Abstract
Monocytes are an important component in the innate immune system. However, studies to date have failed to conclude whether their levels are altered in patients with systemic lupus erythematosus (SLE). We applied the cytodiff counting method and comprehensively measured the circulating levels of distinct white blood cell (WBC) subsets, including CD16+, CD16−, and total monocytes, in 61 SLE patients as well as in 203 age-matched healthy controls (HCs). The absolute number of CD16− monocytes, total monocytes, immature granulocytes, mature neutrophils, total neutrophils, and T cell blasts was significantly higher, that of non-cytotoxic T lymphocytes, cytotoxic T + NK lymphocytes, T + NK lymphocytes, total lymphocytes, basophils, and eosinophils significantly lower (all p < 0.05), but that of CD16+ monocytes, B lymphocytes, B cell blasts, non-B and non-T cell blasts, and total blasts was not statistically different in SLE patients, as compared to HC. Specifically, among all subsets examined, the percentage of CD16− monocytes and total monocytes was the only one that could discriminate active SLE from quiescent SLE (p = 0.033 and 0.026, respectively). SLE patients with lupus nephritis were also associated with higher levels of circulating CD16− monocytes and total monocytes, in comparison with that of controls (both p < 0.0001). This study suggests the significance of distinct WBC subsets, particularly the differential regulations of monocyte subsets, in the pathogenesis and development of SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the excessive production of autoantibodies directed against cell nuclear antigens and involving multiple key components of the immune system. The clinical symptoms of SLE are heterogeneous and vary greatly among patients [1].
Human blood monocyte subsets exhibit differential surface expression of various Fc receptors for immunoglobulin G (IgG) (FcγRs). CD16 (FcγRIII) is one of the FcγRs, which can activate FcγRs by its cytoplasmic region. In humans, the CD16 receptor exhibits high affinity binding to demonstrate IgG1 and IgG3, which leads to phagocytosis, release of inflammatory mediators, and clearance of immune complexes [2]. Monocytes, including CD16+ and CD16− monocytes, are a critical component of the innate immune response and have been shown to play a role in the development of SLE [3]. CD16+ monocytes produce large amounts of TNF-α and IL-1β and are considered to be pro-inflammatory. CD16− monocytes express high levels of CCR2 and CD93 and have the ability to phagocytose [4, 5]. However, to date, the proportions of monocyte subsets in SLE patients remain controversial [6,7,8,9,10,11].
Cytodiff is a flow cytometric counting method developed by Beckman Coulter (Miami, FL, USA) that uses a five-color/six-antibody cocktail to enable automatic counting of distinct white blood cell (WBC) subsets including B lymphocytes, non-cytotoxic T lymphocytes, cytotoxic T + NK lymphocytes, natural killer (NK) lymphocytes, T + NK lymphocytes, total lymphocytes, CD16− monocytes, CD16+ monocytes, total monocytes, immature granulocytes, mature neutrophils, total neutrophils, eosinophils, basophils, B cell blasts, T cell blasts, and non-B and non-T cell blasts [12]. Cytodiff is superior to traditional electronic counters that can only identify five subsets of WBCs, namely lymphocytes, monocytes, neutrophils, eosinophils, and basophils. The performance of cytodiff is also superior to manual counting, which, although generally accepted as the reference method for obtaining leukocyte differentials, is time-consuming, labor-intensive, and difficult to standardize [13].
Methods
Patients and healthy controls
This retrospective study was approved by the Ethics Committee of Peking Union Medical College Hospital (Beijing, China), and written informed consent was obtained from all participants. A cohort of 61 SLE patients admitted into the Peking Union Medical College Hospital from Jan. 2014 to Nov. 2014 were recruited into this study. The diagnosis of SLE was established following the Systemic Lupus International Collaborating Clinic (SLICC) Revision of the American College of Rheumatology (ACR) Classification Criteria for SLE [14]. Patients with other autoimmune diseases including rheumatoid arthritis, type 1 diabetes, or primary Sjögren’s syndrome were excluded. The SLE Disease Activity Index (SLEDAI) was used to assess the activity of lupus for each patient upon enrollment. A total of 24 active rheumatoid arthritis (RA) patients were also collected. A total of 203 healthy controls (HC) were enrolled into this study during their routine physical examination at the same hospital; these individuals were healthy and had no autoimmune disorders or family history of SLE. Peripheral blood sample was taken from each participant, and medical records on clinical examination were collected for further analysis
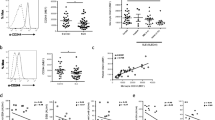
The percentage of CD16− monocytes by the cytodiff flow cytometry in SLE patients and healthy controls (HC). a The percentage of CD16− monocytes was higher in SLE patients (7.01%) than in HC (5.67%, p < 0.0001). b The mean number of CD16− monocytes (0.43 × 103/μL) in SLE patients was higher than that in HC (0.33 × 103/μL, p < 0.0001). c The percentage of CD16− monocytes was higher in active SLE patients (7.64%) than in inactive SLE and HC (5.54%, 5.67%; p = 0.037, p < 0.0001, respectively). d The absolute number of CD16− monocytes was higher in active SLE patients (0.45 × 103/μL) than that HC (0.33 × 103/μL, p < 0.0001)
The percentage of monocytes between SLE patients who took low-dose or moderate-dose to high-dose prednisone. a The percentage of CD16+ monocytes was higher in low-dose group (1.21%) than that in moderate-dose to high-dose group (0.32%, p = 0.006). b The percentage of CD16− monocytes was higher in low-dose group (9.88%) than that in moderate-dose to high-dose group (6.79%, p = 0.016). c The percentage of total monocytes was higher in low-dose group (10.12%) than in moderate-dose to high-dose group (7.00%, p = 0.0013). d The percentage of cytotoxic T + NK lymphocytes was higher in low-dose group (1.90%) than that in moderate-dose to high-dose group (0.56%, p = 0.035)
Flow cytometry analysis
The flow cytometry analysis was performed with a five-color flow cytometer (FC500, Beckman Coulter) by a technician blind to the clinical information of each patient, according to the manufacturer’s instructions. The cytodiff five-color/six-antibody cocktail (Beckman Coulter) included FITC-conjugated anti-CD36, PE-conjugated anti-CD2, PE-conjugated anti-CD294, ECD-conjugated anti-CD19, PC5-conjugated anti-CD16, and PC7-conjugated anti-CD45-PC7. Each sample was prepared on the cell preparator (Beckman Coulter) with a “lyse no wash” protocol. One hundred microliters of whole blood was mixed with 10 μL of cytodiff reagent for 20 min before red blood cells were lysed with Versalyse solution (Beckman Coulter). The auto-gating strategy was based on side-scatter graph (SSC), and specific gates were established as described by Faucher et al. [13]. The absolute number for each cell subset was calculated according to the percentage of each subset and the total number of WBC measured using the Automatic Blood Cell Counter (LH570, Beckman Coulter).
Statistical analysis
Statistical analysis was performed by MATLAB R 2014a and GraphPad Prism software version 5.0. The comparison of SLE or active RA with HC groups was performed using Mann-Whitney U test. The Kruskal-Wallis test followed by Dunn’s post hoc test was used to compare the differences among multiple groups. A two-tailed p value of < 0.05 was considered statistically significant.
Results
Clinical characteristics of study subjects
A total of 61 SLE patients (including 52 females), 24 active RA patients (including 17 females), and 203 HC (including 93 females) were recruited into this study. The median age (interquartile range (IQR)) for SLE was 32.85 (25.50–40.00) years, and those for RA and HC were 51.00 (48.00–61.75) and 42.00 (31.00–52.00) years (p > 0.05). The general clinical characteristics of all SLE patients are summarized in Table 1. These patients presented a wide range of clinical symptoms, from malar rash, discoid rash, oral ulcers, alopecia, arthritis, serositis, renal disorder, neurological disorder, to hematological disorder. Of note, 42 (68.85%) SLE patients had lupus nephritis. And the invasive pathological biopsy was made in seven SLE patients, with one patient LN III, two LN IV-V, and four LN IV. The majority (96.72%) of SLE patients tested positive for anti-nuclear antibody (ANA), while only 44.26% were positive for anti-double-stranded DNA (dsDNA) antibody. When assessed for lupus activity, 14 patients (22.95%) had inactive SLE (inSLE; SLEDI < 4) and the remaining 77.05% active SLE (aSLE). Seven SLE patients (11.48%) were treated with low-dose prednisone (< 7.5 mg/day [15]), while the remaining were treated with moderate-dose to high-dose prednisone.
Comparison of total monocytes and monocyte subsets among SLE, active RA, and HC
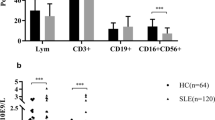
The total WBC count in SLE patients was 7.92 × 103/μL whole blood, which was significantly higher than that in HC (6.11 × 103/μL whole blood; p = 0.0092). Cytodiff flow cytometric analysis showed that the percentage of CD16− monocytes and total monocytes was higher in SLE patients than in HC (p < 0.0001 for both; Table 2, Fig. 1). But the median (IQR) percentage of CD16− monocytes in active RA was 6.06% (5.06–6.65%). Comparing with that of HC [5.67% (4.82–6.40%)], the difference was not statistically significant (p = 0.22). Also, the comparison of the percentage of CD16+ monocytes and total monocytes between active RA and HC did not reach statistical significance (p = 0.15 and 0.30, respectively). But the mean number of CD16− monocytes (0.43 × 103/μL) and total monocytes (0.48 × 103/μL) in SLE patients was significantly higher than the corresponding number (0.33 × 103/μL for CD16− monocytes, p < 0.0001; 0.35 × 103/μL for total monocytes, p < 0.0001) in HC. However, there were no significant differences in the percentage or absolute number of CD16+ monocytes between SLE and HC (all p > 0.05; Table 2).
Comparison of total lymphocyte and lymphocyte subpopulations between SLE and HC
SLE patients had a significantly lower percentage and absolute count of non-cytotoxic T lymphocytes, cytotoxic T + NK lymphocytes, T + NK lymphocytes, and total lymphocytes, as compared with HC (all, p < 0.05). But neither the percentage nor the absolute number of B lymphocytes was significantly different between SLE and HC (all p > 0.05; Table 2).
Comparison of total neutrophils and granulocyte subpopulations between SLE and healthy controls
The proportion and absolute number of eosinophils and basophils decreased, whereas that of immature neutrophils, mature neutrophils, and total neutrophils increased in SLE patients, as compared to HC (all p < 0.05; Table 2).
Comparison of distinct WBC subsets by SLE activity
Among the WBC subsets showing significant differences between SLE patients and HC, we chose nine subsets (CD16− monocytes, total monocytes, cytotoxic T + NK lymphocytes, T + NK lymphocytes, eosinophils, basophils, immature neutrophils, mature neutrophils, and total neutrophils) to further analyze their variations according to disease activity. As shown in Fig. 1 and supplementary Fig. 1, only the percentage of CD16− monocytes and total monocytes presented a significant difference between aSLE and inSLE (p = 0.033 and 0.026, respectively). The number of CD16− monocytes, as well as that of total monocytes, mature neutrophils, and total neutrophils, was significantly higher in aSLE patients than in HC (p < 0.01), but not between inSLE patients and HC (p > 0.05). The number of the other five subsets was not only significantly different between aSLE and HC but also between inSLE and HC (p < 0.01; supplementary Fig. 2).
Comparison of leukocytes in SLE patients divided by nephritis
We also analyzed the variations of WBC subsets by nephritis, which is defined by the presence of lupus nephritis (LN) (supplementary Figs. 3 and 4). We found that all nine subsets were significantly different between LN patients and HC, as well as between non-LN patients and HC (p < 0.05), but not between LN and non-LN patients (p > 0.05).
Comparison of leukocytes in SLE patients divided by dose of prednisone
By comparison between high-dose and low-dose group, we found that the low-dose group had high percentage of CD16+ monocytes, CD16− monocytes, total monocytes, and cytotoxic T + NK lymphocytes (all p < 0.05, Fig. 2). High-dose group tended to have higher percentage of mature neutrophil and total neutrophil comparing to low-dose group (68.75 vs. 67.71%, 71.41 vs. 67.88%, respectively), but the difference did not have statistical significance.
Discussion
Conflicting studies have been published regarding the variations in monocytes in SLE patients [6,7,8,9,10,11]. In this study, we showed that the absolute number of CD16− as well as total monocytes, but not CD16+ monocytes, was significantly increased in SLE patients than in healthy controls. Consistent with our findings, Burbano et al. found an increased percentage and absolute number of CD16− monocytes in active SLE patients [6]. In contrast, another study on six female SLE patients showed decreased proportions of CD16− monocytes in SLE patients as compared to HC [10], while Li et al. detected no difference in monocyte subsets between SLE patients and healthy individuals [9]. Multiple factors may contribute to the inconsistent observations regarding monocytes between SLE patients and HC, including variations in the enrollment criteria, in flow cytometric gating strategies, and/or in sample size. With respect to CD16+ monocytes, this study, as well as three other studies [7,8,9], failed to reveal any statistically significant differences between SLE patients and healthy individuals. In disagreement, Burbano et al. showed reduced CD16+ monocytes in SLE patients [8], while Jiang et al. identified increased CD16+ monocytes in six SLE patients [13]. Also, a study with 10 SLE patients suggested the expansion of CD16+ monocytes in SLE patients [11]. This discrepancy may be attributed to the small sample size, different including criterion and diverse dose of prednisone. Supporting the significance of CD16+ monocytes in autoimmune diseases including SLE, studies have shown that CD16+ monocytes were associated with elevated ESR and CRP in RA patients [16], correlated with an increasing risk of subclinical coronary artery atherosclerosis in RA [17], and glucocorticoid treatment decreased the number of CD16+ monocytes in a dose-related manner [18]. In our study, not only the number of CD16+ monocytes but also other cells (CD16− monocytes, total monocytes, and cytotoxic T + NK lymphocytes) also decreased in high-dose group.
Monocytes represent an essential arm of the innate immune system with a multitude of immunological functions including antigen presentation, phagocytosis, cytokine production, and T cell modulation [5, 19]. In mouse models of SLE, monocytes bearing activating Fc receptors were pivotal to the development of immune complexes mediating glomerulonephritis [20]. Defective clearance of immune complexes is an indicator of “defective” monocyte function which may play a role in tissue and organ damage in SLE. Conversely, aberrant activation of monocytes/macrophages may also contribute to the pathogenesis of SLE [21, 22]. Disease activity and proliferative glomerular LN lesions are associated with accumulation of CD16+ monocytes in glomeruli of active LN [23]. Increased CD64 expression on circulating monocytes was related with systemic inflammation and renal disease in SLE patients [9]. In this study, although we showed that the number of CD16− monocytes and total monocytes were significantly higher in active SLE than in HC, or in SLE patients with LN than in HC, we failed to detect statistical differences of these two parameters between LN and non-LN patients, suggesting that monocytes and their subsets are not a sensitive marker for renal involvement of SLE patients. But, the percentage of CD16− monocytes was higher in active SLE patients than that in inactive SLE patients. It implied that the endocytosis and phagocytosis of immune complexes may play a vital role in the initiation and development of active SLE patients.
Given that all patients received steroid treatments, it is not known whether steroid therapy may have an effect on the number of distinct WBC subsets. Also, the effect of other immunosuppressant should be taken into consideration. Although we comprehensively explored the alterations of distinct circulating WBC subsets in SLE patients, the underlying mechanisms leading to these alterations and their biological significance in the development and treatment responses of SLE remain to be further investigated.
In summary, by using the cytodiff differential counting strategy, we demonstrated for the first time that distinct WBC subsets are differentially regulated in Chinese SLE, with the circulating number of CD16− monocytes, total monocytes, immature granulocytes, mature neutrophils, total neutrophils, and T cell blasts significantly elevated, non-cytotoxic T lymphocytes, cytotoxic T + NK lymphocytes, T + NK lymphocytes, total lymphocytes, basophils, and eosinophils potently reduced, while CD16+ monocytes, B lymphocytes, B cell blasts, non-B and non-T cell blasts, and total blasts not dramatically altered in SLE patients, as compared to HC. This study supports the significance of leukocytes, in particular, monocytes in SLE development and paves the way for future studies in SLE.
References
D’Cruz DP, Khamashta MA, Hughes GR (2007) Systemic lupus erythematosus. Lancet 369:587–596
Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN et al (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80
Katsiari CG, Liossis SN, Sfikakis PP (2010) The pathophysiologic role of monocytes and macrophages in systemic lupus erythematosus: a reappraisal. Semin Arthritis Rheum 39:491–503
Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC (2012) The three human monocyte subsets: implications for health and disease. Immunol Res 53:41–57
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Burbano C, Vasquez G, Rojas M (2014) Modulatory effects of CD14+CD16++ monocytes on CD14++CD16- monocytes: a possible explanation of monocyte alterations in systemic lupus erythematosus. Arthritis Rheum (Hoboken, NJ) 66:3371–3381
Henriques A, Ines L, Carvalheiro T, Couto M, Andrade A, Pedreiro S et al (2012) Functional characterization of peripheral blood dendritic cells and monocytes in systemic lupus erythematosus. Rheumatol Int 32:863–869
Cairns AP, Crockard AD, Bell AL (2002) The CD14+ CD16+ monocyte subset in rheumatoid arthritis and systemic lupus erythematosus. Rheumatol Int 21:189–192
Li Y, Lee PY, Sobel ES, Narain S, Satoh M, Segal MS et al (2009) Increased expression of FcgammaRI/CD64 on circulating monocytes parallels ongoing inflammation and nephritis in lupus. Arthritis Res Ther 11:R6
Jiang W, Zhang L, Lang R, Li Z, Gilkeson G (2014) Sex differences in monocyte activation in systemic lupus erythematosus (SLE). PLoS One 9:e114589
Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B (2015) Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep 5:13886
Roussel M, Benard C, Ly-Sunnaram B, Fest T (2010) Refining the white blood cell differential: the first flow cytometry routine application. Cytometry A 77:552–563
Faucher JL, Lacronique-Gazaille C, Frebet E, Trimoreau F, Donnard M, Bordessoule D et al (2007) “6 markers/5 colors” extended white blood cell differential by flow cytometry. Cytometry A 71:934–944
Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR et al (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686
Duru N, van der Goes MC, Jacobs JW, Andrews T, Boers M, Buttgereit F et al (2013) EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis 72:1905–1913
Wijngaarden S, van Roon JA, Bijlsma JW, van de Winkel JG, Lafeber FP (2003) Fcgamma receptor expression levels on monocytes are elevated in rheumatoid arthritis patients with high erythrocyte sedimentation rate who do not use anti-rheumatic drugs. Rheumatology (Oxford) 42:681–688
Winchester R, Giles JT, Nativ S, Downer K, Zhang HZ, Bag-Ozbek A et al (2016) Association of Elevations of specific T cell and monocyte subpopulations in rheumatoid arthritis with subclinical coronary artery atherosclerosis. Arthritis Rheum (Hoboken, NJ) 68:92–102
Sumegi A, Antal-Szalmas P, Aleksza M, Kovacs I, Sipka S, Zeher M et al (2005) Glucocorticosteroid therapy decreases CD14-expression and CD14-mediated LPS-binding and activation of monocytes in patients suffering from systemic lupus erythematosus. Clin Immunol 117:271–279
Schmidl C, Renner K, Peter K, Eder R, Lassmann T, Balwierz PJ et al (2014) Transcription and enhancer profiling in human monocyte subsets. Blood 123:e90–e99
Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K et al (2007) Clearance deficiency and systemic lupus erythematosus (SLE). J Autoimmun 28:114–121
Li Y, Lee PY, Reeves WH (2010) Monocyte and macrophage abnormalities in systemic lupus erythematosus. Arch Immunol Ther Exp 58:355–364
Byrne JC, Ni Gabhann J, Lazzari E, Mahony R, Smith S, Stacey K et al (2012) Genetics of SLE: functional relevance for monocytes/macrophages in disease. Clin Dev Immunol 2012:582352
Nakatani K, Yoshimoto S, Iwano M, Asai O, Samejima K, Sakan H et al (2010) Fractalkine expression and CD16+ monocyte accumulation in glomerular lesions: association with their severity and diversity in lupus models. Am J Physiol Renal Physiol 299:F207–F216
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants No. 81373188 and 81172857 to Y.-Z. L. and 81302592 to S.-L. Z.), the National Science Technology Pillar Program in the 12nd 5-Year Plan (No. 2014BAI07B00), and the Capital Health Research and Development of Special Grants (No. 2014-1-4011, to Y.-Z. L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Wu, Z., Zhang, S., Zhao, L. et al. Upregulation of CD16− monocyte subsets in systemic lupus erythematous patients. Clin Rheumatol 36, 2281–2287 (2017). https://doi.org/10.1007/s10067-017-3787-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3787-2