Abstract
The objective of this study was to evaluate prevalence, initial risk factors, and outcomes in Henoch-Schönlein purpura nephritis (HSPN) patients in Latin America. Two hundred ninety-six patients (validated EULAR/PRINTO/PRES HSP criteria) were assessed by demographic data, clinical/laboratorial involvements, and treatments in the first 3 months after diagnosis. They were followed-up in a Latin American tertiary center and were divided in two groups: with and without nephritis. Persistent non-nephrotic proteinuria, nephrotic proteinuria, and acute/chronic kidney injury were also systematically evaluated at 1, 5, 10, and 15 years after diagnosis. HSPN was evidenced in 139/296 (47%) in the first 3 months. The median age at diagnosis was significantly higher in HSPN patients compared without renal involvement [6.6 (1.5–17.7) vs. 5.7 (0.9–13.5) years, p = 0.022]. The frequencies of persistent purpura (31 vs. 10%, p < 0.0001), recurrent abdominal pain (16 vs. 7%, p = 0.011), gastrointestinal bleeding (25 vs. 10%, p < 0.0001), and corticosteroid use (54 vs. 41%, p = 0.023) were significantly higher in the former group. Logistic regression demonstrated that the independent variables associated with HSNP were persistent purpura (OR = 3.601; 95% CI (1.605–8.079); p = 0.002) and gastrointestinal bleeding (OR = 2.991; 95% CI (1.245–7.183); p = 0.014). Further analysis of patients without HSPN in the first 3 months revealed that 29/118 (25%) had persistent non-nephrotic proteinuria and/or hematuria in 1 year, 19/61 (31%) in 5 years, 6/17 (35%) in 10 years and 4/6 (67%) in 15 years after diagnosis. None of them had chronic kidney injury or were submitted to renal replacement therapy. The present study observed HSPN in almost one half of patients in the first months of disease, and HSPN was associated with persistent purpura and gastrointestinal bleeding. One fourth of patients had nephritis only evidenced during follow-up without severe renal manifestations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Henoch-Schönlein purpura (HSP), also named immunoglobulin A vasculitis, is the most frequent primary vasculitis affecting children and adolescents [1,2,3,4,5,6,7]. This disease is characterized by cutaneous, articular, gastrointestinal, and renal involvements [1,2,3].
Renal alterations observed in HSP nephritis (HSPN) patients are self-limited and usually with transitory microscopic hematuria and/or low-grade proteinuria in the first 3 months after diagnosis [1, 6]. HSPN may have acute, recurrent, or chronic course and is considered the main risk factor for poor outcome [6, 8,9,10]. Deterioration or new development of nephritis in HSP patients have been described during follow-up, generally using clinical definition of 1990 American College of Rheumatology (ACR) classification criteria studies [10,11,12,13].
In 2010, validated and international classification criteria for children and adolescents HSP patients were proposed by European League Against Rheumatism (EULAR), Paediatric Rheumatology International Trials Organisation (PRINTO) and Paediatric Rheumatology European Society (PRES) [14]. Data of initial risk factors associated with HSPN using EULAR/PRINTO/PRES criteria were reported by European and Asiatic HSP populations [6, 15,16,17,18,19], and rarely evaluating short and medium-term outcomes [19]. To our knowledge, there is no study assessing initial risk factors associated with HSPN and outcomes, using these new criteria, in a cohort of children and adolescents with this primary vasculitis in Latin America.
Therefore, the objectives of the present study were to evaluate initial risk factors associated with HSPN and outcomes in pediatric patients with HSP assessed in 1, 5, 10, and 15 years after diagnosis.
Patients and methods
Data from 322 children and adolescents with HSP followed at the Pediatric Rheumatology Department of our University Hospital during a 32-year period (January 1983 to December 2015) were retrospectively assessed. Twenty-six patients were excluded due to incomplete medical charts. The remaining 296 patients fulfilled validated EULAR/PRINTO/PRES criteria for HSP patients and were evaluated [14]. Demographic data, clinical manifestations, laboratory exams, and treatments were systematically evaluated in the first 3 months after disease diagnosis, and in 1, 5, 10, and 15 years after diagnosis. The Ethics Committee of our University Hospital approved this study, and informed consent was obtained from all patients and their legal guardians.
HSPN was defined according to the presence of hematuria (> 5 red blood cells/high power field), red blood cell casts in urinary sediment, and/or proteinuria > 0.1 g/m2/day [1, 14]. Nephrotic syndrome was characterized by edema, serum albumin < 2.5 g/L, and proteinuria > 1 g/m2/day [1]. High blood pressure was defined as systolic and/or diastolic blood pressures ≥ 95th percentile for gender, age, and height on ≥ 3 occasions [20]. Acute kidney injury was diagnosed by sudden increase in serum creatinine above 2 mg/dl [21] or by modified RIFLE criteria (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) [22]. Chronic kidney insufficiency was defined as structural or functional kidney abnormalities for ≥ 3 months, with decreased glomerular filtration rate < 60 ml/min/1.73 m2 [23]. Renal replacement therapy (hemodialysis, peritoneal dialysis, hemofiltration, and renal transplantation) was also assessed. Pregnancy and/or surgery after diagnosis were evaluated as trigger factors for HSPN relapse [10, 19].
Renal biopsy was performed in HSPN patients with severe (nephrotic syndrome, acute, or chronic renal disease) and/or persistent renal alterations (hematuria or proteinuria > 0.1 g/m2/day with > 3 months) [1]. Kidney biopsy was classified according to histological findings proposed by the International Study of Kidney Disease in Children (ISKDC): grade I (minor glomerular abnormalities), grade II (pure mesangial proliferation), grade III [minor glomerular abnormalities or mesangial proliferation, with crescents/segmental lesions (sclerosis, adhesion, thrombosis, and necrosis) in < 50% of the glomeruli], grade IV (same as grade III but with crescents/segmental lesions in 50–75% of the glomeruli), grade V (same as grade III but with crescents/segmental lesions in > 75% of the glomeruli), and grade VI [membranoproliferative-like lesions and tubulointerstitial lesions (interstitial inflammation or fibrosis and tubular loss)] [24].
HSP patients were divided in two groups: with and without HSPN at the first 3 months. Additionally, persistent non-nephrotic proteinuria, nephrotic proteinuria, and renal insufficiency were also evaluated in 1, 5, 10, and 15 years after diagnosis in two groups: patients with HSPN and HSP patients without renal involvement.
Demographic data included gender, age at diagnosis, disease duration, and body mass index (BMI). BMI was characterized by weight in kilograms divided by the square of the body height (m2). Recurrent purpura/petechiae was defined as new cutaneous lesions after total recovery and persistent purpura/petechiae, as skin lesions persisting for ≥ 1 month [1]. Arthritis was defined by joint swelling or joint pain with limitation on motion. Arthralgia was characterized by joint pain without joint edema or limitation on motion [14], and recurrent arthritis as new arthritis after total recovery [1].
Abdominal pain was determined as diffuse abdominal colicky with acute onset, and severe abdominal pain as the presence of at least one of the following: abdominal angina, bowel intussusception, and gastrointestinal bleeding. Recurrence was defined as new abdominal pain after complete resolution. Abdominal Doppler ultrasound was performed to evaluate severe abdominal involvement [1].
Scrotal involvement was defined by the presence of scrotal edema and pain/tenderness in physical examination and/or testicular Doppler ultrasound abnormalities [25, 26]. Neuropsychiatric involvement was defined as the presence of at least one neuropsychiatric manifestation, such as headache, seizure, hemiparesis, aphasia, cortical blindness, and impaired consciousness [27].
Current HSP treatment data were also recorded: corticosteroid (prednisone, prednisolone, and/or intravenous methylprednisolone), intravenous immunoglobulin (IVIG), azathioprine, cyclosporine, intravenous cyclophosphamide (IVCYC), plasmapheresis, angiotensin converting enzyme inhibitors, and angiotensin II receptor blockade.
Statistical analysis
The sample size provided power of 80% to find differences from 10 to 13% among the groups with and without HSPN (Graphpad StatMate 1.01). Results were presented as median (range) or mean ± standard deviation (SD) for continuous variables and number (%) for categorical variables. Mann-Whitney or t tests were used to compare continuous variables between the two study groups (with and without HSPN). For categorical variables, the differences were evaluated by Fisher’s exact test. Multivariate analysis was carried out by Backward Stepwise logistic regression. In the regression model, the dependent variable was the presence of HSPN and the independent variables were those with 20% in univariate analysis. For all statistical tests, p value less than 0.05 were considered of statistical significance.
Results
HSPN was diagnosed in 139/296 (47%) in the first 3 months after diagnosis. Of them, isolated hematuria was observed in 19% of HSPN patients, isolated proteinuria in 47%, and both (hematuria and proteinuria) in 26%. Nephrotic syndrome was identified in 4/139 (0.03%), acute kidney injury in 5/139 (0.04%), and none had chronic kidney insufficiency. The median disease PHS duration was 2.5 years (range 0.1–15.9).
Table 1 shows demographic data, clinical/laboratorial involvements, and treatments in with and without HSPN patients in the first 3 months after diagnosis. The median age at HSP diagnosis was significantly higher in HSPN patients compared to those without this complication [6.6 (1.5–17.7) vs. 5.7 (0.9–13.5) years, p = 0.022]. The frequencies of persistent purpura/petechiae (31 vs. 10%, p < 0.0001), recurrent abdominal pain (16 vs. 7%, p = 0.011), gastrointestinal bleeding (25 vs. 10%, p < 0.0001), and corticosteroid use (54 vs. 41%, p = 0.023) were significantly higher in the former group. No differences were evidenced regarding male gender, BMI, arterial hypertension, increased serum IgA, IVCYC, and IVIG use in HSPN patients compared to those without renal involvement (p > 0.05) (Table 1).
Table 2 presents multivariate analysis by logistic regression in HSP patients in the first 3 months after diagnosis. The logistic regression model showed that only persistent purpura (OR = 3.601; 95% CI 1.605–8.079; p = 0.002) and gastrointestinal bleeding (OR = 2.991; 95% CI 1.245–7.183; p = 0.014) were associated with HSPN.
Regarding follow-up, 172/296 (58%) had HSPN during disease course. None of them had pregnancy and/or surgery, as trigger factor of nephritis. Kidney biopsies were performed in 16/172 (9%) HSP, and ISKDC histological findings showed grade I in 3/16 (19%), grade II in 12/16 (75%), and grade III in 1/16 (6%).
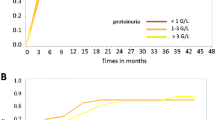
Regarding abnormalities at follow-up, further analysis of 139 HSP patients who presented nephritis in the first 3 months revealed that hematuria, cell cast, persistent non-nephrotic proteinuria, nephrotic proteinuria, and acute kidney injury were respectively observed in 1 year [35/88 (40%), 7/88 (8%), 46/88 (52%), 1/88 (1%), and 2/88 (2%)], in 5 years [16/47 (34%), 6/47 (13%), 25/47 (53%), 1/47 (2%), and 1/47 (2%)], in 10 years [6/20 (30%), 0/20 (0%), 9/20 (45%), 1/20 (5%), and 1/20 (5%)], and in 15 years [1/6 (17%), 0/6 (0%), 1/6 (17%), 0/6 (0%), and 0/6 (0%)]. None of them was submitted to renal replacement therapy: hemodialysis, peritoneal dialysis, and hemofiltration and/or kidney transplantation.
In addition, regarding abnormalities at follow-up in 118/157 (75%), HSP patients without initial nephritis showed renal involvement in 33/118 (28%) HSP patients during follow-up. Of them, 22/33 (67%) presented nephritis in 1 year, 8/33 (24%) in 5 years, 2/33 (6%) in 10 years, and 1/33 (3%) in 15 years. Of 118 HSP patients, 12/118 (10%) had hematuria, 4/118 (3%) cell cast, and 14/118 (12%) persistent non-nephrotic proteinuria in 1 year; 9/61 (15%) had hematuria, 2/61 (3%) cell cast, and 14/61 (23%) persistent non-nephrotic proteinuria in 5 years; 4/17 (23%) had hematuria, 2/17 (12%) cell cast, and 5/17 (29%) persistent non-nephrotic proteinuria in 10 years; and 2/6 (33%) had hematuria, 1/6 (17%) cell cast, and 3/6 (50%) persistent non-nephrotic proteinuria in 15 years after follow-up. None of them had nephrotic syndrome, acute kidney injury, and chronic renal disease and was submitted to renal replacement therapy. Angiotensin converting enzyme inhibitors and/or angiotensin II receptor blockade was used in the majority of HSPN patients with persistent non-nephrotic proteinuria.
Discussion
The present study observed HSPN in almost one half of patients in the first 3 months after diagnosis, and HSPN was associated with persistent purpura and gastrointestinal bleeding. One fourth of patients had nephritis only evidenced during follow-up without severe renal manifestations.
One of the strengths of this study was the inclusion of a large cohort of HSP patients followed in a tertiary center of Latin America that fulfilled the validated EULAR/PRINTO/PRES criteria [14]. The medium and long-term follow-up was relevant, since nephritis may occur solely during disease course [10,11,12]. Moreover, the use of standardized database was also important. However, the main weaknesses of our study were the retrospective design, with potential missing data, and the absence of serum galactose IgA1 analysis [28].
At disease onset, nephritis occurred from 20 to 80% of HSP patients [1, 17, 29, 30], as observed herein. The majority of our HSP patients presented mild renal abnormalities, whereas nephrotic syndrome and acute renal injury were rarely observed at the beginning of this primary vasculitis, as also previously reported [31].
Furthermore, these findings indicated low grades of severity ISKDC classification and with the absence of renal replacement therapy in our patients. Indeed, the very low frequency of end-stage renal disease may be related to the short and medium period of disease follow-up. Goldstein et al. showed progressive renal insufficiency with long-term disease duration in a UK HSP population [10, 11].
We confirmed that persistent purpura and gastrointestinal bleeding were associated with HSPN in 3 months after onset using EULAR/PRINTO/PRES criteria. These results were similar to studies carried out with European and Asiatic HSP populations; however, they used non-validated HSP criteria for children and adolescents [6, 15, 32,33,34].
Importantly, one fourth of HSP patients without initial nephritis showed additional renal alteration at medium-term assessment. Subclinical renal abnormalities, with persistent non-nephrotic proteinuria and/or hematuria, suggested mild renal alterations. The prognosis was favorable in our patients with absence of severe renal manifestations during follow-up. This aspect may suggest different pathogenesis and genetic abnormalities in our population. Further long-term and prospective study will be necessary in Latin America to evaluate these findings.
Even mild renal abnormalities may result to chronic kidney failure after long-term follow-up [10, 35, 36]. Indeed, persistent proteinuria in adult with HSP may induce tubulointerstitial fibrosis [37], and angiotensin converting enzyme inhibitors and/or angiotensin II receptor blockade should be indicated in children with proteinuria [38]. A very unusual absence of chronic renal failure/end-stage kidney disease was observed in the present study during follow-up, contrasting with a systematic review that long-term renal impairment occurred in 5% of HSP patients who had abnormal urinary findings [39]. Therefore, rigorous monitoring of renal involvement should be performed during childhood and adulthood in HSP patients.
In conclusion, in the present study, initial HSPN was associated with persistent purpura and gastrointestinal bleeding. Nephritis was also evidenced during follow-up in HSP patients without severe renal manifestations; however due to retrospective nature of this study, further prospective study will be necessary.
Funding statement
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 303422/2015-7 to CAS), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2015/03756-4 to CAS), Federico Foundation (to CAS), and by Núcleo de Apoio à Pesquisa “Saúde da Criança e do Adolescente” da USP (NAP-CriAd) to CAS.
Change history
29 January 2018
One of the author’s name on this article was incorrectly spelled as “Sylvia C. L. Fahrat” . The correct spelling is “Sylvia C. L. Farhat” and is now presented correctly in this article. The original article has been corrected.
References
de Almeida JL, Campos LM, Paim LB, Leone C, Koch VH, Silva CA (2007) Renal involvement in Henoch-Schönlein purpura: a multivariate analysis of initial prognostic factors. J Pediatr 83:259–266
Ozen S, Acar-Ozen NP (2017) Recent advances in childhood vasculitis. Curr Opin Rheumatol 29(5):530–534. https://doi.org/10.1097/BOR.0000000000000424
Júnior CR, Yamaguti R, Ribeiro AM, Melo BA, Campos LA, Silva CA (2008) Hemorrhagic vesicle-bullous lesions in Henoch-Schönlein purpura and review of literature. Acta Reumatol Port 33(4):452–456
Jauhola O, Ronkainen J, Koskimies O, Ala-Houhala M, Arikoski P, Holtta T, Jahnukainen T, Rajantie J, Ormala T, Nuutinen M (2010) Clinical course of extrarenal symptoms in Henoch-Schönlein purpura: a 6-month prospective study. Arch Dis Child 95(11):871–876. https://doi.org/10.1136/adc.2009.167874
Suehiro RM, Soares BS, Eisencraft AP, Campos LM, Silva CA (2007) Acute hemorrhagic edema of childhood. Turk J Pediatr 49(2):189–192
Chan H, Tang YL, Lv XH, Zhang GF, Wang M, Yang HP, Li Q (2016) Risk factors associated with renal involvement in childhood Henoch-Schönlein purpura: a meta-analysis. PLoS One 11(11):e0167346. https://doi.org/10.1371/journal.pone.0167346
Terreri MT, Campos LM, Okuda EM, Silva CA, Sacchetti SB, Marini R, Ferriani VP, Ventura MH, Fernandes T, Sato JO, Fernandes EC, Len C, Barbosa C, Lotito AP, Santos MC, Aikawa NE, Facó M, Piotto D, Bugni V, Kozu KT, Romanelli PR, Sallum AM, Febronio M, Fraga M, Magalhães CS (2013) Profile of paediatric rheumatology specialists and services in the state of São Paulo. Rev Bras Reumatol 53(4):346–351
Feng D, Huang WY, Hao S, Niu XL, Wang P, Wu Y, Zhu GH (2017) A single-center analysis of Henoch-Schonlein purpura nephritis with nephrotic proteinuria in children. Pediatr Rheumatol Online J 15(1):15. https://doi.org/10.1186/s12969-017-0146-4
Soylemezoglu O, Ozkaya O, Ozen S, Bakkaloglu A, Dusunsel R, Peru H, Cetinyurek A, Yildiz N, Donmez O, Buyan N, Mir S, Arisoy N, Gur-Guven A, Alpay H, Ekim M, Aksu N, Soylu A, Gok F, Poyrazoglu H, Sonmez F, Turkish Pediatric Vasculitis Study Group (2009) Henoch-Schönlein Nephritis: a nationwide study. Nephron Clin Pract 112(3):c199–c204. https://doi.org/10.1159/000218109
Goldstein AR, White RH, Akuse R, Chantler C (1992) Long-term follow-up of childhood Henoch-Schonlein nephritis. Lancet 339(8788):280–282. https://doi.org/10.1016/0140-6736(92)91341-5
Ronkainen J, Nutinen M, Koskimies O (2002) The adult kidney 24 years after childhood Henoch-Schönlein purpura: a retrospective cohort study. Lancet 360(9334):666–670. https://doi.org/10.1016/S0140-6736(02)09835-5
Butani L, Morgenstern BZ (2007) Long-term outcome in children after Henoch-Schönlein purpura nephritis. Clin Pediatr 46(6):505–511. https://doi.org/10.1177/0009922806298896
Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, Edworthy SM, Fauci AS, Leavitt RY, Lie JT (1990) The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum 33(8):1114–1121
Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, Rigante D, Cantarini L, Hilario MO, Silva CA, Alegria M, Norambuena X, Belot A, Berkun Y, Estrella AI, Olivieri AN, Alpigiani MG, Rumba I, Sztajnbok F, Tambic-Bukovac L, Breda L, al-Mayouf S, Mihaylova D, Chasnyk V, Sengler C, Klein-Gitelman M, Djeddi D, Nuno L, Pruunsild C, Brunner J, Kondi A, Pagava K, Pederzoli S, Martini A, Ruperto N, for the Paediatric Rheumatology International Trials Organisation (PRINTO) (2010) EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: final classification criteria. Ann Rheum Dis 69(5):798–806. https://doi.org/10.1136/ard.2009.116657
Zhao YL, Liu ZJ, Bai XM, Wang YC, Li GH, Yan XY (2015) Obesity increases the risk of renal involvement in children with Henoch-Schönlein purpura. Eur J Pediatr 174(10):1357–1363. https://doi.org/10.1007/s00431-015-2547-z
Elmas AT, Tabel Y (2016) Platelet counts in children with Henoch-Schonlein purpura—relationship to renal involvement. J Clin Lab Anal 3:71–74
Mao Y, Yin L, Huang H, Zhou Z, Chen T, Zhou W (2014) Henoch-Schönlein purpura in 535 Chinese children: clinical features and risk factors for renal involvement. J Int Med Res 42(4):1043–1049. https://doi.org/10.1177/0300060514530879
Nickavar A, Mehrazma M, Lahouti A (2012) Clinicopathologic correlations in Henoch-Schonlein nephritis. Iran J Kidney Dis 6(6):437–440
Wang X, Zhu Y, Gao L, Wei S, Zhen Y, Ma Q (2016) Henoch-Schonlein purpura with joint involvement: analysis of 71 cases. Pediatr Rheumatol 14(1):20. https://doi.org/10.1186/s12969-016-0080-x
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114(2):555–576. https://doi.org/10.1542/peds.114.2.S2.555
Chan JC, Williams DM, Roth KS (2002) Kidney failure in infants and children. Pediatr Rev 23(2):47–60. https://doi.org/10.1542/pir.23-2-47
Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL (2007) Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int 71(10):1028–1035. https://doi.org/10.1038/sj.ki.5002231
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39:S1–266
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE (2009) The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 76:534–545
Modi S, Mohan M, Jennings A (2016) Acute scrotal swelling in Henoch-Schönlein purpura: case report and review of the literature. Urol Case Rep 6:9–11. https://doi.org/10.1016/j.eucr.2016.01.004
Ha TS, Lee JS (2007) Scrotal involvement in childhood Henoch-Schönlein purpura. Acta Paediatr 96(4):552–555. https://doi.org/10.1111/j.1651-2227.2006.00173.x
Pacheva IH, Ivanov IS, Stefanova K et al (2017) Central nervous system involvement in Henoch-Schonlein purpura in children and adolescents. Case Rep Pediatr 2017:5483543
Kiryluk K, Moldoveanu Z, Sanders JT, Eison TM, Suzuki H, Julian BA, Novak J, Gharavi AG, Wyatt RJ (2011) Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int 80(1):79–87. https://doi.org/10.1038/ki.2011.16
Chen JY, Mao JH (2015) Henoch-Schönlein purpura nephritis in children: incidence, pathogenesis and management. World J Pediatr 11(1):29–34. https://doi.org/10.1007/s12519-014-0534-5
Hetland LE, Susrud KS, Lindahl KH, Bygum A (2017) Henoch-Schönlein purpura: a literature review. Acta Derm Venereol 2017:27
Calviño MC, Llorca J, García-Porrúa C, Fernández-Iglesias JL, Rodriguez-Ledo P, González-Gay MA (2001) Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine 80(5):279–290. https://doi.org/10.1097/00005792-200109000-00001
Anil M, Aksu N, Kara OD, Bal A, Anil AB, Yavaşcan O, Un B (2009) Henoch-Schönlein purpura in children from western Turkey: a retrospective analysis of 430 cases. Turk J Pediatr 51(5):429–436
Shin JI, Park JM, Shin YH, Hwang DH, Kim JH, Lee JS (2006) Predictive factors for nephritis, relapse, and significant proteinuria in childhood Henoch-Schönlein purpura. Scand J Rheumatol 35(1):56–60. https://doi.org/10.1080/03009740510026841
Sano H, Izumida M, Shimizu H, Ogawa Y (2002) Risk factors of renal involvement and significant proteinuria in Henoch-Schönlein purpura. Eur J Pediatr 161(4):196–201. https://doi.org/10.1007/s00431-002-0922-z
Coppo R, Andrulli S, Amore A et al (2006) Predictors of outcome in Henoch-Schönlein nephritis in children and adults. Am J Kidney Dis 47(6):993–1003. https://doi.org/10.1053/j.ajkd.2006.02.178
Tudorache E, Azema C, Hogan J, Wannous H, Aoun B, Decramer S, Deschênes G, Ulinski T (2015) Even mild cases of paediatric Henoch-Schönlein purpura nephritis show significant long-term proteinuria. Acta Paediatr 104(8):843–848. https://doi.org/10.1111/apa.12723
Kim CH, Lim BJ, Bae YS et al (2014) Using the Oxford classification of IgA nephropathy to predict long-term outcomes of Henoch-Schönlein purpura nephritis in adults. Mod Pathol 27(7):972–982. https://doi.org/10.1038/modpathol.2013.222
Tudorache E, Azema C, Hogan J et al (2014) Even mild cases of paediatric Henoch-Schönlein purpura nephritis show significant long-term proteinuria. Acta Paediatr 104:843–848
Narchi H (2005) Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child 90(9):916–920. https://doi.org/10.1136/adc.2005.074641
Author information
Authors and Affiliations
Ethics declarations
The Ethics Committee of our University Hospital approved this study, and informed consent was obtained from all patients and their legal guardians
Disclosures
None.
Additional information
One of the author’s name on this article was incorrectly spelled as “Sylvia C. L. Fahrat”. The correct spelling is “Sylvia C. L. Farhat” and is now presented correctly in this article.
Rights and permissions
About this article
Cite this article
Buscatti, I.M., Casella, B.B., Aikawa, N.E. et al. Henoch-Schönlein purpura nephritis: initial risk factors and outcomes in a Latin American tertiary center. Clin Rheumatol 37, 1319–1324 (2018). https://doi.org/10.1007/s10067-017-3972-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3972-3