Abstract
Rheumatoid arthritis is considered a T-lymphocyte-mediated disease. However, studies have focussed on CD4 T-lymphocytes, ignoring CD8 T-lymphocytes despite the latter being found abundantly in the synovium. Specifically, there is little data of the effect of methotrexate, the gold-standard DMARD, on various CD8 cytokine T-lymphocyte subsets and conflicting data on CD4 subsets. In this prospective study, patients with active rheumatoid arthritis, who were 18 to 65 years of age, were treated with methotrexate (up to 25 mg per week) for 24 weeks. At baseline and 24 weeks, frequencies of CD8+IFNγ+, CD8+IL17+, CD8+IL4+, corresponding CD4 subsets and plasma levels of IFNγ, IL-12, IL-10, IL-4 and IL-17 were determined by flow cytometry. These are summarised as median (IQR = interquartile range, 25th–75th percentile) and paired data compared using Wilcoxon signed rank test. This study included 67 patients (F/M = 4:1) with rheumatoid arthritis, 57 (85%) being RF positive and 20 receiving prednisolone at baseline. Mean (± SD) dose of methotrexate at 24 weeks was 22.9 ± 3.0 mg per week. On treatment with methotrexate, there was a significant (p = 0.04) decline in CD8+IFNγ+ cells from 37.2 (IQR 19.4–60.2) to 22.7% (IQR 8.5–49.7) and a marginal increase in CD8+IL17+ cells from 0.3 (IQR 0.1–0.6) to 0.4 (IQR 0.2–1.2), p = 0.006. There was no significant change in the other subsets. There was also a significant decline in circulating levels of IL-12, IL-10 and IL-17 and marginal increase in IL-4. On evaluating by response, non-responders but not responders had a significant increase in CD8+IL17+ (p = 0.01). There is a significant decline of CD8+IFNγ+ T cells and marginal increase in CD8+IL17+ T cells after methotrexate. Change in Tc1 subset may be mediated through reduction in IL-12 levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis is a multi-factorial disease that involves activation of many pathways driven by numerous cells. Among these, T-lymphocytes have been considered the master conductors of the orchestra of inflammation. CD4 T-lymphocytes particularly the CD4+IFNγ+ cells (Th1 subset) have been implicated in human and most animal models [1,2,3]. In addition, since their discovery, CD4+IL17+ (Th17) have also been implicated in the pathogenesis of RA. However, CD8 cells, that constitute a large proportion of the synovial compartment and are increased in circulation of early RA patients, have largely been ignored in its pathogenesis [4, 5]. CD8 T-lymphocytes are not a homogenous group and, similar to the paradigm of CD4 T-lymphocytes, can also be divided by cytokine production into CD8+IFNγ+, CD8+IL4+ and CD8+IL17+ cells [6, 7]. Some studies have found higher CD8+IFNγ+ in active RA, as compared to quiescent RA, but there is little data on the other subsets [8].
Methotrexate was developed in 1950s as a better folate analogue than aminopterin and was first used in arthritis in the 1960s [9]. It has been in regular use in rheumatoid arthritis since pivotal randomised controlled trials in the 1980s and its subsequent FDA approval [10, 11]. It is the current benchmark and gold standard in rheumatoid arthritis. Apart from its anti-folate action and anti-proliferative properties, it acts by increasing extracellular adenosine, which mediates its anti-inflammatory properties. It has an effect on a wide variety of cells, from neutrophils, mast cells to lympocytes [12, 13]. The effect of methotrexate on T-lympocytes has been an active area of research. However, surprisingly, there is little data on its effect on CD8 cytokine subsets and conflicting data on CD4 cytokine subsets.
We hypothesised that changes in the CD4+IFNγ+ (and CD4+IL17+) and CD8+IFNγ+ subsets and possibly a rise in CD4+IL-4+ and CD8+IL4+ and CD8+IL17+ subsets could be the cellular accompaniments of methotrexate action in rheumatoid arthritis.
Materials and methods
Subjects
Study participants were 18–65 years old, fulfilled the 1987 American College of Rheumatology (ACR) Criteria for rheumatoid arthritis (RA) [14], had active disease and had not received methotrexate in the last 6 months. Active disease was defined as modified disease activity score (28 joints) using three variables (DAS28-3) > 3.2 [15]. Key exclusion criteria were pre-existing renal insufficiency, liver disease or a recent serious infection (last 4 weeks). This study was approved by institutional ethics committee of our institute, and a written informed consent was obtained from all participants. Procedures followed in this study involving human studies were in accordance with ethical standards of the responsible committee on human experimentation and with the Declaration of Helsinki.
Treatment
Patients received oral methotrexate at a dose of 15 mg per week for first 4 weeks, followed by 20 mg per week for the next 4 weeks and thereafter a dose of 25 mg per week for the next 16 weeks. This escalation schedule was subject to tolerance of the drug by the patient and absence of laboratory abnormalities in the form of transaminitis or cytopenias. All patients received folic acid at a dose of 5 mg twice a week, 2 and 4 days after methotrexate.
Clinical assessments
Patients were followed up with monthly visits for assessing tolerance and disease activity. In addition, erythrocyte sedimentation rate (Westergren), complete blood counts and serum transaminase levels were obtained from the laboratory. Disease activity was assessed using the modified disease activity score using 28 joints and 3 variables (DAS28-3) that was calculated using the formula [0.56 × √(TJC28) + 0.28 × √(SJC28) + 0.70 × ln (ESR)] 1.08 + 0.16, where TJC28 = tender joint count, SJC28 = swollen joint count in a 28-joint count and ESR = erythrocyte sedimentation rate [15]. It does not include patient global assessment on the visual analogue score, which is a difficult concept to understand in our patients. This has been found to be equivalent to the DAS28 [15]. At the end of 24 weeks, patients were categorised based on the European League Against Rheumatism (EULAR) guidelines for response [16]. For the purpose of this study, good and moderate responders (as per the EULAR criteria) were designated as “responders” and compared with non-responders.
Determination of CD4 and CD8 T-lymphocyte subsets
At baseline and 24 weeks, 5 ml venous blood was obtained by venesection in a vacutainer containing lithium heparin. This was transported to the laboratory in 2 h where peripheral blood mononuclear cells were isolated by density gradient centrifugation using Ficoll-1077 (HiMedia Laboratories, India). Briefly, blood was mixed with an equal volume of phosphate-buffered saline (PBS) and carefully layered on equal volume of Ficoll-1077 in a tube. This was centrifuged (with brake off) at 400×g at room temperature for 30 min. The upper plasma layer was discarded, and the mononuclear cell layer at plasma-Ficoll interface was carefully aspirated by a pipette and put in another tube. This was washed twice with 3× volumes of PBS and then sedimented at 300 and 200 g respectively. Cells were then suspended in RPMI and counted, and adjusted to 1 × 106 cells per millilitre.
Intracellular cytokine production was detected after stimulation with phorbol-12-myristate-13-acetate (PMA) (a protein kinase C activator) and ionomycin (Ca2+ ionophore) as per published protocols [17]. This leads to the activation of a T-lymphocyte (mimicking the actual activation caused on TCR engagement and co-stimulation). In brief, PBMC cell suspension of 1 × 106 cells was stimulated with 50 ng/ml of phorbol-12-mirystate-13-acetate (PMA; Sigma, St. Louis, MO, USA) and 1 μg/ml of ionomycin (Sigma, St. Louis, MO, USA) for 5.5 h at 37 °C in complete RPMI (with HEPES). In addition, protein transport inhibitor Brefeldin (1 μl; GolgiPlug, BD Bioscience, CA, USA) was added to prevent extracellular transport of cytokines. Cells were washed with PBS, and surface staining was done by incubating with anti-CD3-PE-Cy7, anti-CD8-PerCP and anti-CD4-PE (BD Bioscience, CA, USA) for 20 min at room temperature. Subsequently, the cells were fixed and permeabilised using 250 μl of cytofix/cytoperm solution (BD Cytofix Fixation Buffer, BD Biosciences, CA, USA) for 20 min.
After fixation and permeabilization, cells were washed and resuspended in PBS (with 1% fetal bovine serum) and kept overnight at 4 °C. Subsequently, they were suspended in 1 ml perm-wash buffer for 15 min at room temperature. They were centrifuged, and approximately 100 μl perm-wash buffer left in which they were resuspended by vortexing. This was divided into two tubes; in the first tube, anti-human IFNγ-FITC and IL-4-BV421 and in the second tube anti-human IFNγ-FITC and IL-17-BV421 were added and incubated for 30 min in the dark. The cells were then washed and re-suspended in perm-wash buffer for acquisition.
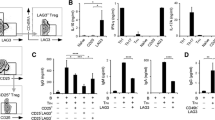
Acquisition was done on BD FACS Aria, and a total of 30,000 events were acquired in each tube, and analysed using software (BD FACS Diva, Becton Dickinson). Initially, PBMCs were gated using surface and side scatter characteristics followed by sequential gating (drilling down) of CD3+ (PE-CY7) positive cells, followed by gating of CD4+ (PE) and CD8+ (PerCP) positive cells, followed by opening a dot plot of IFNγ (FITC) and IL-17 or IL-4 (BV421). Positive events were identified using FMO controls. Frequencies of CD3+CD4+IFNγ+, CD3+CD4+IL4+ and CD3+CD4+IL17+ and CD3+CD8+IFN-γ, CD3+CD8+IL4+ and CD3+CD8+IL17+ as percentage positive cells were enumerated (Fig. 1). These are referred to without the CD3+ marker henceforth.
Measurement of circulating cytokines
Plasma was separated by centrifugation from lithium heparin anti-coagulated blood. Levels of IL-17A, IFN-γ, IL-12, IL-10 and IL-4 were determined by the use of human-enhanced sensitivity flex set (BD Biosciences, CA, USA) as per the manufacturer’s instruction. This assay has a detection range of 274–2,00,000 pg/ml. In brief, capture beads for these cytokines (from individual flex sets) were mixed in microcentrifuge tubes and reconstituted standards (provided) or samples added and incubated for 2 h at room temperature. After this, human detection reagent (part A) was added to each tube, shaken and incubated for 2 h at room temperature. Then, supernatant was aspirated, and tubes were washed twice with wash buffer. Subsequently, enhanced sensitivity detection reagent (part B) was added to each tube, shaken and incubated for 1 h at room temperature. Again, supernatant was aspirated, and tubes were washed twice with wash buffer and finally resuspended in the same for acquisition. Acquisition was done on BD FACS Aria (BD Bioscience, USA), and analysis was done on FCAP Array Software (BD Bioscience, USA).
Statistical analysis
Collected data was entered into MS Excel spreadsheet, coded appropriately and cleaned from any inadvertent errors. This was then transferred to SPSS for Windows version 19 (SPSS Inc., Chicago, IL, USA), where analysis was carried out. Normally distributed samples were presented as means and standard deviation and non-normal as median (interquartile range; 25th and 75th percentile). To compare paired non-normal data, Wilcoxon signed rank test was used. To compare independent groups, Student’s t test was used to compare normal variables in two different groups (responder and non-responder) and Mann-Whitney U test were used for non-normal variables. A two-sided p value of < 0.05 was considered statistically significant.
Results
Baseline characteristics
This study included 67 patients with rheumatoid arthritis, a majority of whom were females (54/67). They had mean (± SD) age and disease duration of 42.9 ± 11.9 and 2.6 ± 2.4 years respectively, and a majority were rheumatoid factor positive (57, 85%). Mean (± SD) modified disease activity score (three variables) at baseline was 5.9 ± 0.9. At baseline, 20 patients were receiving low-dose steroids and four were on other DMARDs (all sulfasalazine).
Effect of methotrexate on T-lymphocyte subsets (CD4 and CD8)
The mean (± SD) dose of methotrexate at completion of study, i.e. 24 weeks was 22.9 (± 3.0) mg per week. The mean (95% confidence interval) modified disease activity score (three variables) declined by − 1.1 (− 1.3 to − 0.8) to a value of 4.8 (± 1.0) at 24 weeks. There was no change in the frequencies of the Th1, Th2 or Th17 subsets between baseline and 24 weeks post-treatment (Table 1). However, the frequency of CD8+IFNγ+ (Tc1 subset) declined significantly after 24 weeks (p = 0.047). Also there was a slight but significant rise in the CD8+IL17+ (Tc17 subset) (p = 0.006) (Fig. 2).
Effect of methotrexate on cytokine levels
There was a significant fourfold to fivefold fall in levels of IL-12 and IL-17 after 24 weeks of treatment with methotrexate. In addition, the level of IL-10 declined by a log-fold after 24 weeks. In contrast, there was a slight but significant rise in the level of IL-4 after 24 weeks (Fig. 3). However, there was no significant change in the level of interferon-γ after treatment with methotrexate (Table 2).
Response to methotrexate
Among 61 patients (in whom this detail was available), there were 34 responders and 27 non-responders to methotrexate at 24 weeks. In non-responders (but not responders), there was a significant rise in the proportion of CD4+IL17+ and CD8+IL17+ (Th17 and Tc17 subsets). There was a significant decline in the IL-12 and IL-10 in responders but not non-responders to methotrexate. In both groups, there was a significant fall in levels of IL-17 and rise in IL-4 (Table 3).
Discussion
This study found a significant decline in the circulating CD8+IFNγ+ subset and a marginal increase in CD8+IL17+ subset after 24 weeks of treatment with methtorexate in rheumatoid arthritis. There was no significant change in the CD4 subsets. Also, there was significant decline in circulating IL-12, IL-17 and IL-10 levels and a mild increase in IL-4 level.
CD8 T-lymphocytes are known to have a major role in protection against viral infections and tumours and may mediate damage in certain autoimmune diseases like multiple sclerosis, type 1 diabetes mellitus and vitiligo [4, 18]. This is mediated through the classical subset called the cytotoxic T-lymphocytes or Tc1 (CD8+IFNγ+) subset [7]. In RA, CD8 T-lymphocytes are abundant in the synovial fluid and synovium [19] and show oligoclonality, suggesting an antigen-driven stimulation [20, 21]. Indeed, one study found the majority of oligoclonal T-lymphocytes in the synovial compartment to be CD8 T-lymphocytes [20]. CD8+IFNγ+ cells have also been shown to be crucial for the formation of ectopic germinal centres in rheumatoid arthritis synovium, and depleting leads to disintegration of the germinal centres [22]. Even in some animal models, CD8 cells seem to be crucial to pathogenesis. In collagen-induced arthritis, frequency of CD8−/− DBA/1 mice getting arthritis is significantly reduced [23]. Similarly, in the K/BxN mouse model, monoclonal anti-CD8 therapy led to disease amelioration [24].
The decline in CD8+IFNγ+ cells after 24 weeks of methotrexate in our study is similar to that observed by Schuerwegh et al. after 12 months of methotrexate and steroid therapy [25]. That was a smaller study on 20 patients, all of whom received steroids, and methotrexate was used in a dose of 15 mg per week. These suggest that methotrexate, which is the gold-standard benchmark drug in RA, may be working through a reduction in specifically the CD8+IFNγ+ cells. A major limitation is that we have not explored the actual cytotoxic ability in terms of granzyme or perforin expression or carried out in vitro cytotoxicity assays.
Recently, Carvalheiro et al. in a cross-sectional study found that CD8 T-lymphocytes in active RA patients exhibited an active effector phenotype with elevated expression of pro-inflammatory cytokines like IFNγ, whereas patients in remission had lower CD8 cytokine production by patients in remission [8]. Also, even biological agents like abatacept have been shown to decrease the CD8+IFNγ+ subset [26]. Thus, CD8+IFNγ+ seem to be associated with disease activity (and maybe mediating it), and reduction in this subset may not be specifically related to methotrexate treatment, but rather control of disease activity.
The reduction in CD8+IFNγ+ cells may be mediated by the reduction in IL-12 levels by methotrexate found in this study. A reduction in IL-12 gene expression in isolated PBMC after methotrexate treatment has been reported earlier as well [27]. IL-12 has been shown to be important for driving the antigen-activated CD-8 cells into fully functional effectors (Tc1 subset) [28, 29]. The marginal increase in IL-4, found in this study, could also contribute to a reduction in these cells. A previous study also found an increase in gene expression of IL-4 on methotrexate treatment [30]. However, without direct cytokine blocking experiments, it is not possible to prove their role in changes in CD8+IFNγ+ cells.
This study also found a marginal increase in CD8+IL17+ cells, particularly in the responders (along with increase in CD4+IL17+ cells). Previously, CD8+IL17+ cells have been shown to be non-functional and not displaying granzyme or displaying any CTL in vitro [6, 31]. Indeed, mice with lacking both T-bet and eomdesodermin, in whom CD8 cells secreted only IL-17 but not IFNγ, were found to get fatally virally infected, suggesting a lack of effective protection by these cells [32]. Thus, their increase on methotrexate therapy may not be associated with higher cytotoxicity. However, they have also been shown to transform to Tc1 in vivo [6]. Again, the limitation in our study is the lack of data on perforin and granzyme in this subset. This study also found a fall in IL-10 levels with methotrexate treatment. This may be as a result of reduction in CD8+IL10+ cells as has been shown to be increase in RA patients in the synovial fluid, although not studied by us [33]. It would be interesting to look at IL-6 and IL-21/23 levels to look at the mechanism of this change.
The present study did not find any significant change in CD4+ cell subsets (Th1, Th2 and Th17) in patients of RA after treatment with MTX. Previously varying and often opposing results have been found. Some studies have found a reduction in Th1 frequencies [25, 34], whereas other have found a reduction in Th2 after MTX treatment [35]. Studies have also found reduction in circulating Th17 subsets after MTX treatment [36]. Finally, some studies have not found any change [25, 37].
To conclude, our study suggests that there is a significant effect of methotrexate (or indirectly though reduction of disease activity) on CD8+IFNγ+ frequencies that may be mediated by the reduction in IL-12.
References
Cope AP, Schulze-Koops H, Aringer M (2007) The central role of T cells in rheumatoid arthritis. Clin Exp Rheumatol 25(5 Suppl 46):S4–11
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23):2205–2219. https://doi.org/10.1056/NEJMra1004965
Smolen JS, Tohidast-Akrad M, Gal A, Kunaver M, Eberl G, Zenz P, Falus A, Steiner G (1996) The role of T-lymphocytes and cytokines in rheumatoid arthritis. Scand J Rheumatol 25(1):1–4. https://doi.org/10.3109/03009749609082660
Carvalheiro H, da Silva JA, Souto-Carneiro MM (2013) Potential roles for CD8(+) T cells in rheumatoid arthritis. Autoimmun Rev 12(3):401–409. https://doi.org/10.1016/j.autrev.2012.07.011
Petrelli A, van Wijk F (2016) CD8(+) T cells in human autoimmune arthritis: the unusual suspects. Nat Rev Rheumatol 12(7):421–428. https://doi.org/10.1038/nrrheum.2016.74
Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, Anders RA, Trimble CL, Adler AJ, Lin TY, Pardoll DM, Huang CT, Drake CG (2009) Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol 183(11):7161–7168. https://doi.org/10.4049/jimmunol.0900368
Mittrucker HW, Visekruna A, Huber M (2014) Heterogeneity in the differentiation and function of CD8(+) T cells. Arch Immunol Ther Exp 62(6):449–458. https://doi.org/10.1007/s00005-014-0293-y
Carvalheiro H, Duarte C, Silva-Cardoso S, da Silva JA, Souto-Carneiro MM (2015) CD8+ T cell profiles in patients with rheumatoid arthritis and their relationship to disease activity. Arthritis Rheumatol 67(2):363–371. https://doi.org/10.1002/art.38941
Black RL, O’Brien WM, Vanscott EJ, Auerbach R, Eisen AZ, Bunim JJ (1964) Methotrexate therapy in psoriatic arthritis; double-blind study on 21 patients. JAMA 189:743–747
Weinblatt ME (2013) Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc 124:16–25
Weinblatt ME, Coblyn JS, Fox DA, Fraser PA, Holdsworth DE, Glass DN, Trentham DE (1985) Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med 312(13):818–822. https://doi.org/10.1056/NEJM198503283121303
Cronstein BN (2005) Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 57(2):163–172. https://doi.org/10.1124/pr.57.2.3
Chan ES, Cronstein BN (2010) Methotrexate—how does it really work? Nat Rev Rheumatol 6(3):175–178. https://doi.org/10.1038/nrrheum.2010.5
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324. https://doi.org/10.1002/art.1780310302
Fransen J, van Riel PL (2005) The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 23(5 Suppl 39):S93–S99
Fransen J, van Riel PL (2009) The disease activity score and the EULAR response criteria. Rheum Dis Clin N Am 35(4):745–757, vii-viii. https://doi.org/10.1016/j.rdc.2009.10.001
Maino VC, Picker LJ (1998) Identification of functional subsets by flow cytometry: intracellular detection of cytokine expression. Cytometry 34(5):207–215. https://doi.org/10.1002/(SICI)1097-0320(19981015)34:5<207::AID-CYTO1>3.0.CO;2-J
Gravano DM, Hoyer KK (2013) Promotion and prevention of autoimmune disease by CD8+ T cells. J Autoimmun 45:68–79. https://doi.org/10.1016/j.jaut.2013.06.004
Steiner G, Tohidast-Akrad M, Witzmann G, Vesely M, Studnicka-Benke A, Gal A, Kunaver M, Zenz P, Smolen JS (1999) Cytokine production by synovial T cells in rheumatoid arthritis. Rheumatology (Oxf) 38(3):202–213. https://doi.org/10.1093/rheumatology/38.3.202
Masuko-Hongo K, Sekine T, Ueda S, Kobata T, Yamamoto K, Nishioka K, Kato T (1997) Long-term persistent accumulation of CD8+ T cells in synovial fluid of rheumatoid arthritis. Ann Rheum Dis 56(10):613–621. https://doi.org/10.1136/ard.56.10.613
Fitzgerald JE, Ricalton NS, Meyer AC, West SG, Kaplan H, Behrendt C, Kotzin BL (1995) Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol 154(7):3538–3547
Kang YM, Zhang X, Wagner UG, Yang H, Beckenbaugh RD, Kurtin PJ, Goronzy JJ, Weyand CM (2002) CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J Exp Med 195(10):1325–1336. https://doi.org/10.1084/jem.20011565
Tada Y, Ho A, Koh DR, Mak TW (1996) Collagen-induced arthritis in CD4- or CD8-deficient mice: CD8+ T cells play a role in initiation and regulate recovery phase of collagen-induced arthritis. J Immunol 156(11):4520–4526
Raposo BR, Rodrigues-Santos P, Carvalheiro H, Agua-Doce AM, Carvalho L, Pereira da Silva JA, Graca L, Souto-Carneiro MM (2010) Monoclonal anti-CD8 therapy induces disease amelioration in the K/BxN mouse model of spontaneous chronic polyarthritis. Arthritis Rheum 62(10):2953–2962. https://doi.org/10.1002/art.27729
Schuerwegh AJ, van Offel JF, Bridts CH, Stevens WJ, De Clerck LS (2001) Influence of longterm therapy with methotrexate and low dose corticosteroids on type 1 and type 2 cytokine production in CD4+ and CD8+ T lymphocytes of patients with rheumatoid arthritis. J Rheumatol 28(8):1793–1799
Scarsi M, Zanotti C, Chiarini M, Imberti L, Piantoni S, Frassi M, Tincani A, Airo P (2014) Reduction of peripheral blood T cells producing IFN-gamma and IL-17 after therapy with abatacept for rheumatoid arthritis. Clin Exp Rheumatol 32(2):204–210
Hobl EL, Mader RM, Erlacher L, Duhm B, Mustak M, Broll H, Hogger P, Kalipciyan M, Jilma B (2011) The influence of methotrexate on the gene expression of the pro-inflammatory cytokine IL-12A in the therapy of rheumatoid arthritis. Clin Exp Rheumatol 29(6):963–969
Cox MA, Harrington LE, Zajac AJ (2011) Cytokines and the inception of CD8 T cell responses. Trends Immunol 32(4):180–186. https://doi.org/10.1016/j.it.2011.01.004
Cui W, Joshi NS, Jiang A, Kaech SM (2009) Effects of signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine 27(15):2177–2187. https://doi.org/10.1016/j.vaccine.2009.01.088
Constantin A, Loubet-Lescoulie P, Lambert N, Yassine-Diab B, Abbal M, Mazieres B, de Preval C, Cantagrel A (1998) Antiinflammatory and immunoregulatory action of methotrexate in the treatment of rheumatoid arthritis: evidence of increased interleukin-4 and interleukin-10 gene expression demonstrated in vitro by competitive reverse transcriptase-polymerase chain reaction. Arthritis Rheum 41(1):48–57. https://doi.org/10.1002/1529-0131(199801)41:1<48::aid-art7>3.0.co;2-k
Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, Chou AH, Chang SR, Hsiao KN, Yu FW, Chen HW (2007) Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol 82(2):354–360. https://doi.org/10.1189/jlb.0207111
Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL (2008) Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321(5887):408–411. https://doi.org/10.1126/science.1159806
Berner B, Akca D, Jung T, Muller GA, Reuss-Borst MA (2000) Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol 27(5):1128–1135
Herman S, Zurgil N, Langevitz P, Ehrenfeld M, Deutsch M (2008) Methotrexate selectively modulates TH1/TH2 balance in active rheumatoid arthritis patients. Clin Exp Rheumatol 26(2):317–323
Rudwaleit M, Yin Z, Siegert S, Grolms M, Radbruch A, Braun J, Sieper J (2000) Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor alpha, increase of interleukin 10, and predicted by the initial concentration of interleukin 4. Ann Rheum Dis 59(4):311–314. https://doi.org/10.1136/ard.59.4.311
Yue C, You X, Zhao L, Wang H, Tang F, Zhang F, Zhang X, He W (2010) The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol Int 30(12):1553–1557. https://doi.org/10.1007/s00296-009-1179-x
Shen H, Xia L, Lu J, Xiao W (2010) Infliximab reduces the frequency of interleukin 17-producing cells and the amounts of interleukin 17 in patients with rheumatoid arthritis. J Investig Med 58(7):905–908. https://doi.org/10.231/JIM.0b013e3181eb9895
Acknowledgements
Methotrexate used in this study was provided by Zydus Synovia (Zydus Cadila Healthcare Ltd).
Funding
This study was supported by funding from Fast Track Young Scientist Scheme under Science and Engineering Research Board, Department of Science and Technology, Government of India by vide grant number SB/YS/LS-41/2013 dated June 09, 2013. Amit Sandhu was supported by Indian Council of Medical Research by the Junior Research Fellowship scheme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Sandhu, A., Ahmad, S., Kaur, P. et al. Methotrexate preferentially affects Tc1 and Tc17 subset of CD8 T lymphocytes. Clin Rheumatol 38, 37–44 (2019). https://doi.org/10.1007/s10067-018-4011-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4011-8