Abstract
Pulmonary arterial hypertension (PAH) is an increasingly recognized complication of systemic lupus erythematosus (SLE). This study aims to estimate the point prevalence of PAH and identify risk factors for PAH in a large cohort of hospitalized SLE patients. We have collected the medical records of patients hospitalized with SLE at the First Affiliated Hospital of Anhui Medical University and Anhui Provincial Hospital. Resting transthoracic echocardiography (TTE) was used to estimate pulmonary artery pressure (PAP) and PAH was defined as systolic PAP (PASP) > 30 mmHg. Patients with other connective tissue diseases, aPL syndrome, left heart disease, valvular heart disease, congenital heart disease, HIV, and portal hypertension were excluded because of diseases affecting the PAP. We assessed potential risk factors for PAH such as thrombogenic factors, SLE clinical manifestations, laboratory abnormalities and disease activity. Ninety-five were diagnosed with PAH of 1639 patients with SLE. The presence of high fibrinogen, serositis, and thrombocytopenia were significantly higher in patients with PAH than in those without PAH (all P < 0.05). Multivariate logistic regression found the associations between high fibrinogen (OR = 1.629), serositis (OR = 2.866), and thrombocytopenia (OR = 1.825) with PAH. The point prevalence of PAH was 5.8% in our cohort of patients with SLE. The significant association of high fibrinogen, serositis, and thrombocytopenia with PAH suggested that hypercoagulable state, organ damage, and hematological abnormality may all contribute to the development of PAH in SLE. This is important, as it is treatable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic multi-organ autoimmune disease affecting predominantly women of childbearing age [1]. Cardiac and pulmonary involvements are serious complications with high morbidity and mortality rate, especially pulmonary arterial hypertension (PAH), which is the third leading cause of death in SLE patients [2]. The early symptoms of PAH are often mild and are like many other conditions, such as dyspnea, fatigue, and dizziness. Due to the absence of apparent signs or symptoms of illness, it means that PAH is often not diagnosed until the disorder is relatively advanced [3,4,5]. The clinical classification of PAH is intended to categorize multiple clinical conditions into five groups according to their similar clinical presentation, pathological findings, hemodynamic characteristics, and treatment strategy [6]. PAH can be associated with a number of other conditions, which together account for most other cases, including systemic sclerosis and SLE; also, it is a rare but relatively well-documented complication of human immunodeficiency virus (HIV) with estimated prevalence in HIV patients being 0.5% [7]. Estimates of the prevalence of PAH in SLE patients vary from 0.5 to 43% [8,9,10,11,12,13,14,15,16]. The data was from retrospective studies of large groups of patients over a period of 5–10 years or cross-sectional studies involving small numbers of patients. The wide variability in the prevalence rates reflects the various definitions of PAH used, the different PAH diagnostic approaches, the patients’ selection criteria, and the number of patients involved.
Increase of pulmonary vascular resistance in PAH is associated with various mechanisms, including vasoconstriction, loss of distal arteries, proliferative and obstructive remodeling of the pulmonary vessel wall, microthrombosis, and inflammation [17,18,19,20]. In situ thrombosis is also an important hallmark of PAH and participator in the worsening process of the disease. A recent study demonstrated that patients with PAH have a pro-thrombotic and hypercoagulable phenotype [20].
The non-specific nature of symptoms such as palpitations, fatigue, and syncope related with PAH could result in a delay in the diagnosis of SLE-PAH. This reveals a need for appropriate screening methods to detect PAH as early as possible. As we all knew, right heart catheterization (RHC) is the gold standard method to diagnose PAH. However, this test is invasive and expensive, which makes it unsuitable for use as a screening tool [21]. In contrast, transthoracic echocardiography (TTE) test has been demonstrated to be a sensitive and safe tool to screen for PAH [22, 23]. TTE has been used to investigate the prevalence of PAH in patients with connective tissue diseases [23]. Therefore, we set out to investigate the prevalence of PAH in our SLE cohort and to assess risk factors with this technique, including thrombogenic factors, SLE clinical manifestations, laboratory findings, and disease activity, which may play vital roles in the development of PAH in SLE patients.
Materials and methods
Patient recruitment
The protocol for our study was consistent with provisions of the World Medical Association Declaration of Helsinki, and informed consent was obtained per subject before enrollment. Overall medical records of patients hospitalized with SLE at the First Affiliated Hospital of Anhui Medical University and Anhui Provincial Hospital were collected. All patients fulfilled at least four of the SLE classification criteria of American College of Rheumatology [24]. Patients were excluded if they presented with overlapping systemic sclerosis, rheumatoid arthritis, polymyositis, or other connective tissue diseases. Moreover, patients with aPL syndrome, left heart disease, valvular heart disease, congenital heart disease, HIV, and portal hypertension were also excluded for their influence on PAH diagnosis. A final sample of 1639 SLE patients contributed to the analyses according to these criteria. This study was launched in January 2011; the cut-off for this study was December 2015.
Outcomes
Eligible patients had TTE examinations performed at rest by experienced cardiologists or echocardiographers. Repeat TTE was done to confirm the diagnosis of PAH by a single experienced cardiologist who was blinded to the treatment details. PAH was defined as a pulmonary arterial systolic pressure (PASP) of > 30 mmHg at rest as measured by TTE [25, 26].
Thrombogenic risk factor assessment
The levels of plasma coagulation fibrinogen and D-dimer were measured by the routine analyses (high fibrinogen defined as > 4 μg/ml, high D-dimer defined as > 0.5 μg/ml). Diabetes mellitus was considered to be present if fasting plasma glucose > 7.0 mmol/l or current diabetic therapy. Also, if a physician recorded the diagnosis in the medical record the patient had ever been prescribed lipid-lowering medication, or the fasting plasma cholesterol level measured was > 200 mg/dl, we may deem the patient with hypercholesterolemia.
Clinical manifestations and laboratory abnormalities
Clinical manifestations of SLE patients such as lupus nephritis; mucocutaneous manifestations; vasculitis, myositis, arthritis, and neuropsychiatric manifestations; and serositis were recorded. Laboratory abnormalities, including thrombocytopenia (< 100 × 109/L) and leukopenia (< 4.0 × 109/L) as well as levels of C3 and C4 and erythrocyte sedimentation rate (ESR) were also retrieved from the medical records (low C3 defined as < 0.85 mg/ml, low C4 defined as < 0.12 mg/ml, high ESR defined as > 20 mm/h). Autoantibody levels were measured and included anti-double-stranded DNA (anti-dsDNA), anti-Smith (anti-Sm), anti-SSA/Ro, anti-SSB/La, anti-ribonucleoprotein (anti-RNP), and anti-ribosomal RNP (anti-rRNP) antibodies. SLE disease activity was evaluated by SLE Disease Activity Index (SLEDAI) score [27]. Active lupus disease was defined as SLEDAI score ≥ 8. Data on demographic information and corticosteroids or immunosuppressive drugs (use in the past month or not) was also nailed down.
Statistical analysis
Frequencies and proportions were reported for categorical variables and mean ± SD or medians with range were reported for continuous variables, depending on their distribution. Chi-squared tests or Fisher’s exact tests were employed to compare categorical data, and Mann-Whitney U test was used to compare quantitative data between different groups.
Multiple logistic regression models were applied to assess independent variables of PAH in SLE patients; results were presented as odds ratio (OR) along with their 95% confidence intervals. Variables associated with the dependent variable at univariate analyses (probability threshold, P < 0.05) were included in the multivariate regression models. All information was analyzed by SPSS 13.0 (Chicago, Illinois, USA).
Results
General information
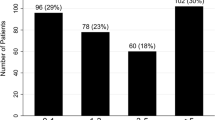
One thousand six hundred thirty-nine patients with SLE were studied and 1499 patients (91.5%) were female. The median (range) age was 36 (13–78) years old and disease duration was 1.5 (0–30) years. A total of 95 SLE patients with PAH were included into our research and the prevalence of PAH was 5.8%. As shown in Table 1, no difference was observed in age, gender, smoking history, weight, or disease duration between SLE patients with PAH and without PAH.
Thrombogenic factors
Thrombogenic factors were compared between patients with PAH and without PAH in Table 2. The incidences of high fibrinogen (χ2 = 5.331, P = 0.021) showed significant statistical differences in patients with PAH and without PAH. The presence of other thrombogenic factors was not significantly different between the groups.
Clinical manifestations
Clinical characteristics and SLE disease activity were compared between patients with PAH and without PAH in Table 3. The presence of serositis was significantly associated with PAH (χ2 = 26.130, P < 0.001). No significant differences were found in the way of other clinical manifestations between two groups (all P > 0.05).
Laboratory findings
As shown in Table 4, the incidence of thrombocytopenia was significantly higher in the patients with PAH (χ2 = 6.428, P = 0.011). Interestingly, we also found that positive anti-Sm autoantibodies seemed to be a protective factor for PAH in SLE patients (χ2 = 4.274, P = 0.039). We tended to believe change of appearance of anti-Sm autoantibodies would raise much more attention for diagnosis and treatment. Therefore, this variable was not included in the multivariate regression model. Rates of leukocytopenia, hypocomplementemia, anti-cardiolipin (ACL) antibody isotypes, and other autoantibodies were not significantly different between the groups (all P > 0.05).
Lupus activity and corticosteroids or immunosuppressive drugs
The presence of lupus activity and corticosteroids or immunosuppressive drugs were higher in patients with PAH compared with those without PAH, but they did not achieve statistical significance (P = 0.623 for lupus activity; P = 0.838 for corticosteroids or immunosuppressive drugs). It was displayed in Table 5 with detail.
Independent associated variables for PAH in SLE patients
Multivariate analysis revealed that the presence of high fibrinogen (OR = 1.629), serositis (OR = 2.866), and thrombocytopenia (OR = 1.825) were independent significant risk factors for PAH in patients with SLE (all P < 0.05), as shown in Table 6.
Discussion
In our study, the possible prevalence rate of PAH in Chinese patients with SLE was 5.8%, which is higher than that reported by others previously [13]. This may be because all subjects in our study were equipped with more severe symptoms requiring hospitalization, thereupon obtained detailed clinical examinations. Anyway, regular TTE screening for PAH would increase hospitalization days and aggravate the economic burden for SLE patients. Identifying a subgroup of SLE patients at higher risk of PAH to undergo screening would decrease the cost.
In this study, we investigated the risk factors for PAH from a rheumatoid standpoint. We found the presence of serositis was an independent risk factor. Although there was a consensus that serositis is a common pathological feature of PAH [13, 28], it remained unclear how it plays the role in PAH. Some support the view that the presence of serositis contributes to disease activity in patients with SLE, since serositis is usually a reflection of SLE activity. However, lupus activity was not significantly associated with the development of PAH in our cohort of SLE. The alternate view is that serositis is an epiphenomenon of pulmonary pathophysiological change. Anyway, we could suggest that SLE patients experiencing a disease flare should be screened for PAH in clinical practice, especially if they also have serositis.
Thrombosis is a common hallmark of PAH [20, 29, 30]. Abnormalities of blood coagulation factors, anti-thrombotic factors, and the fibrinolytic system were believed to play important roles in aggravating the thrombotic state in patients with PAH [30]. It has been reported that endothelial cell dysfunction may promote the development of PAH through interfering with the balance between pro-coagulant and anti-coagulant systems [31]. Moreover, tissue factor was significantly increased in the pulmonary vasculature of rats with pulmonary hypertension induced by monocrotaline and pneumonectomy. The upregulated tissue factor was also observed in the pulmonary vasculature of patients with PAH [32]. Furthermore, the upregulated levels of fibrinopeptide A, a surrogate of fibrin production, and dysregulated activity of von Willebrand factor, which is important for the interaction between platelets and endothelial cells, have been observed in patients with PAH [33]. In this study, we have confirmed that high fibrinogen was given great placement in predicting PAH in patients with SLE. These physiologic changes are thought to be the rationale for the use of oral anticoagulation to treat PAH in patients with SLE.
The results of this study also revealed that thrombocytopenia was associated with the risk of PAH in SLE patients. However, it is not clear whether thrombocytopenia originates due to defects in platelet production and/or because of abnormal platelet activation. Recently, a study of 22 patients with PAH and 25 healthy controls reported that the mean platelet volume (MPV) value, which is a well-studied marker of abnormal platelet activity, was upregulated in patients with PAH, and this increase was negatively associated to total thrombocyte count, indicating platelet consumption in the periphery [34]. There are some proposed mechanisms for increased platelet activation in patients with SLE. Firstly, systemic inflammation in patients with SLE might cause platelet activation. Inflammation is a crucial feature of SLE, and upregulated expression of cytokines, such as interleukin-6 (IL-6), was observed in patients with SLE [35]. Megakaryocytes play an important role in regulating platelet size. IL-6 mediated megakaryocyte ploidy, resulting in the production of more reactive and larger platelets [36, 37]. Moreover, inflammatory response can cause pulmonary vascular endothelial dysfunction, which leads to platelet activation [31]. Since their strategic role in inducing thrombotic obstruction of pulmonary arteries and regulating vessel remodeling, platelet abnormalities and abnormal platelet activation are considered to be important mediators of disease progression in patients with PAH [38]. Further studies are needed to fully understand the role of platelets in the establishment and progression of PAH in patients with SLE, so that we can better design and test a therapeutic approach.
Some limitations also should be considered in this study. First, to better correlate disease pathology with risk factors, it is ideal to collect clinical data at the time of disease diagnosis. However, it is challenging to obtain these concurrent data in practice. In our study, although a subset of clinical data was obtained when making diagnosis, others were obtained at relapse or remission stage. These patients were on medications, including a subset of patients who were taking corticosteroids or immunosuppressive drugs (use in the past month or not). This is clearly a cofounding variable and a limitation of our study because we did not examine the concurrent data. The second is related to the selection of study population. Further study with other ethnic population should be undertaken. Third, the diagnosis of PAH was based on an indirect method using TTE. Therefore, the accuracy of our prevalence data should be confirmed in a further study that utilizes RHC to definitively diagnose PAH. Finally, because of the cross-sectional nature of this study, the temporal sequence cannot be determined. Longitudinal studies are necessary to demonstrate our findings.
Conclusions
In summary, our data revealed the low prevalence of PAH in Chinese patients with SLE. We have also provided solid evidence that SLE patients with PAH was associated with high level of fibrinogen and the presence of serositis as well as thrombocytopenia, which suggests that hypercoagulable state, organ damage, and hematological abnormality may all contribute to the development of PAH in the patients with SLE. In the light of these results, we recommend screening by TTE for this complication majoring in high-risk groups such as those with hypercoagulable phenotype, serositis, and thrombocytopenia.
References
Chen W, Li XP, Zhai ZM, Tao JH, Wang GS, Zhang H, Qian L (2007) The expression of Treg cells and related cytokines in patients with systemic lupus erythematosus. Chin J Dis Contr Prev 1:11
Qian J, Wang Y, Huang C, Yang X, Zhao J, Wang Q, Tian Z, Li M, Zeng X (2016) Survival and prognostic factors of systemic lupus erythematosus-associated pulmonary arterial hypertension: a PRISMA-compliant systematic review and meta-analysis. Autoimmun Rev 15(3):250–257. https://doi.org/10.1016/j.autrev
Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G (2006) Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173(9):1023–1030. https://doi.org/10.1164/rccm.200510-1668OC
Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD (2010) Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137(2):376–387. https://doi.org/10.1378/chest.09-1140
Gaine SP, Rubin LJ (1998) Primary pulmonary hypertension. Lancet 352(9129):719–725. https://doi.org/10.1016/S0140-6736(98)02111-4
Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A (2004) Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43(12 Suppl S):5S–12S. https://doi.org/10.1016/j.jacc
Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, Gressin V, Clerson P, Sereni D, Simonneau G (2008) Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 177(1):108–113
Simonson JS, Schiller NB, Petri M, Hellmann DB (1989) Pulmonary hypertension in systemic lupus erythematosus. J Rheumatol 16(7):918–925
Winslow TM, Ossipov MA, Fazio GP, Simonson JS, Redberg RF, Schiller NB (1995) Five-year follow-up study of the prevalence and progression of pulmonary hypertension in systemic lupus erythematosus. Am Heart J 129(3):510–515
Johnson SR, Gladman DD, Urowitz MB, Ibanez D, Granton JT (2004) Pulmonary hypertension in systemic lupus. Lupus 13(7):506–509. https://doi.org/10.1191/0961203303lu1051oa
Quismorio FP Jr, Sharma O, Koss M, Boylen T, Edmiston AW, Thornton PJ, Tatter D (1984) Immunopathologic and clinical studies in pulmonary hypertension associated with systemic lupus erythematosus. Semin Arthritis Rheum 13(4):349–359
Perez-Penate GM, Rua-Figueroa I, Julia-Serda G, Leon-Marrero F, Garcia-Quintana A, Ortega-Trujillo JR, Erausquin-Arruabarrena C, Rodriguez-Lozano C, Cabrera-Navarro P, Ojeda-Betancor N, Gomez-Sanchez MA (2016) Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol 43(2):323–329. https://doi.org/10.3899/jrheum.150451
Li M, Wang Q, Zhao J, Li Z, Ye Z, Li C, Li X, Zhu P, Wang Z, Zheng Y, Zhang M, Tian Z, Liu Y, He J, Zhang F, Zhao Y, Zeng X (2014) Chinese SLE Treatment and Research group (CSTAR) registry: II. Prevalence and risk factors of pulmonary arterial hypertension in Chinese patients with systemic lupus erythematosus. Lupus 23(10):1085–1091. https://doi.org/10.1177/0961203314527366
Choi JH, Joo SJ, Kim J (2016) Determining the necessity for right heart catheterization in pulmonary hypertension associated with connective tissue diseases assessed by echocardiography. Int J Rheum Dis 19(1):65–73. https://doi.org/10.1111/1756-185X.12769
Wang H, Guo X, Lai J, Wang Q, Tian Z, Liu Y, Li M, Zhao J, Zeng X, Fang Q (2016) Predictors of health-related quality of life in patients with systemic lupus erythematosus associated pulmonary arterial hypertension. Clin Exp Rheumatol 34(2):291–295
Huang C, Li M, Liu Y, Wang Q, Guo X, Zhao J, Lai J, Tian Z, Zhao Y, Zeng X (2016) Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine (Baltimore) 95(10):e2761. https://doi.org/10.1097/MD.0000000000002761
Leopold JA, Maron BA (2016) Molecular mechanisms of pulmonary vascular remodeling in pulmonary arterial hypertension. Int J Mol Sci 17(5):761. https://doi.org/10.3390/ijms17050761
Sung YK, Yuan K, de Jesus Perez VA (2016) Novel approaches to pulmonary arterial hypertension drug discovery. Expert Opin Drug Discov 11(4):407–414. https://doi.org/10.1517/17460441.2016.1153625
O'Callaghan DS, Savale L, Montani D, Jais X, Sitbon O, Simonneau G, Humbert M (2011) Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 8(9):526–538. https://doi.org/10.1038/nrcardio.2011.104
Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F (2011) Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 8(8):443–455. https://doi.org/10.1038/nrcardio.2011.87
McGoon M, Gutterman D, Steen V, Barst R, McCrory DC, Fortin TA, Loyd JE (2004) Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 126(1 Suppl):14S–34S. https://doi.org/10.1378/chest.126.1_suppl.14S
Hsu VM, Moreyra AE, Wilson AC, Shinnar M, Shindler DM, Wilson JE, Desai A, Seibold JR (2008) Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. J Rheumatol 35(3):458–465
Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C (2005) The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum 52(7):2125–2132. https://doi.org/10.1002/art.21131
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25(11):1271–1277
Galie N, Torbicki A, Barst R, Dartevelle P, Haworth S, Higenbottam T, Olschewski H, Peacock A, Pietra G, Rubin LJ, Simonneau G (2005) Guidelines on diagnosis and treatment of pulmonary arterial hypertension. Rev Esp Cardiol 58(5):523–566
Ruiz-Irastorza G, Garmendia M, Villar I, Egurbide MV, Aguirre C (2013) Pulmonary hypertension in systemic lupus erythematosus: prevalence, predictors and diagnostic strategy. Autoimmun Rev 12(3):410–415. https://doi.org/10.1016/j.autrev
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35(6):630–640
Zhao J, Bai W, Zhu P, Zhang X, Liu S, Wu L, Ma L, Bi L, Zuo X, Sun L, Huang C, Tian X, Li M, Zhao Y, Zeng X (2016) Chinese SLE Treatment and Research group (CSTAR) registry VII: prevalence and clinical significance of serositis in Chinese patients with systemic lupus erythematosus. Lupus 25(6):652–657. https://doi.org/10.1177/0961203315625460
Roldan T, Landzberg MJ, Deicicchi DJ, Atay JK, Waxman AB (2016) Anticoagulation in patients with pulmonary arterial hypertension: an update on current knowledge. J Heart Lung Transplant 35(2):151–164. https://doi.org/10.1016/j.healun
Tournier A, Wahl D, Chaouat A, Max JP, Regnault V, Lecompte T, Chabot F (2010) Calibrated automated thrombography demonstrates hypercoagulability in patients with idiopathic pulmonary arterial hypertension. Thromb Res 126(6):e418–e422. https://doi.org/10.1016/j.thromres.2010.08.020
Berger G, Azzam ZS, Hoffman R, Yigla M (2009) Coagulation and anticoagulation in pulmonary arterial hypertension. Israel Med Assoc J 11(6):376–379
White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB (2007) Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293(3):L583–L590
Johnson SR, Granton JT, Mehta S (2006) Thrombotic arteriopathy and anticoagulation in pulmonary hypertension. Chest 130(2):545–552
Guvenc TS, Erer HB, Ilhan S, Zeren G, Ilhan E, Karakus G, Sayar N, Orhan AL, Eren M (2012) Comparison of mean platelet volume values among different causes of pulmonary hypertension. Cardiol J 19(2):180–187. https://doi.org/10.5603/Cj.2012.0031
Murakami M, Nishimoto N (2011) The value of blocking IL-6 outside of rheumatoid arthritis: current perspective. Curr Opin Rheumatol 23(3):273–277. https://doi.org/10.1097/BOR.0b013e3283456797
Debili N, Masse JM, Katz A, Guichard J, Breton-Gorius J, Vainchenker W (1993) Effects of the recombinant hematopoietic growth factors interleukin-3, interleukin-6, stem cell factor, and leukemia inhibitory factor on the megakaryocytic differentiation of CD34+ cells. Blood 82(1):84–95
Burstein SA, Downs T, Friese P, Lynam S, Anderson S, Henthorn J, Epstein RB, Savage K (1992) Thrombocytopoiesis in normal and sublethally irradiated dogs: response to human interleukin-6. Blood 80(2):420–428
James White R, Lannan KL, Phipps RP (2014) Drug discovery in pulmonary arterial hypertension: attacking the enigmatic root of a deadly weed. Drug Discov Today 19(8):1226–1229. https://doi.org/10.1016/j.drudis.2014.04.008
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Ethics approval
This study was conducted with the approval of the ethics committee of Anhui Medical University and according to the Declaration of Helsinki principles.
Rights and permissions
About this article
Cite this article
Xu, SZ., Yan Liang, Li, XP. et al. Features associated with pulmonary arterial hypertension in Chinese hospitalized systemic lupus erythematosus patients. Clin Rheumatol 37, 1547–1553 (2018). https://doi.org/10.1007/s10067-018-4056-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4056-8