Abstract
Previous studies of the occurrence of interstitial lung disease (ILD) in undifferentiated connective tissue diseases (UCTD) were conducted in patients admitted to Respiratory Medicine Units. The aim of the present prospective study was to investigate lung involvement in UCTD patients admitted to a Rheumatology Unit. Eighty-one consecutive UCTD patients were enrolled in the study. Each patient underwent history and physical examination, routine laboratory investigations, antinuclear antibody (ANA) profiling, B-mode echocardiography, and lung function study according to previously reported methods. Lung high resolution computed tomography (HRCT) was performed in patients who provided informed consent. Six patients (7.4%) had a history of grade II dyspnea. Three of them had a DLCO ranging from 42 to 55% of the predicted value; and a HRCT-documented ILD with a non-specific interstitial pneumonia (NSIP) pattern. Symptoms in the other three patients were due to cardiac disease. None of the 75 asymptomatic patients, had relevant findings at physical examination, 26/75 had a DLCO <80% (<70% in 10 cases). Of these, 3 of the 30 patients who underwent lung HRCT were affected by NSIP-ILD. Six of the 81 enrolled were affected by ILD, which was symptomatic in three patients. A higher percentage of patients had a reduced DLCO. The latter finding may reflect a preradiographic ILD or a preechocardiographic pulmonary vascular disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interstitial lung disease (ILD) represents a distinct manifestation of many connective tissue diseases (CTDs). Its prevalence is highest in Systemic Sclerosis (SSc), but it also occurs in a significant percentage of patients with Mixed Connective Tissue disease, Systemic Lupus Erythematosus, Sjögren’s Syndrome, Polymyositis Dermatomyositis, and Rheumatoid Arthritis [1, 2].
In recent years, various studies reported that some ILD patients present features suggestive of an autoimmune origin even though they do not meet the classification criteria for any major CTD. Such a condition has been considered as ILD secondary to undifferentiated CTD (UCTD) [3, 4] and otherwise referred to as lung dominant CTD [5], autoimmune featured ILD (AIF-ILD) [6], and interstitial pneumonia with autoimmune features (IPAF) [7].
Previous studies of ILD secondary to UCTD were conducted in patients admitted to Respiratory Medicine Units for evaluation of an ILD and investigated for features consistent with either a major CTD or a UCTD. The aim of our study was to investigate lung involvement in consecutive patients with “stable” UCTD admitted to a Rheumatology Unit.
Materials and methods
Patients
UCTD patients consecutively admitted to the outpatient clinic of the Rheumatology Unit of the University of Campania “Luigi Vanvitelli” (previously named Second University of Naples) from 1st January 2013 to 31st December 2015 were enrolled in the study, after giving their written informed consent.
UCTD was diagnosed according to Mosca et al. [8] and Doria et al. [9]. Briefly, patients had to (a) present signs and symptoms suggestive of a CTD without fulfilling the criteria of any definite CTD [8]; (b) be antinuclear antibodies (ANA) positive; (c) do not have either any CTD marker autoantibody (i.e., anti-dsDNA, anti-Sm, anti-ribosomal P protein, anti-Scl-70, anticentromere, anti-La/SSB, anti-Jo1, anti-Mi-2) or any manifestation distinctive of any major CTD (i.e., malar rash, subacute CLE, chronic CLE, skin sclerosis, heliotrope rash, Gottron’s plaques, erosive arthritis, alopecia) [9]; and (d) have a disease duration as assessed from the onset of the 1st symptom/sign ≥3 years. Therefore, our patients were affected by “stable UCTD,” according to Mosca et al. [10].
Routine assessment
According to standard clinical practice and to ensure a correct classification, each patient with a suspected UCTD underwent a detailed history and physical examination to identify: (I) any of the above-listed exclusion criteria [8, 9]; (II) symptoms/signs of ILD, namely dyspnea, graded according to the New York Heart Association, and bibasilar crackles; (III) routine laboratory investigations, namely, blood cell count, urinalysis, blood urea nitrogen, serum creatinine, alanine aminotransferase, aspartate aminotransferase, erythrosedimentation rate, serum protein electrophoresis, serum C3 and C4 concentration; (IV) nailfold videocapillaroscopy (NVC), (V) ANA screening and profiling; and (VI) B-mode echocardiography and lung function testing.
Autoantibody profile assessment
Autoantibodies were investigated in sera collected at the first visit, stored at −20 °C and heated at 56 °C for 30 min before testing. ANA and ACA were determined by indirect immunofluorescence on HEp-2 cells (Astra srl, Pavone Canavese, Italy), using fluorescent anti-human gammaglobulin as conjugate, and a 1:160 serum dilution as cut-off value. Anti-Scl-70, anti-SSA, anti-SSB, anti-Sm, anti-Jo1, and anti-U1RNP were identified with commercially available ELISA kits (Chematil srl, Angri, Italy), using 25 U/ml as cut-off value. Anti-RNA polymerase III, anti-fibrillarin, and anti-Pm-Scl were investigated by EliA Varelisa Phadia test (Germany) using 150 U/ml as cut-off value. Anti-Th/To were investigated by western blotting technique (Arnika srl, Milan, Italy). Anti-DNA ds antibodies were identified by IIF on Crithidia luciliae at a serum dilution of 1:10 (Astra srl) [11].
Nailfold videocapillaroscopy assessment
Patients were investigated for typical microvascular alterations (megacapillaries and/or avascular areas) by NCV. It was carried out while the patients were seated with their hands positioned at heart level, and at a room temperature of 22–25 °C. A drop of immersion oil was applied to the nailfold to increase the translucency of the keratin layer. All the fingers were examined by the same physician (R.I.) who is experienced in NCV using a videocapillaroscope (Videocap 25-DS Medica; Freiburg, Germany) with optical probes of ×200. The degree of capillary enlargement on a scale of 0–3 and capillary loss (grades A–D) were considered. Megacapillaries (capillary enlargement ≥2) and/or avascular areas (capillary loss grade ≥C) were considered a scleroderma pattern [12].
Assessment of internal organ involvement
All patients underwent B-mode echocardiography and lung function study. Echocardiographic examination was performed as described elsewhere [13]. Pulmonary function tests including forced vital capacity (FVC), total lung capacity (TLC), forced expiratory volume 1/FVC (FEV1/FVC), and diffusing capacity for carbon monoxide (DLCO), expressed as percentages of predicted values based on age, sex and height and corrected for hemoglobin level, were performed according to techniques approved by the American Thoracic Society (ATS) [14]. The percentage predicted DLCO value reported was the average of at least two acceptable tests meeting the reproducibility requirements of ATS [15]. The detection of either a DLCO or a FVC <80% of the respective predicted values in the absence of smoking, obstructive lung disease, and/or cardiac involvement was considered indicative of possible ILD [16,17,18,19].
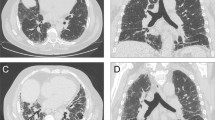
Patients giving additional consent underwent a high resolution computed tomography of lungs (Lung HRCT). Chest HRCT images were obtained with 1-mm collimation and 10-mm intervals at maximal end- inspiratory phase with the patient in a supine position using a high spatial frequency algorithm. If needed, prone scans were added to distinguish gravity-related changes from structural abnormalities. According to Fischer et al. [19], the presence of a non-specific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), organizing pneumonia (OP), and lymphoid interstitial pneumonia (LIP) were considered consistent with a diagnosis of ILD.
Follow-up
Each patient was annually investigated for clinical manifestations, laboratory findings and lung function. Patients with a baseline HRCT-detected ILD and those experiencing deterioration in lung function at spirometry were invited to undergo HRCT and B-mode echocardiography with estimation of pulmonary systolic arterial pressure.
Statistical analysis
We used the SPSS for Windows software (version 16.0) for statistical analysis. Categorical data were analyzed by contingency table (chi square). A p value <0.05 was considered significant.
The study was approved by the ethics committee of the University of Campania “Luigi Vanvitelli”.
Results
Lung findings at baseline
Eighty-one consecutive Caucasian UCTD patients were enrolled in this study. Most patients were female (90.1%), and the median age was 40 years (from 33 to 48); the median disease duration was 6 years (from 5 to 11).
Table 1 summarizes the main demographic, clinical and laboratory features of the patients enrolled. The most frequent clinical manifestations related to CTD were arthralgias/arthritis (70.3%), asthenia (51.8%), Raynaud’s phenomenon (49.3%), myalgias (46.9%), and ocular and/or oral dryness (43.2%).
Six patients presented effort dyspnea, all of them grade II. Three of these patients had bibasilar crackles and a DLCO value of 42, 44, and 55%, respectively. One had a mild restrictive pattern of the volume flow curve namely, TLC <80%, FVC >80% of the predicted value and FEV1/FVC >70%. The other two patients had a normal volume flow curve: TLC and FVC >80% and FEV1/FVC >70% of the predicted values. All three presented a bibasilar NSIP pattern at lung HRCT. These patients, accounting for 3.7% of our series, were considered to have symptomatic ILD secondary to UCTD. The other three dyspnoic patients did not present either basilar crackles or ILD findings at HRCT or a DLCO <70% of the predicted value and were affected by hypertensive cardiomyopathy (arterial hypertension, cardiac hypertrophy, and diastolic dysfunction), which accounted for effort dyspnea.
Of the remaining 75 UCTD asymptomatic patients, none had relevant findings at physical examination, all had TLC and FVC >80% of the predicted values; one of them presented a mild obstructive pattern of the volume flow curve and FEV1/FVC <70%. Twenty-six patients had a DLCO <80%, which was <70% in 10 cases. Sixteen of these 26 patients had findings that could account for the reduced DLCO (11 were smokers, 4 were affected by pulmonary vascular disease detected by echocardiography, and 1 had chronic obstructive pulmonary disease). The remaining 10 did not present any feature that could explain the reduced DLCO.
In summary, a definite, i.e., HRCT-documented ILD was identified in 6 out of 81 consecutive UCTD patients (7.4%), 3 of whom had a DLCO <70% as the only altered physiologic parameter, and only one had a DLCO <70% and TLC <80%. On the other hand, an altered DLCO, unexplained by any other cause, was detected in 10 further patients, 2 of whom presented a DLCO value <70%.
Relationships between lung alterations and other disease findings
Statistical analysis did not reveal any relationships between lung involvement features, i.e., HRCT-documented ILD or isolated DLCO, and any disease feature including disease duration, Raynaud’s phenomenon, anti-SSA positivity and altered inflammation indices.
Follow-up evaluation
After a follow-up ranging from 1 to 2 years, the three patients with ILD in the symptomatic group still presented normal FVC values, moderate reduction of DLCO values, and a NSIP pattern at HRCT. They were treated with azathioprine, which resulted in stabilization of lung function (i.e., ΔFVC and ΔDLCO <10%) and no progression of the radiologic extension. Seven of the 10 UCTD asymptomatic patients with an aberrant DLCO values unexplained by any other cause at baseline remained stable and three improved (i.e., increased >15% of the basal value).
Discussion
Kinder et al. first investigated 75 ILD patients admitted to a ILD Center, 28 of whom had features compatible with a diagnosis of UCTD. They found that a ground-glass pattern consistent with an NSIP was more frequent in UCTD-ILD than in idiopathic ILD, and that 88% of the 75 patients found to have a ILD with a NSIP pattern at lung HRCT, had autoimmune features consistent with a diagnosis of UCTD [3].
Suda et al. [20] compared the lung features of 22 patients affected by UCTD-NSIP with those of 25 patients affected by primary NSIP and found a female preponderance and a predominantly lymphocyte infiltration as detected by bronchoalveolar lavage analysis in women.
We investigated UCTD patients admitted to a Rheumatology Unit. Our patients presented lung symptoms and signs and physiologic and imaging findings milder than those detected in patients admitted to Pneumology Units [3,4,5,6,7]. When asked, only 6/75 patients declared grade II effort dyspnoea. Of these, only three were affected by ILD as witnessed by an NSIP pattern at HRCT and characterized by a reduction of DLCO in all three cases.
Of the 75 remaining patients, HRCT, carried out in 30, revealed ILD in 3 other patients in whom FVC was >80% of the predicted value in all and DLCO was <70% in one case only. Therefore, ILD was identified in 3 out 6 symptomatic patients and in 3 of 75 asymptomatic patients for a prevalence of 7.4%.
Unexpectedly, we found a decreased DLCO unexplained by smoking or obstructive pulmonary disease or clinically or echocardiographically detected cardiac or pulmonary vascular disease in 10 other patients (12.3%).
DLCO does not strictly reflect ILD since it can be influenced by vascular pulmonary disease, smoking and obstructive disease [15]. Nevertheless, it is noteworthy that 20/73 patients with SSc (27%) admitted to Pittsburgh Unit with an isolated reduction of DLCO developed a restrictive pattern during the follow-up and, more importantly, 22% of them already presented pulmonary fibrosis as detected by chest x-ray at baseline and 43% had this feature at a 5.4-year follow-up [16]. Notably, DLCO has been recently demonstrated to provide the best overall estimate of HRCT-measured SSc-ILD disease in the absence of pulmonary hypertension [21]. In that context, we detected a preradiographic alveolitis in 13/29 patients with SSc, presenting with a reduced DLCO as the only altered parameter and investigated by bronchoalveolar lavage analysis [22]. Given the differences in the evolution between SSc-ILD [21,22,23,24] and UCTD-ILD [2, 4] and because data from SSc patients cannot be estrapolated to UCTD, we treated our patients with azathioprine. Indeed, the 3 symptomatic patients with UCTD-ILD responded to azathioprine therapy, whereas DLCO values remained stable or improved in the 10 asymptomatic patients with abnormal values at baseline in the absence of CT-detected ILD.
Recently, Ferri et al. [25] compared demographic and clinical data of patients with IPF and IPAF admitted to a Respiratory Unit with those of UCTD patients attending a Rheumatology Unit and hypothesized that IPAF and UCTD might be two clinical variants of the same systemic autoimmune disorder, and that the difference in the prevalence of ILD might at least in part due to selection bias between patients referred to different specialties.
Nosographic controversies are still present in this topic point of view. Nevertheless, from a clinical point of view, our data suggest that lung involvement be investigated in patients with UCTD admitted to Rheumatology Units. From a pathophysiologic point of view, the finding of an isolated reduction of DLCO should be investigated to determine its origin (vascular, alveolar wall infiltration), clinical impact and treatment, if necessary.
References
Fischer A, du Bois R (2012) Interstitial lung disease in connective tissue in connective tissue disorders. Lancet 280:689–698
Wells AU, Denton CP (2014) Interstitial lung disease in connective tissue disease: mechanisms and management. Nat Rev Rheumatol 10:728–739
Kinder BW, Collard HR, Koth L, Daikh DI, Wolters PJ, Elicker B et al (2007) Idiopathic nonspecific interstitial pneumonia lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med 176:691–697
Kinder BW, Shariat C, Collard HR, Koth LL, Wolters PJ, Golden JA et al (2010) Undifferentiated connective tissue disease-associated interstitial lung disease: changes in lung function. Lung 188:143–149
Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM (2010) Connective tissue disease associated interstitial lung disease: a call for clarification. Chest 138:251–256
Vij R, Noth I, Strek ME (2011) Autoimmune-featured interstitial lung disease: a distinct entity. Chest 140:1292–1299
Fischer A, Antoniou KM, Brown KK, Cadranel J, Corte TJ, du Bois RM, ERS/ATS Task Force on Undifferentiated Forms of CTD-ILD et al (2015) An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 46:976–987
Mosca M, Neri R, Bombardieri S (1999) Undifferentiated connective tissue diseases (UCTD): review of the literature and proposal for preliminary classification criteria. Clin Exp Rheumatol 17:615–620
Doria A, Mosca M, Gambari PF, Bombardieri S (2005) Defining unclassifiable connective tissue diseases: incomplete, undifferentiated, or both? J Rheumatol 32:213–215
Mosca M, Tani C, Vagnani S, Carli L, Bombardieri S (2005) The diagnosis and classification of undifferentiated connective tissue diseases. J Autoimmun 48-49:50–52
Valentini G, Marcoccia A, Cuomo G, Vettori S, Iudici M, Bondanini F, Santoriello C, Ciani A, Cozzolino D, De Matteis GM, Cappabianca S, Vitelli F, Spanò A (2014) Early systemic sclerosis: analysis of the disease course in patients with marker autoantibody and/or capillaroscopic positivity. Arthritis Care Res (Hoboken) 66(10):1520–1527
Maricq HR (1981) Widefield capillary microscopy: technique and rating scale for abnormalities seen in scleroderma and related disorders. Arthritis Rheum 24:1159–1165
Maione S, Cuomo G, Giunta A et al (2005) Echocardiographic alterations in systemic sclerosis. A longitudinal study. Semin Arthritis Rheum 34:721–727
American Thoracic Society (1995) Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique-1995 update. Am J Respir Crit Care Med 152:2185–2198
American Thoracic Society (1995) Standardization of spirometry—1995 update. Am J Respir Crit Care Med 152:1107–1136
Steen VD, Owens GR, Fino GJ, Rodnan GP, Medsger TA (1985) Pulmonary involvement in systemic sclerosis (scleroderma). Arthritis Rheum 28:759–767
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R et al (2005) Interpretative strategies for lung function tests. Eur Respir J 26:948–968
Staitieh BS, Ioachimescu O (2017) Interpretation of pulmonary function tests: beyond the basics. J Investig med 65:301–310
Fischer A, Brown KK (2015) Interstitial lung disease in undifferentiated forms of connective tissue disease. Arthritis Care Res (Hoboken) 67:4–11
Suda T, Kono M, Nakamura Y, Enomoto N, Kaida Y, Fujisawa T et al (2010) Distinct prognosis of idiopathic nonspecific interstitial pneumonia (NSIP) fulfilling criteria for undifferentiated connective tissue disease (UCTD). Respir Med 104:1527–1534
Steen VD, Graham G, Conte C, Owens G, Medsger TA Jr (1992) Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum 35:765–770
Iudici M, Cuomo G, Vettori S, Bocchino M, Sanduzzi Zamparelli A, Cappabianca S et al (2015) Low-dose pulse cyclophosphamide in interstitial lung disease associated with systemic sclerosis (SSc-ILD): efficacy of maintenance immunosuppression in responders and non-responders. Semin Arthritis Rheum 44:437–444
Tashkin DP, Volkmann ER, Tseng CH, Kim HJ, Goldin J, Clements P et al (2016) Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann Rheum Dis 75:374–381
Khanna D, Seibold JR, Wells A, Distler O, Allanore Y, Denton C et al (2010) Systemic sclerosis-associated interstitial lung disease: lessons from clinical trials, outcome measures, and future study design. Curr Rheumatol Rev 6:138–144
Ferri C, Manfredi A, Sebastiani M, Colaci M, Giuggioli D, Vacchi C et al (2016) Interstitial pneumonia with autoimmune features and undifferentiated connective tissue disease: our interdisciplinary rheumatology-pneumology experience, and review of the literature. Autoimmun Rev 15:61–70
Acknowledgements
The authors thank Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
None.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Riccardi, A., Irace, R., Di Stefano, I. et al. Lung involvement in “stable” undifferentiated connective tissue diseases: a rheumatology perspective. Clin Rheumatol 36, 1833–1837 (2017). https://doi.org/10.1007/s10067-017-3704-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3704-8