Abstract
The use of hydroxychloroquine (HCQ) in Primary Sjögren’s Syndrome (pSS) has been assessed in different studies over the last years, with conflicting results regarding its efficacy in sicca syndrome and extraglandular manifestations (EGM). The goal of this study was to compare the incidence rate of EGM in pSS patients with and without HCQ therapy.
We performed a multicenter retrospective study, including patients with pSS (European classification criteria) with at least 1 year of follow-up. Subjects with concomitant fibromyalgia, autoimmune hepatitis, primary biliary cirrhosis, and primary sclerosing cholangitis were excluded. Demographics and pSS characteristics were recorded. The EGM were defined by EULAR-SS disease activity index (ESSDAI). Patients were divided into two groups according to their use or not of HCQ therapy. We evaluated the use of HCQ and its relationship to EGM. HCQ therapy was defined as the continuous use of the drug for at least 3 months. A descriptive analysis of demographics and pSS characteristics was performed. We compared the incidence of EGM between groups defined by HCQ therapy using chi2 test or Fisher’s exact test. A total of 221 patients were included (97.3% women), mean age, 55.7 years (SD 14). Mean age at diagnosis, 48.8 years (SD 15); median disease duration, 60 months (IQR 35–84). One hundred and seventy patients (77%) received HCQ. About half of the patients had at least one EGM during the course of the disease, 20% of them developed an EGM before the onset of the sicca syndrome and 26% simultaneously with dryness symptom. Overall, EGM were less frequent in those on HCQ therapy (36.5% vs 63.5%, p < 0.001). Considering each EGM individually, the following manifestations were more frequent in the non-treated group: arthritis (p < 0.001), fatigue (p < 0.001), purpura (p = 0.01), Raynaud phenomenon (p = 0.003), and hypergammaglobulinemia (p = 0.006). Immunosuppressive treatment was indicated on 28 patients (12.7%), 13 of which were receiving also HCQ. The first reason for those treatments was the presence of arthritis in 12/28 patients (42.8%), and the drug used in all the cases was methotrexate. Only three patients required immunosuppressive therapy with cyclophosphamide, due to the presence of glomerulonephritis, vasculitis, and interstitial lung disease. None of the patients received biologic therapy. The lower incidence of EGM was observed in patients on HCQ therapy supports its efficacy in pSS. However, further large scale prospective studies are needed to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sjögren’s syndrome (SS) is a chronic systemic autoimmune disease characterized by a clinical spectrum of sicca symptoms and multiple extraglandular manifestations (EGM). Primary Sjögren syndrome (pSS) occurs in the absence of another underlying rheumatic disorder, whereas secondary Sjögren syndrome is associated with other underlying rheumatic diseases.
Systemic or extraglandular symptoms are divided in non-visceral (cutaneous and musculoskeletal) and visceral (pulmonary, cardiovascular, renal, gastrointestinal, endocrinological, and neurological, among others), and the most feared one is an increased prevalence of lymphoproliferative disease [1, 2].
The classical therapeutic approach is based on the symptomatic treatment of sicca complex and on the use of different immunomodulatory and immunosuppressive agents for EGM. These drugs must be adjusted to the specific organ or organs involved, with limited data from controlled trials to guide the treatment [3,4,5]. Different drugs, with different mechanisms of action have been tested in this disease, mainly those that already showed effectiveness in other rheumatic diseases such as methotrexate, leflunomide [6], and more recently cyclosporine [7].
Hydroxychloroquine (HCQ) is an immunomodulatory drug widely used in pSS, especially for patients with fatigue, arthralgia, arthritis or myalgia. However, there is too little evidence supporting its administration. Most literature consists of open-label, retrospective studies, with small samples [8, 9]. A recently published double-blind and placebo-controlled study assessed hydroxychloroquine effect on fatigue and sicca symptoms in 120 patients, and no statistical difference was found between both groups [10].
Taking all this into account, and because there is no publication assessing the influence of HCQ therapy on EGM in pSS patients, we performed a multicenter retrospective study, with the objective of comparing the incidence rate of EGM in pSS patients with and without HCQ therapy.
Methods
A retrospective review of pSS patient clinical histories of different Rheumatology centers of Argentina was made. The study included patients meeting the EULAR Criteria 2002 for pSS [11] with at least 1 year of follow-up. Patients with fibromyalgia, autoimmune hepatitis, primary biliary cirrhosis, or primary sclerosing cholangitis were excluded.
Age, gender, and pSS disease duration were recorded. HCQ therapy was defined as the continuous use of the drug for at least 3 months. We also recorded the date of the onset of HCQ therapy, whether or not the drug was discontinued, the reason for the discontinuation, and the discontinuation and restart dates for each course of therapy. Patients without HCQ therapy were also included as a control group.
Always taking in account their onset date, we assessed the following EGM: arthritis, fatigue, purpura, Raynaud phenomenon, pericarditis, myocarditis, interstitial lung disease, lymphocytic alveolitis, pulmonary fibrosis, pleural effusion, pulmonary hypertension, renal tubular acidosis, glomerulonephritis, interstitial cystitis, peripheral neuropathy, central nervous system (CNS) vasculitis, lymphadenopathies, lymphoma, hypocomplementemia, hypergammaglobulinemia, cryoglobulinemia, and anemia of chronic disease.
The requirement of immunomodulatory, immunosuppressive, and/or biological therapy was specified. For each therapy, we recorded the drug—high dose corticosteroid therapy (an equivalent dose to prednisone equal or higher to 40 mg, methotrexate, azathioprine, leflunomide, cyclophosphamide, cyclosporine, mycophenolate mofetil, anti-TNFα, anti-CD20, or another biological agent—the date of therapy onset, and the reason for therapy administration.
The current use or not of HCQ was recorded in every patient showing an EGM and/or requiring immunomodulatory or immunosuppressive therapy.
The study was approved by the institutional review board or ethics committee at each participating site and was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
Statistical analysis
We described patient and disease characteristics using proportions, means, medians, standard deviation (SD), and interquartile range (IQR). Extraglandular manifestation (EGM) prevalence and distribution were estimated. We compared the proportion of EGM in groups with and without HCQ therapy using chi-square test or Fisher’s exact test for categorical variables, and Student t test, or Mann-Whitney test for continuous variables. Differences were considered statistically significant at p values < 0.05. Statistical analyses were performed using Stata Version 11.0 (StataCorp LP, College Station, TX, USA).
Results
A total of 221 pSS patients from 7 rheumatology private and public centers of Argentina were included. Two hundred and fifteen patients (97.3%) were women, with a mean age at diagnosis of 48.8 years (SD 14) and median disease duration of 60 months (IQR 35–84). One hundred and seventy patients (77%) received HCQ therapy, from which 25 (17%) had an intermittent use or discontinuation of the drug. The main reasons for the discontinuation were visual field disturbances and the patient’s own decision for the lack of symptom improvement. Among the patients that received HCQ continuously (n = 145), the median treatment duration was 45 months (IQR = 25–76).
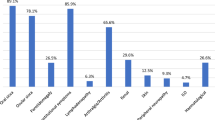
One hundred and fifteen patients (52%) showed EGM during the course of the disease. The most frequently reported manifestations were arthritis in 49 patients (22.1%), hypergammaglobulinemia in 47 (21.2%), and fatigue in 30 (13.6%). Less frequently reported ones were as follows: Raynaud phenomenon (n = 20; 9%), hypocomplementemia (n = 18; 8.1%), anemia of chronic disease (n = 17; 7.7%), lymphadenopathies (n = 14; 6.3%), and peripheral neuropathy (n = 10; 4.5%). Fewer than 5% of the manifestations were cutaneous, pulmonary, and/or renal; lymphoma was reported only in two patients (0.9%) (Table 1).
Considering the time of EGM presentation relative to diagnosis, we found that 58 out of 115 patients (50.4%) presented one EGM after pSS diagnosis with a median time of 4 years (IQR, 1.3–5). In 30 patients (26%), the pSS diagnosis was concomitant with EGM presentation. The remaining 20% referred one or more EGM some years before pSS diagnosis, with a median time between EGM and diagnosis of 4 years (IQR, 2–9). The most frequently reported EGM before pSS diagnosis were: arthritis in 8 out of 26 patients, Raynaud phenomenon in 5, and lymphadenopathies in 4. Less frequent ones were peripheral neuropathy, purpura, and polyclonal hypergammaglobulinemia. For four patients, no presentation time was recorded.
Regarding HCQ therapy, most of the 115 patients with one or more EGM (n = 73; 63.5%) did not use HCQ as basal therapy. Conversely, only 25 (23.6%) of the 106 without EGM did not receive HCQ therapy, and this difference was statistically significant (p < 0.001) (Fig. 1). We compared patients with EGM with or without HCQ therapy and we found a statistically significant lower use for the following manifestations: arthritis (22.4%, p < 0.001), fatigue (16.7%, p < 0.001), purpura (18.2%, p < 0.01), Raynaud phenomenon (25%, p = 0.003), and hypergammaglobulinemia (38.3%, p = 0.006) (Table 2).
There were two cases of malignant lymphoproliferative disease: a mucous associated lymphoid tissue lymphoma (MALT) and a Hodgkin lymphoma. Although both patients were on HCQ therapy at the time of diagnosis, their treatment was discontinuous. Regarding these patients, both women had an early diagnosis (35 years) of pSS and its onset was with sicca symptoms and associated EGM. They had also arthritis, fatigue, lymphadenopathies, Raynaud phenomenon, hypergammaglobulinemia, and anemia of chronic disease (some of them considered bad prognosis factors), preceding the diagnosis of lymphoma by years. In addition, both patients received methotrexate for arthritis management. The time between the onset of pSS and the diagnosis of MALT and Hodgkin disease was 5 and 15 years, respectively.
Out of the 221 patients analyzed, 28 (12.7%) required immunosuppressive and/or immunomodulatory therapy, and 13 of them were already on HCQ therapy. The first reason to initiate another treatment was the presence of arthritis in 12/28 patients (42.8%), and methotrexate was the drug used in all the cases. Only three patients required immunosuppressive treatment with cyclophosphamide for glomerulonephritis, interstitial lung disease, and vasculitis. The reasons leading to the administration of other immunosuppressive and/or immunomodulatory treatments were: cutaneous vasculitis, lymphopenia, glomerulonephritis, and ophthalmological manifestations (scleritis and ocular pemphigus). No patients required biological therapy.
Discussion
Antimalarial drugs have shown beneficial effects in many autoimmune diseases, like induction of remission in combination with other disease modifying drugs in rheumatoid arthritis or an immunomodulatory and cardioprotective effect in patients with systemic lupus erythematous [12,13,14,15].
However, currently, the use of HCQ in pSS is based in expert recommendations and in few studies with a low level of evidence. There are very few publications assessing HCQ use in a double-blind, randomized, and placebo-controlled studies. The first of them was a crossover study conducted by Kruise A. et al. [16] in 1993, and included only 19 pSS patients followed during 2 years. The study assessed sicca symptoms and EGM, and found statistically significant differences in the group with HCQ therapy, but the effects were only a reduction in the erythrocyte sedimentation rate (ESD) and in the levels of immunoglobulins (Ig) G and M. The second study was a randomized controlled trial, conducted 20 years later by Gottenberg J. et al. [10], assessed 120 pSS patients without HCQ, rituximab or cyclophosphamide therapy and without EGM in the last 6 months. The primary objective was to reach a 30% improvement at 24th week of therapy at least in two of following: sicca symptoms, fatigue, and arthralgia (measured with a visual analogue scale). The study analyzed also validated activity indexes like ESSDAI (EULAR Sjögren’s syndrome disease activity index), ESSPRI (EULAR Sjögren’s syndrome patient reported index), quality of life, and objective tests like Schirmer test, unstimulated whole saliva flow rate, ESD, IgG, IgM, and IgA levels. The only statistically significant difference observed in this study was a reduction of ESD at 24th week in the group on HCQ therapy.
In 2016, Chang et al. [17] published the third and most recent controlled trial on HCQ efficacy. The primary goal of the study was to assess the effect of HCQ on ocular dryness and secondarily on systemic inflammation using VSG, IL-17, IL-6, BAFF, and LTh-17. The results showed that HCQ 300 mg daily for 12 weeks apparently had no clinical effect on ocular dryness or systemic inflammation.
Other papers that evaluated HCQ therapy in pSS were retrospective studies with a small number of patients, primarily assessing sicca symptoms, with contradictory results [18–22].
In 1998, Fox et al. [18] found a significant reduction of total immunoglobulin G in a group of pSS patients on HCQ therapy. Another study of the same author from 1996 [19] reported a sustained improvement of the symptoms (painful eyes, painful mouth) and a reduction of systemic manifestations (arthralgias, myalgias) after HCQ therapy. An open-label study by Tishler et al. [20] found that HCQ could achieve a significant reduction of some salivar inflammatory markers in pSS patients. In 2011, Yavuz et al. [21] showed that HCQ could relieve signs and symptoms of ocular dryness in pSS and reduce BAFF in lacrimal fluid. However, as mentioned before, so far, no controlled study has shown the efficacy of HCQ therapy. This finding agrees with the only meta-analysis conducted on the subject and recently published in 2017 by Shi-Qin Wang et al. [22]. This meta-analysis included two randomized controlled trials, one double-blind, crossover study, and an open-label retrospective study, with a total of 215 pSS patients. For subjective symptoms, like mouth dryness, the efficacy of HCQ therapy was slightly higher than placebo (grouped proportion = 47.9 vs 42.6%), and it was superior to placebo for ocular dryness (50.6 vs 46.4%). Its efficacy was also superior for pain (48.9 vs 35.8%). Instead, the response was higher to placebo in patients with fatigue (35.9 vs. 51.4%). With regard to the objective indexes, the meta-analysis could only measure the grouped weighted mean difference for ESR and Schirmer test, and found that HCQ therapy achieved a statistically significant reduction of ESR (p < 0.05), with no difference in Schirmer test (p = 0.97).
In summary, based on the literature, the efficacy of HCQ therapy for the relief of sicca symptoms and EGM in pSS patients is currently extremely limited. Therefore, our first goal was to assess the EGM in pSS patients and its association with HCQ therapy.
Of the 221 patients included, we observed an incidence of EGM of 51%, a little lower than the frequency of 80% of EGM in 41 pSS patients reported by Zazzetti et al. [23] in Argentina. In 2011, the Sjögren Study Group of the Sociedad Argentina de Reumatología (GESSAR) described clinical characteristics of 284 pSS patients and reported a higher frequency of EGM than those of our study in all the EGM evaluated; this may be explained by the different definitions used for each EGM. On the other hand, our results were similar to those of the Spanish cohort of 921 patients of Ramos-Casals M. et al. in 2014 [24] that reported a 40% of systemic involvement. At the end of 2015, the same authors in association with the EULAR-SS Task Force made a systematic revision of 233 studies on EGM in pSS. The objective was to describe epidemiological characteristics of each manifestation, on the base of EULAR-SS disease activity index (ESSDAI), to obtain clear consensus definitions about systemic involvement in this pathology [25]. Regarding articular and cutaneous involvement, prevalence proportions are similar to our results; on the other hand, on pulmonary and renal involvement, theirs were higher than ours (pulmonary interstitial disease, 16 vs 3%; glomerulonephritis, 4 vs 1%). As we mentioned before, these differences could be related to the absence of consensus about definitions for EGM in pSS or to the small number of studies with low level of evidence in the literature concerning this disease. Then, in order to get a better understanding of the diagnosis and therapeutic management of this disease, an active collaboration with multicenter records that could improve the prognosis of the patient subgroup with more serious systemic disease could be promoted.
In our study, one or more EGM were reported only in 36.5% of patients on HCQ therapy, while we found EGM in 63.5% of those without HCQ therapy, and this difference was statistically significant (p < 0.001). On the other hand, most of the patients (76.4%) that did not develop any EGM during the course of the disease had received HCQ therapy. It is important to highlight that most of our patients (66%) initiated HCQ therapy in the moment the diagnosis of pSS was confirmed, with the occurrence of any previous EGM.
As it was demonstrated for RA and SLE, we found that arthritis was less frequent in patients on HCQ therapy. Unlike few previous reports, the same was found for fatigue, purpura, Raynaud phenomenon, and hypergammaglobulinemia; and this could reinforce the immunomodulatory role of HCQ in pSS. Although the observed differences do not reach statistical significance for the remaining EGM, the frequency was always lower in the group on HCQ therapy. Only two of our patients developed a malignant lymphoproliferative disease, one of the most feared complications of pSS, too low number for making a correct analysis. Even though both patients were on HCQ therapy, they had also a more aggressive disease, an early age onset, associated with bad prognosis factors and both had presented different EGM with the institution of different treatments.
Recently, Brito-Zerón P. together with a EULAR-SS group has published clinical recommendations for an early diagnosis of pSS [26]. In this revision, they describe a presentation subgroup of the disease where EGM preceding sicca symptoms, that they called “hidden Sjögren.” In our study, we found that almost 20% of the patients showed EGM before the onset of sicca symptoms and another 26% presented with concurrent EGM and sicca symptoms. These findings agree with the new guidelines of the disease, which pose the suspicion and early diagnosis, without relying only on sicca symptoms, but keeping in mind the systemic nature of pSS.
The study’s retrospective design could pose, among others, some deficiencies like the missing data and a lack of more objective and measurable evaluations (like activity indexes). The most important limitation of this study is that we have no data for patients without EGM. Because the use of this drug in pSS patients is a usual practice, we must highlight that more than half of our patients with EGM did not use HCQ. Even though we recognize these limitations, we consider that it is, so far, one of the few studies that assess the association between EGM and HCQ therapy in patients with pSS. In addition, we highlight that the number of patients was big (n = 221) and the mean duration of the disease in our patients (48 months) was long enough to allow the occurrence of EGM.
In conclusion, we emphasize the positive results found with HCQ therapy in patients with pSS in our study; however, further large scale prospective studies are needed to confirm these findings.
References
Ramos-Casals M, Brito-Zeron P, Siso-Almirall A, Bosch X (2012) Primary Sjögren syndrome. BMJ 344. https://doi.org/10.1136/bmj.e3821
Fox RI (2005) Sjögren’s syndrome. Lancet 366:321–331. https://doi.org/10.1016/S0140-6736(05)66990-5
Brito-Zerón P, Sisó-Almirall A, Bové A, Kostov BA, Ramos-Casals M (2013) Primary Sjögren syndrome: an update on current pharmacotherapy options and future directions. Expert Opin Pharmacother 14:279–289. https://doi.org/10.1517/14656566.2013.767333
Mavragani CP, Moutsopoulos NM, Moutsopoulos HM (2006) The management of Sjögren’s syndrome. Nat Clin Pract Rheumatol 2:252–261. https://doi.org/10.1038/ncprheum0165
Ter Borg EJ, Kelder JC (2016) Polyarthritis in primary Sjögren’s syndrome represents a distinct subset with less pronounced B cell proliferation a Dutch cohort with long-term follow-up. Clin Rheumatol 35:649–655. https://doi.org/10.1007/s10067-016-3175-3
Scagliusi P, Minenna G, D’Amore M, Scagliusi A (2005) New therapeutic perspectives in Sjögren syndrome: leflunomide. Recenti Prog Med 96:194
Kedor C, Zernicke J, Hagemann A, Gamboa LM, Callhoff J, Burmester GR, Feist E (2016) A phase II investigator-initiated pilot study with low-dose cyclosporine A for the treatment of articular involvement in primary Sjögren’s syndrome. Clin Rhreumatol 35:2203–2210. https://doi.org/10.1007/s10067-016-3360-4
Patel R, Shahane A (2014) The epidemiology of Sjögren’s syndrome. Clin Epidemiol 6:247–255. https://doi.org/10.2147/CLEP.S47399
Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG (2012) Topical and systemic medications for the treatment of primary Sjögren’s syndrome. Nat Rev Rheumatol 8:399–411. https://doi.org/10.1038/nrrheum.2012.53
Gottenberg JE, Ravaud P, Puéchal X, Le Guern V et al (2014) Effects of hydroxychloroquine on symptomatic improvement in primary Sjögren syndrome: the JOQUER randomized clinical trial. JAMA 312:249–258. https://doi.org/10.1001/jama.2014.7682
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL et al (2002) Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61:554–558
The Canadian Hydroxychloroquine Study Group (1991) A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 324:150–154
Wallace D (1994) Antimalarial agents and lupus. Rheum Dis Clin N Am 20:243–263
Saunders SA, Capell HA, Stirling A, Vallance R, Kincaid W, McMahon AD, Porter DR (2008) Triple therapy in early active rheumatoid arthritis: a randomized, single-blind, controlled trial comparing step-up and parallel treatment strategies. Arthritis Rheum 58:1310–1317
Willis R, Seif AM, McGwin G Jr, Martinez-Martinez LA, González EB, Dang N, Papalardo E, Liu J, Vilá LM, Reveille JD, Alarcón GS, Pierangeli SS (2012) Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus 21:830–835. https://doi.org/10.1177/0961203312437270
Kruize AA, Hené RJ, Kallenberg CG, van Bijsterveld OP, van der Heide A, Kater L, Bijlsma JW (1993) Hydroxychloroquine treatment for primary Sjögren’s syndrome: a two year double blind crossover trial. Ann Rheum Dis 52:360–364
Yoon CH, Lee HJ, Lee EY, Lee EB, Lee WW, Kim MK, Wee WR (2016) Effect of hydroxychloroquine treatment on dry eyes in subjects with primary Sjögren’s syndrome: a double-blind randomized control study. J Korean Med Sci 31:1127–1135. https://doi.org/10.3346/jkms.2016.31.7.1127
Fox RI, Chan E, Benton L, Fong S, Friedlaender M, Howell FV (1988) Treatment of primary Sjögren’s syndrome with hydroxychloroquine. Am J Med 85:62–67
Fox R, Dixon R, Guarrasi V, Krubel S (1996) Treatment of primary Sjögren’s syndrome with hydroxychloroquine: a retrospective, open-label study. Lupus 5(Suppl 1):S31–S36
Tishler M, Yaron I, Shirazi I, Yaron M (1999) Hydroxychloroquine treatment for primary Sjögren’s syndrome: its effect on salivary and serum inflammatory markers. Ann Rheum Dis 58:253–256
Yavuz S, Asfuroğlu E, Bicakcigil M, Toker E (2011) Hydroxychloroquine improves dry eye symptoms of patients with primary Sjögren’s syndrome. Rheumatol Int 31:1045–1049. https://doi.org/10.1007/s00296-010-1415-4
Wang SQ, Zhang LW, Wei P, Hua H (2017) Is hydroxychloroquine effective in treating primary Sjogren’s syndrome: a systematic review and meta-analysis. BMC Musculoskeletal Disord 18:186. https://doi.org/10.1186/s12891-017-1543-z
Zazzetti F, Rivero M, Duartes Noe DE, Gallacher A, Schiel A, Khoury MC, Laborde HA, Barreira JC (2010) Frequency of systemic manifestations in patients with primary Sjogren’s syndrome in Argentina. Reumatol Clin 6:299–302. https://doi.org/10.1016/j.reuma.2010.01.004
Ramos-Casals M, Brito-Zeron P, Solans R, Camps MT, Casanovas A et al (2014) Systemic involvement in primary Sjögren’s syndrome evaluated by the EULAR-SS disease activity index: analysis of 921 Spanish patients. Rheumatology (Oxford) 53:321–331. https://doi.org/10.1093/rheumatology/ket349
Ramos-Casals M, Brito-Zeron P, Seror R, Bootsma H, Bowman S et al (2015) Characterization of systemic disease in primary Sjögren’s syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 54:2230–2238. https://doi.org/10.1093/rheumatology/kev200
Brito-Zerón P, Theander E, Baldini C, Seror R, Retamozo S et al (2016) Early diagnosis of primary Sjögren’s syndrome: EULAR-SS task force clinical recommendations. Expert Rev Clin Immunol 12:137–156. https://doi.org/10.1586/1744666X.2016.1109449
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The following authors have declared conflicts of interest:
Rafael Chaparro del Moral is Biotherapeutics Medical Manager at AbbVie.
Federico Zazzetti is Immunology Medical Manager at Janssen.
Sofía Velez is Senior Medical Science Liaison at Janssen.
The other remaining authors declare no conflicts of interest.
Ethical approval
The study was approved by the institutional review board or ethics committee at each participating site and was conducted in accordance with the principles of the Declaration of Helsinki.
Statement of informed consent
All patients provided written informed consent.
Rights and permissions
About this article
Cite this article
Demarchi, J., Papasidero, S., Medina, M.A. et al. Primary Sjögren’s syndrome: Extraglandular manifestations and hydroxychloroquine therapy. Clin Rheumatol 36, 2455–2460 (2017). https://doi.org/10.1007/s10067-017-3822-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3822-3