sabio et al. [16] reported no significant difference in number of prednisone (27% vs. 18%, p = 0.669), hydroxychloroquine (27% vs. 36%, p = 0.722), and other immunosuppressive drugs use (azathioprine, methotrexate, and mycophenolate mofetil) (15% vs. 15%, p = 0.09) between PSS patients with PWV > 9.5 m/s and PWV ≦ 9.5 m/s. Similar finding applies to Gravani et al. [15] as they did not find a statistically significant difference in current steroid dose (1.3 ± 2.3 mg vs. 1.1 ± 2.1 mg) and total steroid dose (7.8 ± 17 g vs. 6.6 ± 11.3 g) between PSS patients with IMT > 0.9 mm and IMT ≦ 0.9 mm. Meanwhile, there were only 13.7% and 9.6% of PSS patients on steroids and immunosuppressive drugs, respectively, in Demirci et al. [20] study. Lastly, Atzeni et al. [14] reported the majority of PSS patients were being treated with hydroxychloroquine 400 mg per day, and only a few of them received azathioprine (6 patients), methotrexate (4 patients), and steroids (7 patients).
At present, there is a lack of effective treatment for patients with PSS. The efficacies of DMARDs and biologic agents are based on researches on patients with secondary Sjogren’s syndrome, such as those with SLE or RA [38]. Due to increasing evidence of the pathogenic role of B cells in PSS, rituximab was studied in several randomized controlled trials but with mixed results as some reported improvement of salivary flow and VAS for pain, fatigue, and oral dryness, or vice versa [38]. Belimumab, a B-cell activating factor (BAFF) inhibitor, was showed to improve at least two of five disease indicators, including pain, dryness, fatigue, systemic activity, and B cell biomarkers in 60% of the patients in an open-labeled trial (BELISS), yet no change in salivary flow and Schirmer’s test [38, 39]. As a type I interferon pathway modulator, the efficacy of hydroxychloroquine is not as promising as shown in a randomized controlled trial (JOQUER) compared to the results of open-labeled studies [38, 39]. However, these studies did not assess the improvement of cardiovascular parameters. Thus, further studies evaluating improvement of PWV and IMT with rituximab or belimumab therapy could be considered.
Conclusion
In conclusion, our study suggests that PSS is associated with arterial stiffness and subclinical atherosclerosis. More large-scale studies need to be conducted to find the correlation of subclinical atherosclerosis in PSS with the cardiovascular or cerebrovascular event, and also the pathophysiology of increased arterial stiffness in PSS. Randomized controlled trials or prospective cohort studies to explore the benefit of statins, controlling cardiovascular risk factors or disease activity could potentially help providers in improving patients’ general health and avoiding progression of atherosclerosis.
References
Gyongyosi M, Pokorny G, Jambrik Z, Kovacs L, Kovacs A, Makula E, Csanady M (1996) Cardiac manifestations in primary Sjogren's syndrome. Ann Rheum Dis 55(7):450–454
Sung MJ, Park SH, Kim SK, Lee YS, Park CY, Choe JY (2011) Complete atrioventricular block in adult Sjogren's syndrome with anti-Ro autoantibody. Korean J Intern Med 26(2):213–215
Scofield RH (2011) Vasculitis in Sjogren's syndrome. Curr Rheumatol Rep 13(6):482–488
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H et al (2012) American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 64(4):475–487
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE et al (2002) Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European consensus group. Ann Rheum Dis 61(6):554–558
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135
Higgins JPT, Green S (eds) (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from http://handbook.cochrane.org
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Sterne JA, Egger M (2001) Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54(10):1046–1055
Akyel A, Tavil Y, Yayla C, Tufan A, Kaya A, Tezcan ME, Ozturk MA, Boyaci B (2012) Endothelial dysfunction in primary Sjogren syndrome. West Indian Med J 61(9):870–872
Atzeni F, Sarzi-Puttini P, Signorello MC, Gianturco L, Stella D, Boccassini L et al (2014) New parameters for identifying subclinical atherosclerosis in patients with primary Sjogren's syndrome: a pilot study. Clin Exp Rheumatol 32(3):361–368
Gravani F, Papadaki I, Antypa E, Nezos A, Masselou K, Ioakeimidis D, Koutsilieris M, Moutsopoulos HM, Mavragani CP (2015) Subclinical atherosclerosis and impaired bone health in patients with primary Sjogren's syndrome: prevalence, clinical and laboratory associations. Arthritis Res Ther 17(1):99
Sabio JM, Sanchez-Berna I, Martinez-Bordonado J, Vargas-Hitos JA, Navarrete-Navarrete N, Exposito Ruiz M et al (2015) Prevalence of and factors associated with increased arterial stiffness in patients with primary Sjogren's syndrome. Arthritis Care Res (Hoboken) 67(4):554–562
Vaudo G, Bocci EB, Shoenfeld Y, Schillaci G, Wu R, Del Papa N et al (2005) Precocious intima-media thickening in patients with primary Sjogren's syndrome. Arthritis Rheum 52(12):3890–3897
Zardi EM, Basta F, Afeltra A (2016) Levels of vitamin D, disease activity and subclinical atherosclerosis in post-menopausal women with Sjogren's syndrome: does a link exist? In Vivo 30(5):721–725
Zardi EM, Sambataro G, Basta F, Margiotta DP, Afeltra AM (2014) Subclinical carotid atherosclerosis in elderly patients with primary Sjogren syndrome: a duplex Doppler sonographic study. Int J Immunopathol Pharmacol 27(4):645–651
Sezis Demirci M, Karabulut G, Gungor O, Celtik A, Ok E, Kabasakal YI (2016) There an increased arterial stiffness in patients with primary Sjogren's syndrome? Intern Med 55(5):455–459
Valim V, Gerdts E, Jonsson R, Ferreira GA, Brokstad KA, Brun JG et al (2016) Atherosclerosis in Sjogren's syndrome: evidence, possible mechanisms and knowledge gaps. Clin Exp Rheumatol 34(1):133–142
Rongen GA, van Ingen I, Kok M, Vonkeman H, Janssen M, Jansen TL (2018) Vasodilator function worsens after cessation of tumour necrosis factor inhibitor therapy in patients with rheumatoid arthritis only if a flare occurs. Clin Rheumatol 37(4):909–916
Izzo JL Jr (2004) Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol 19(4):341–352
Davies JI, Struthers AD (2003) Pulse wave analysis and pulse wave velocity: a critical review of their strengths and weaknesses. J Hypertens 21(3):463–472
Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA et al (2006) Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam study. Circulation 113(5):657–663
Imura T, Yamamoto K, Kanamori K, Mikami T, Yasuda H (1986) Non-invasive ultrasonic measurement of the elastic properties of the human abdominal aorta. Cardiovasc Res 20(3):208–214
Kurum T, Yildiz M, Soy M, Ozbay G, Alimgil L, Tuzun B (2005) Arterial distensibility as determined by carotid-femoral pulse wave velocity in patients with Behcet's disease. Clin Rheumatol 24(2):134–138
Chiang CH, Liu CJ, Chen PJ, Huang CC, Hsu CY, Chan WL, Huang PH, Chen TJ, Lin SJ, Chen JW, Leu HB (2014) Primary Sjogren's syndrome and risk of ischemic stroke: a nationwide study. Clin Rheumatol 33(7):931–937
Chiang CH, Liu CJ, Chen PJ, Leu HB, Hsu CY, Huang PH et al (2013) Primary Sjogren's syndrome and the risk of acute myocardial infarction: a nationwide study. Zhonghua Minguo Xin Zang Xue Hui Za Zhi 29(2):124–131
Kang JH, Lin HC (2010) Comorbidities in patients with primary Sjogren's syndrome: a registry-based case-control study. J Rheumatol 37(6):1188–1194
Ramagopalan SV, Pakpoor J, Seminog O, Goldacre R, Graham L, Goldacre MJ (2013) Risk of subarachnoid haemorrhage in people admitted to hospital with selected immune-mediated diseases: record-linkage studies. BMC Neurol 13:176
Zoller B, Li X, Sundquist J, Sundquist K (2012) Risk of subsequent ischemic and hemorrhagic stroke in patients hospitalized for immune-mediated diseases: a nationwide follow-up study from Sweden. BMC Neurol 12:41
Bartoloni E, Baldini C, Schillaci G, Quartuccio L, Priori R, Carubbi F, Bini V, Alunno A, Bombardieri S, de Vita S, Valesini G, Giacomelli R, Gerli R (2015) Cardiovascular disease risk burden in primary Sjogren's syndrome: results of a population-based multicentre cohort study. J Intern Med 278(2):185–192
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352(16):1685–1695
Krasnokutsky S, Romero AG, Bang D, Pike VC, Shah B, Igel TF, Dektiarev I, Guo Y, Zhong J, Katz SD, Pillinger MH (2018) Impaired arterial responsiveness in untreated gout patients compared with healthy non-gout controls: association with serum urate and C-reactive protein. Clin Rheumatol 37(7):1903–1911
Biesbroek PS, Heslinga SC, van de Ven PM, Peters MJL, Amier RP, Konings TC, Maroules CD, Ayers C, Joshi PH, van der Horst-Bruinsma IE, van Halm VP, van Rossum AC, Nurmohamed MT, Nijveldt R (2018) Assessment of aortic stiffness in patients with ankylosing spondylitis using cardiovascular magnetic resonance. In: Clin Rheumatol, vol 37, pp 2151–2159
Cicek OF, Bayram NA, Ayhan H, Erten S, Aslan AN, Sari C et al (2014) Assessment of the relationship between aortic stiffness and left ventricular functions with echocardiography in patients with Sjogren's syndrome. Int J Rheum Dis 17(6):658–663
Mariette X, Criswell LA (2018) Primary Sjogren's syndrome. N Engl J Med 378(10):931–939
Nocturne G, Cornec D, Seror R, Mariette X (2016) Use of biologics in Sjogren's syndrome. Rheum Dis Clin N Am 42(3):407–417
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(DOCX 11 kb)
Rights and permissions
About this article
Cite this article
Yong, W.C., Sanguankeo, A. & Upala, S. Association between primary Sjogren’s syndrome, arterial stiffness, and subclinical atherosclerosis: a systematic review and meta-analysis. Clin Rheumatol 38, 447–455 (2019). https://doi.org/10.1007/s10067-018-4265-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4265-1