Abstract
Our previous experiments found that lysyl oxidase-like 2 (LOXL2) may be a useful preclinical serological marker for pulmonary fibrosis in the mouse model. The role of LOXL2 in rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is still unclear. We investigated whether serum LOXL2 levels are associated with RA-ILD patients. The levels of serum LOXL2 were measured by enzyme-linked immunosorbent assay in 49 RA-ILD patients (21 patients with ILD disease duration < 3 months; 28 patients with ILD disease duration > 3 months), 43 RA patients without ILD and 20 normal healthy controls. We assessed the correlations between the serum LOXL2 levels and clinical variables. Serum LOXL2 levels were significantly higher in RA patients than in normal healthy controls (326.79 ± 192.56 vs. 53.27 ± 35.86 pg/ml, P < 0.01). No significant difference was present between the RA-ILD group and RA without ILD group (298.87 ± 219.85 vs. 358.60 ± 152.16 pg/ml, P = 0.13). Notably, the serum LOXL2 levels were significantly higher in patients with ILD disease duration < 3 months than in those with ILD disease duration > 3 months (462.71 ± 208.97 vs. 175.99 ± 130.55 pg/ml, P < 0.01) or without ILD (462.71 ± 208.97 vs. 358.60 ± 152.16 pg/ml, P = 0.03). The serum LOXL2 levels in RA-ILD patients significantly correlated with DAS28 (rs = 0.31, P = 0.034), C-reactive protein (rs = 0.41, P = 0.004), rheumatoid factor (rs = 0.41, P = 0.003), forced vital capacity (rs = − 0.39, P = 0.02), and diffusion capacity of the lung for carbon monoxide (rs = − 0.44, P = 0.009). LOXL2 may be involved in the pathogenesis of rheumatoid arthritis-associated interstitial lung disease and might be helpful in early diagnosis of RA-ILD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic, chronic, and autoimmune disease. Interstitial lung disease (ILD) is one of the most common extra-articular manifestations among patients with RA [1]. The estimate of prevalence and incidence of RA-ILD is variable and depends on the diagnostic method and selected population [2,3,4]. Our previous study found that 237 (237/595, 39.8%) patients had interstitial lung disease in 595 rheumatoid arthritis patients [4]. Poor prognosis has been shown in RA-ILD patients; a recent large population-based study revealed that the risk of death for RA-ILD was three times higher than in RA patients without ILD [1]. Early diagnosis is of great value in the treatment of RA-ILD.
Lysyl oxidase-like 2 (LOXL2) belongs to the lysyl oxidase family, and LOXL2’s function is similar to lysyl oxidase in the extracellular matrix (ECM) deposition by promoting cross-linking of collagen in the pathological stroma. LOXL2 protein expression is minor in healthy lung and liver tissue, but strongly expressed in idiopathic pulmonary fibrosis lung tissue and fibrotic liver [5,6,7,8]. Furthermore, LOXL2 has been thought to regulate fibrosis disease-associated extracellular and intracellular cell signaling pathways [9]. The previous research also described that higher baseline serum LOXL2 levels are associated with increased risk for disease progression in two IPF cohorts [10].
However, the role of lysyl oxidase-like 2 in rheumatoid arthritis-associated interstitial lung disease patients is still unknown. Our previous experiments found that lysyl oxidase-like 2 (LOXL2) may be a useful preclinical serological marker for pulmonary fibrosis in the mouse model. We hypothesized that serum LOXL2 has an early diagnostic value for rheumatoid arthritis with interstitial lung disease. This study was designed to investigate the relationship between serum LOXL2 levels and RA-ILD.
Patients and methods
We performed a cross-sectional study involving patients who received medical care at Chao-Yang Hospital, Capital Medical University. The patients were enrolled in our study consecutively during October 2015 to December 2016. The diagnosis, classification, clinical data and serum collection of each patient were collected at the same time.
The inclusion criterion for this study was the fulfillment of 2010 ACR-EULAR classification criteria for RA [11]. Exclusion criteria were other autoimmune and infectious diseases, neoplasm, and lung surgery. The medical records of the cases were obtained from the medical record database. Demographic characteristics, clinical features, inspection, and test data were extracted from the database. The research received ethical approval from Ethics Committee in Chao-Yang Hospital, Capital Medical University, and the principles of the Declaration of Helsinki were followed throughout the study. All participants gave written informed consent.
Taking clinical manifestation and chest HRCT results into consideration, a workgroup which included an experienced rheumatologist and radiologist comprehensively assessed the absence/presence of RA-ILD. RA-ILD was divided into two forms: ILD disease duration < 3 months and ILD disease duration > 3 months. Forty-nine patients with RA-ILD and 43 patients with RA without ILD were enrolled, and sera were obtained from these patients. Control sera were obtained from healthy staff members (n = 20).
Pulmonary function tests
All tests were performed according to American Thoracic Society standards/European Respiratory Society guidelines [12], using Master Screen PFT System (JAEGER, Germany). The results were expressed as percentages of predicted values.
High-resolution computed tomography
All patients had completed HRCT imaging scan of chest, using 1–2-mm-thickness cuts. HRCT scans were obtained with multi-detector CT scanners, including the Light-speed VCT 64-slice multi-detector CT (MDCT) system (GE Healthcare, Little Chalfont, UK), 64-channel dual source CT system (Somatom Definition, Siemens, Germany), the 256-slice MDCT (Brilliance iCT, Philips Medical Systems, Netherlands), and the 16-slice MDCT system (Somatom Emotion, Siemens, Germany). The scans were performed during end inspiration in the supine positions, and those images were evaluated in a blinded manner by two experienced investigators [13].
Measurement of LOXL2
The serum LOXL2 levels were determined using enzyme-linked immunosorbent assay (ELISA) kits (catalog number CSB-EL013041HU, CUSABIO, China) according to the manufacturer’s protocol. Briefly, calibrators, control sera, and patients’ sera (stored samples) were prepared and incubated with a mouse monoclonal antibody against LOXL2 adsorbed onto the microtiter plate wells. After washing, a mouse monoclonal antibody against LOXL2 conjugated to horseradish peroxidase was added, followed by a second washing step and the addition of tetramethylbenzidine substrate. The reaction was terminated by the addition of 2 N sulfuric acid. The absorbance was measured in a microtiter plate reader (Thermo Fisher Scientific, Waltham, MA, USA) set to 450 nm. The assay range was 37.5 to 2400 pg/ml.
Statistical analysis
Analyses were performed by SPSS19.0 (SPSS Inc., Chicago, IL, USA) software. All P values were 2-tailed and P < 0.05 was considered to be statistically significant. Continuous variables were presented in terms of the mean and standard deviation for normally distributed data or the median (P 25, P 75) for non-normally distributed data. Categorical variables were indicated as percentages. Continuous variables were compared using the Student’s t test or Mann-Whitney U test. Categorical variables were compared with the Chi-square test or Fisher’s exact test when the expected values were below 5. The relationships between serum LOXL2 levels and other continuous variables were analyzed using Pearson or Spearman’s rank correlation.
Results
Serum LOXL2 levels were markedly elevated in RA patients with ILD disease duration < 3 months
A total of 92 RA inpatients were identified in this study (49 patients with RA-ILD and 43 patients with RA without ILD). Sixty patients were female and 32 patients were male. The age of the RA patients was 62.70 ± 11.54 years old, and ranged in age from 33 to 95 years. Among the normal health controls (NHCs), 12 were women and 8 were men. The age of the NHCs was 39.15 ± 12.65 years old. There were significant differences between patients with RA and NHCs in terms of age. The NHCs ranged in age from 18 to 60 years. So, we divided the 49 RA-ILD patients into two groups by the age of 60 years: group 1 (less than 60 years old, n = 16) and group2 (above 60 years old, n = 33). The age of the two groups were 51.93 ± 6.31 and 70.93 ± 8.62, respectively, yet no statistic difference of serum level of LOXL2 was found (221.39 ± 192.85 vs. 336.43 ± 224.97 pg/ml, P = 0.086). Moreover, correlation analysis shows there was no significant correlation between serum LOXL2 levels and age in all participants (rs 0.13, P = 0.22).
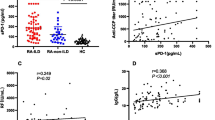
To investigate the association of serum LOXL2 levels with RA, we first compared LOXL2 levels between 92 patients with RA and 20 normal healthy controls (NHCs) by using ELISA (Fig. 1). Serum LOXL2 levels were significantly higher in RA patients than in NHCs (326.79 ± 192.56 vs. 53.27 ± 35.86 pg/ml, P < 0.01). Considering RA is usually complicated with ILD, we next compared serum LOXL2 levels among RA patients with ILD (n = 49, ILD disease duration < 3 months (n = 21) and ILD disease duration > 3 months (n = 28)) and those without ILD (n = 43). And no significant difference was presented between the RA-ILD group and RA without ILD group (298.87 ± 219.85 vs. 358.60 ± 152.16 pg/ml, P = 0.13). However, the serum LOXL2 levels were significantly higher in patients with ILD disease duration < 3 months than in those with ILD disease duration > 3 months (462.71 ± 208.97 vs. 175.99 ± 130.55 pg/ml, P < 0.01) or without ILD (462.71 ± 208.97 vs. 358.60 ± 152.16 pg/ml, P = 0.03) (Fig. 1).
Serum lysyl oxidase-like 2 levels were elevated in rheumatoid arthritis-associated interstitial lung disease patients with ILD disease duration < 3 months. Serum lysyl oxidase-like 2 (LOXL2) levels in 49 RA-ILD patients (21 patients with ILD disease duration < 3 months; 28 patients with ILD disease duration > 3 months), 43 patients without interstitial lung disease (ILD) and 20 normal healthy controls (NHCs) are shown, as measured by enzyme linked immunosorbent assay. The serum LOXL2 levels in patients with ILD disease duration < 3 months were significantly higher
Comparison of clinical manifestations among three groups: ILD disease duration < 3 months, ILD disease duration > 3 months and RA without ILD
We compared clinical manifestations among the three groups: ILD disease duration < 3 months, ILD disease duration > 3 months, and RA without ILD (Table 1). There were no significant differences among all the subsets in terms of age and sex. As for the duration of RA, it lasted dramatically shorter in patients with ILD disease duration < 3 months than that ILD disease duration > 3 months. Although ESR levels showed no significant difference among all the subsets, CRP levels were significantly higher in RA patients with ILD disease duration < 3 months than in those with ILD disease duration > 3 months (P < 0.05). In our study, the anti-CCP antibody titres were high and had no statistical differences among all the subsets. Compared to RA without ILD, higher level of RF was observed in RA with ILD disease duration < 3 months patients (P < 0.05). The levels of IgG, IgM and IgA have no significant difference between all the subsets. Although DAS28 tended to be lower in RA patients with ILD disease duration >3 months, no significant difference has shown. The frequency of usual interstitial pneumonia (UIP) pattern in HRCT was higher in RA patients with ILD disease duration > 3 months (13/28, 46.43%, and 8/21, 38.10%), but there was no significant difference between the two groups (Table 1).
Only 34 participants had pulmonary function test results. Thirteen of 34 were RA with ILD disease duration < 3 months, and the other 21 were RA with ILD disease duration > 3 months patients. Comparison of pulmonary function parameters between the two groups showed that no significant difference was observed in the percent of the predicted value of forced vital capacity (FVC) and diffusion capacity of the lung for carbon monoxide (DLCO). In total, 35 patients with RA (35/92, 38.04%) were treated with a combination of glucocorticoid and disease-modifying anti-rheumatic drugs (DMARDs). The frequency of treatment with a combination of glucocorticoid and DMARDs was higher in RA patients with ILD disease duration < 3 months (9/21, 42.86%), whereas there were no significant differences among all the subsets in terms of treatment (Table 1).
Correlation between serum LOXL2 levels and clinical variables in patients with RA-ILD
The serum LOXL2 levels in RA-ILD patients correlated with DAS28 (rs = 0.31, P = 0.034). Meanwhile, serum LOXL2 levels showed a significantly positive correlation with serum levels of C-reactive protein (rs = 0.41, P = 0.004) and rheumatoid factor (rs = 0.41, P = 0.003). Serum LOXL2 levels showed a negative correlation with pulmonary function parameters of FVC (rs = − 0.39, P = 0.02) and DLCO (rs = − 0.44, P = 0.009). However, there was no significant correlation between serum levels of LOXL2 and ESR or Anti-CCP (Table 2).
Discussion
Our research results show that serum LOXL2 levels were significantly elevated in RA patients with ILD disease duration < 3 months, suggesting that circulation LOXL2 may have predictive value for ILD.
The lysyl oxidase (LOX) family comprises five members: lysyl oxidase (LOX) and four lysyl oxidase-like proteins (LOXL1, LOXL2, LOXL3, and LOXL4). LOX is a copper and lysine tyrosylquinone-dependent amine oxidase. LOXL2 has a similar biological function to LOX, which promotes cross-linking of collagen in the pathological stroma, and plays a crucial role in the extracellular matrix (ECM) deposition and matrix remodeling [9]. The gene of LOXL2 was first discovered in 1997, and the expression of LOXL2 was significantly higher in placenta, prostate, and pancreas, but lower expression in lung, brain, skeletal muscle, and kidney. Previous research reported that dysregulation of LOXL2 has been associated with many diseases, including fibrosis, cancer, and pro-oncogenic angiogenesis diseases [9, 10, 14]. Moreover, LOXL2 were strongly expressed in idiopathic pulmonary fibrosis lung tissue and fibrotic liver. Under pathological conditions, activated fibroblasts can secrete large amounts of LOXL2 [15], and inhibiting LOXL2 can effectively reduce the fibroblast activation, along with a declined level of transforming growth factor-beta [5]. Recent studies demonstrated that LOXL2 not only collaborates with Snail1 to trigger epithelial-to-mesenchymal transition (EMT), but also promotes FAK/Src pathway activation to support EMT [14]. Meanwhile, the expression of LOXL2 can be regulated by transforming growth factor-beta, platelet-derived growth factor, angiotensin II, retinoic acid, and fibroblast growth factor [9].
In bleomycin-induced pulmonary fibrosis mouse model, our research team found that LOXL2 was highly expressed in the lung tissue, and serum LOXL2 levels were significantly higher in the third day following bleomycin instillation. While the histological examination of lung tissues at this time showed as the early stage of alveolar inflammation, collagen fibers stain was negative, and no obvious change in lung computed tomography images (data not yet published). Those results indicate the elevation of serum LOXL2 level was earlier than fibrosis pathological and radiological abnormal changes, which provide a meaningful message that LOXL2 might be a possible predictor for early alveolar inflammation in interstitial lung disease. Our previous research also discovered that LOXL2 levels of serum and lung tissue homogenate were elevated in parallel, which revealed that lung tissue may be a major source of circulation LOXL2 in pulmonary fibrosis disease. The results suggested that LOXL2 may be a sensitive indicator for RA-ILD.
In current study, based on duration of ILD disease, RA-ILD was divided into two forms: ILD disease duration < 3 months and ILD disease duration > 3 months. The LOXL2 levels were significantly higher in patients with ILD disease duration < 3 months than in those with ILD disease duration > 3 months or without ILD, which was consistent with the findings of mouse model in our previous study. Previous research showed that Wilson’s disease and primary biliary cirrhosis patients express LOX and LOXL2 in hepatocytes, along with collagen deposition around the hepatocytes. Especially in Wilson’s disease, LOXL2 expression is up-regulated even before the onset of fibrosis [7]. Thus, the authors speculated that LOX and LOXL2 could be an early diagnostic marker for liver fibrosis in Wilson’s disease. The early rise in serum LOXL2 levels may be associated with pathogenesis of pulmonary interstitial disease. The pathological process of interstitial lung disease can be artificially divided into two stages: the early stage of inflammatory immune response and the later stage of lung fibrosis [16]. With the aggravation of pulmonary fibrosis, patients’ lung function was gradually deteriorated and progressed to respiratory failure eventually. Patients with ILD disease duration < 3 months may be mainly in the early stage of interstitial lung disease, and the pathological changes were alveolar inflammation. As a predictor of alveolar inflammation, LOXL2 expression can up-regulate even before the onset of fibrosis. Therefore, circulating LOXL2 could perhaps be utilized for early diagnostic purposes in RA-ILD.
Interestingly, previous reports showed higher baseline LOXL2 levels associated with increased risk for disease progression in two IPF cohorts [10]. We also found that serum LOXL2 levels were correlated with rheumatoid factor, C-reactive protein, FVC, and DLCO. Considering that LOXL2 may play an essential role in the pathogenesis of RA-ILD, more comprehensive follow-up research could be fulfilled to examine the prognostic potential of serum LOXL2 in the future. It is possible that serum LOXL2 may enhance the predictive value for RA-ILD, along with other promising CTD-ILD biomarkers (e.g., KL-6, surfactant protein-A and D, chitinase-3-like protein 1, or cytokines such as CCL18) [17], and might have prognostic value for helping evaluate disease progression.
This study does have some inevitable limitations. The sample size of our study was small, and the study was designed and enrolled the patients in a single medical care center. There were potential confounding effects on serum LOXL2 levels in RA patients, like treatment. Our research findings need to be validated in distinct RA cohorts, and we will continue to explore the role of LOXL2 in the development of RA-ILD and the value in the clinical application.
In conclusion, these results suggest that serum LOXL2 may play a role in the early stage of RA-ILD. Serum level of LOXL2 is worthy of further investigation and might be helpful in early diagnosis of RA-ILD.
References
Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, Gabriel SE, Matteson EL (2010) Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum 62(6):1583–1591. https://doi.org/10.1002/art.27405
Wang JX, CG D (2015) A retrospective study of clinical characteristics of interstitial lung disease associated with rheumatoid arthritis in Chinese patients. Med Sci Monit 21:708–715. 10.12659/msm.890880
Sokka T, Kautiainen H, Toloza S, Makinen H, Verstappen SM, Lund Hetland M, Naranjo A, Baecklund E, Herborn G, Rau R, Cazzato M, Gossec L, Skakic V, Gogus F, Sierakowski S, Bresnihan B, Taylor P, McClinton C, Pincus T (2007) QUEST-RA: quantitative clinical assessment of patients with rheumatoid arthritis seen in standard rheumatology care in 15 countries. Ann Rheum Dis 66(11):1491–1496. https://doi.org/10.1136/ard.2006.069252
Zhang Y, Li H, Wu N, Dong X, Zheng Y (2017) Retrospective study of the clinical characteristics and risk factors of rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 36(4):817–823. https://doi.org/10.1007/s10067-017-3561-5
Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V (2010) Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 16(9):1009–1017. https://doi.org/10.1038/nm.2208
Ikenaga N, Peng ZW, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, Mikels-Vigdal A, Smith V, Schuppan D, Popov YV (2017) Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. https://doi.org/10.1136/gutjnl-2016-312473
Vadasz Z, Kessler O, Akiri G, Gengrinovitch S, Kagan HM, Baruch Y, Izhak OB, Neufeld G (2005) Abnormal deposition of collagen around hepatocytes in Wilson’s disease is associated with hepatocyte specific expression of lysyl oxidase and lysyl oxidase like protein-2. J Hepatol 43(3):499–507. https://doi.org/10.1016/j.jhep.2005.02.052
Mehal WZ, Iredale J, Friedman SL (2011) Scraping fibrosis: expressway to the core of fibrosis. Nat Med 17(5):552–553. https://doi.org/10.1038/nm0511-552
Moon HJ, Finney J, Ronnebaum T, Mure M (2014) Human lysyl oxidase-like 2. Bioorg Chem 57:231–241. https://doi.org/10.1016/j.bioorg.2014.07.003
Chien JW, Richards TJ, Gibson KF, Zhang Y, Lindell KO, Shao L, Lyman SK, Adamkewicz JI, Smith V, Kaminski N, O'Riordan T (2014) Serum lysyl oxidase-like 2 levels and idiopathic pulmonary fibrosis disease progression. Eur Respir J 43(5):1430–1438. https://doi.org/10.1183/09031936.00141013
Villeneuve E, Nam J, Emery P (2010) 2010 ACR-EULAR classification criteria for rheumatoid arthritis. Rev Bras Reumatol 50(5):481–483
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J (2005) Standardisation of spirometry. Eur Respir J 26(2):319–338. https://doi.org/10.1183/09031936.05.00034805
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Muller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schunemann HJ (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183(6):788–824. https://doi.org/10.1164/rccm.2009-040GL
Cuevas EP, Moreno-Bueno G, Canesin G, Santos V, Portillo F, Cano A (2014) LOXL2 catalytically inactive mutants mediate epithelial-to-mesenchymal transition. Biol Open 3(2):129–137. https://doi.org/10.1242/bio.20146841
Hinz B (2012) Mechanical aspects of lung fibrosis: a spotlight on the myofibroblast. Proc Am Thorac Soc 9(3):137–147. https://doi.org/10.1513/pats.201202-017AW
Bagnato G, Harari S (2015) Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev 24(135):102–114. https://doi.org/10.1183/09059180.00003214
Bonella F, Costabel U (2014) Biomarkers in connective tissue disease-associated interstitial lung disease. Semin Respir Crit Care Med 35(2):181–200. https://doi.org/10.1055/s-0034-1371527
Acknowledgements
Project 81471616 supported by National Nature Science Foundation of China.
Author information
Authors and Affiliations
Contributions
Qiang Fu wrote the manuscript. Yu bai and Yuan Liu collected the clinical data. Qiang Fu and Junfei Zhou completed the ELISA test. Yi Zheng supervised the study.
Corresponding author
Ethics declarations
Disclosures
None.
Ethical approval
The research received an ethical approval from Ethics Committee in Chao-Yang Hospital, Capital Medical University, and the principles of the Declaration of Helsinki were followed throughout the study.
Statement of informed consent
All participants gave written informed consent.
Rights and permissions
About this article
Cite this article
Fu, Q., Bai, Y., Liu, Y. et al. The serum level and significance of lysyl oxidase-like 2 in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol 37, 193–198 (2018). https://doi.org/10.1007/s10067-017-3878-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3878-0