Abstract
There is substantial evidence that aortic aneurysm (AA) may be a manifestation of several systemic rheumatic disorders. However, only several studies have assessed the association between rheumatoid arthritis (RA) and AA. The aim of this study was to evaluate the incidence of AA in RA patients in a case-control study. A retrospective case-control study was performed utilizing the database of Clalit Health Services (CHS), a large healthcare provider organization in Israel. Data available from the CHS database included age, sex, socioeconomic status (SES), and diagnoses of chronic diseases, including AA. Patients over the age of 20 years who were diagnosed with RA (“cases”) were compared with a sample of age- and gender-matched enrollees without RA (“controls”) regarding the prevalence of AA. Chi-square and t tests were used for univariate analysis, and a logistic regression model was used for multivariate analysis. The study included 11,782 RA patients and 57,973 age- and gender-matched controls. The proportion of AA was significantly higher in RA patients (0.72 %) compared to the control group 0.49 % (odds ratio (OR) 1.48, 95 %; confidence interval (CI) 1.15–1.88; p = 0.002). A multivariate analysis that evaluated covariates associated with AA revealed an independent association of AA and RA after adjustment for different factors including age, gender, SES, and smoking status (OR 1.406, 95 %; CI 1.094–1.789; p = 0.006). Our study has demonstrated that AA is more prevalent in patients with RA in comparison with general population. Future large randomized studies are important to identify cardiovascular- and disease-related risk factors for AA formation in RA patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory immune system disorder primarily affecting the joints yet often targets other organs as well including the cardiovascular system. Overall, diverse cardiac manifestations have been described in RA such as pericarditis, myocarditis, cardiac amyloidosis, coronary vasculitis, arrhythmias, and valvular disorders. In addition, RA is a risk factor contributing to the pathogenesis of atherosclerosis leading to higher rates of ischemic heart disease and congestive heart failure resulting in increased higher mortality rate [1–3].
Higher cardiovascular (CV) risk in RA patients is partially mediated by traditional CV comorbidities such obesity, dyslipidemia, type 2 diabetes mellitus (T2DM), metabolic syndrome, hypertension, physical inactivity, advanced age, male gender, family history of CVD, and tobacco use [2, 4, 5]. Nowadays, the importance of RA-specific factors, such as ongoing and long-term inflammation has been recognized to be an additional significant disease-related risk factor [2, 4, 5].
Some studies and isolated case reports demonstrate that aortic involvement may occur as an unusual complication of RA. The described manifestations of aortic involvement in RA consist of aortitis, sometimes to be found only at autopsy, asymptomatic aortic regurgitation, and AA [6–12]. Generally, AA and aortic dissection are rare vascular complications occurring most commonly in elderly men with systemic hypertension and atherosclerosis [13]. Nevertheless, the increased prevalence of cardiovascular risk factors along with the accelerated arthrosclerosis in RA patients raises the hypothesis that these patients may have a higher incidence of AA than previously reported. Studies of AA and aortic dissection in RA patients are scant, and the true incidence of AA in RA patients has not been definitively determined.
The aim of our study is to investigate the coexistence of AA in RA patients in comparison with the general population using the medical database of the largest health maintenance organization in the country, the Clalit Health Services (CHS).
Patients and methods
Study design and participants
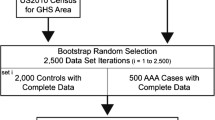
This study is a retrospective case-control study that incorporates data mining techniques and utilizes the Clalit Health Services (CHS) database. CHS is the largest public health insurance organization in Israel, and it provides medical care for approximately 4,400,000 enrollees. The large computerized database of this organization provides unique opportunities for population-based estimates of the incidences of rare events such as AA in RA patients. The potential of this database in population-based studies has been previously described [2, 13–17]. This system ensures virtually complete ascertainment of all clinically recognized cases of RA among the residents of Israel registered in CHS. All diagnosed cases of RA were identified using the computerized diagnostic index. Patients were defined as having RA when there was at least one documented diagnosis of RA in the medical records registered by CHS rheumatologists or CHS physicians in patients diagnosed during hospitalization. Unfortunately, we had no available data regarding the disease activity scores of the RA patients.
The diagnosis of AA was extracted from the CHS chronic diseases registry which is based on data withdrawn from hospital and primary care physicians’ reports. Patients who were diagnosed with RA (“cases”) and enrollees without RA (“controls”) were matched based on age and sex. The prevalence of AA was compared between the study groups in the entire study sample as well as in age, sex, and SES subgroups. Chi-square and t tests were used for univariate analysis. A logistic regression model was used to estimate the association between RA and AA in a multivariate analysis. Statistical analysis was performed using R Statistical Software (version 3.0.3; R Foundation for Statistical Computing, Vienna, Austria).
The study was approved by the institutional review board of the Clalit Health Services at the Soroka Medical Center, in Beer-Sheva.
Results
The study included 11,782 RA patients and 57,973 age- and sex- matched controls. Characteristics of the study population are presented in Table 1.
The proportion of AA was significantly higher in RA patients (0.72 %) compared to the control group 0.49 % (OR 1.48, 95 %; CI 1.15–1.88; p = 0.002). The prevalence of smokers in the RA group (32.8 %) was higher than in the control group (28.8 %).
OR for AA in patients with RA and controls in the entire study sample stratified by age, sex, smoking status, and SES is presented in Table 2. The association between AA and RA was statistically significant among females (0.51 versus 0.31 %; OR 1.64, 95 %; CI 1.16–2.27; p = 0.006). In addition, the age interval 60–79 was also a considerable risk factor for AA in RA population in comparison with controls (0.98 versus 0.64 %; OR 1.55, 95 %; CI 1.13–2.10; p = 0.007). The incidence of AA was higher in RA patients from low SES than in controls subjects (0.75 versus 0.42 %; OR 1.79, 95 %; CI 1.19–2.63; p = 0.006).
A comparison between RA patients with AA and RA patients without AA revealed that AA is more prevalent at older ages within the RA group (OR 1.06, 95 %; CI 1.04–1.08; p < 0.001). Furthermore, smoking was determined to be a risk factor for AA in RA patients (58.8 versus 32.6 %; OR 2.95, 95 %; CI 1.91–4.59; p < 0.001).
A multivariate analysis that evaluated covariates associated with AA revealed an independent association of AA and RA after adjustment for different factors including age, gender, SES, and smoking status (OR 1.406, 95 %; CI 1.094–1.789; p = 0.006) (Table 3).
Discussion
The present study is a case-control study, based on a large cohort of patients with AA and controls from the CHS database. RA was found to be associated with a higher coexistence rate of AA.
To the best of our knowledge, only a small number of studies have addressed this issue and investigated the association between RA and AA. In a recent report, 18 cases of abdominal AA were identified from an AA database of over 1000 patients (prevalence of 0.18 %) [18].
Interestingly, this rate of comorbidity was higher in RA patients compared to patients with other rheumatic conditions such as polymyalgia rheumatica, psoriasis, and giant cell arteritis (GCA). In addition, an increased risk of AA was found primarily in males, in patients over the age of 65 years and in patients with RA lasting more than 5 years from diagnosis [18].
Several isolated case reports have demonstrated an association between AA, aortic dissection, and RA [8, 18–20].
AA may be a manifestation of systemic rheumatic disorders of them vasculitis is the leading reported cause. It seems in vasculitis that the inflammatory condition also enhances atherosclerosis contributing to the formation of AA. For example, it was found that aneurysms of the ascending thoracic aorta were 17-fold more common in patients with GCA than in a control group and abdominal AA was 2.5-fold more frequent [21]. Although stenosis is the hallmark of Takayasu’s disease, AA with a variable distribution along the aorta is a widespread finding [22]. In addition, AA formation secondary to aortitis has been reported in patients suffering from Cogan’s syndrome, relapsing polychondritis and long-standing ankylosing spondylitis [23–25].
AA and aortic dissection may appear rarely as a late complication of systemic lupus erythematosus (SLE) [13, 26, 27]. Along with the improvement in the prognosis of SLE patients, the incidence of these manifestations rises. Recently, the results of a large study including 5018 patients with SLE and 25,090 age- and sex-matched controls demonstrated a higher proportion of AA in SLE patients in comparison with matched controls [13]. A multivariate analysis revealed that SLE was highly associated with AA (OR of 4.5), and this fact reflects the contribution of SLE itself the formation of AA. A similar trend was observed in a previous study that demonstrated a threefold increase in the incidence of AA and aortic dissection in SLE patients compared to healthy subjects [27]. In both studies, risk factors for AA and aortic dissection in SLE patients were older age and gender (males had higher risk than females) [13, 27]. It has been established that the increased risk of AA was attributable to hypertension and the duration of SLE (greater than 3 years) [27].
A meta-analysis of 35 publications on AA in patients with SLE revealed two different pathways for aneurysm development. One of them has been attributed to aortic circulatory disturbances resulting generally from vasculitis and cystic medial degeneration. These disturbances are presumed to play a crucial role in the pathogenesis of fatal non-atherosclerotic thoracic aneurysms. The second pathway described was atherosclerotic abdominal aneurysm formation associated with long-term steroid treatment, and the prognosis for this type of AA was more favorable [26].
The precise pathogenesis of AA formation in RA patients remains undetermined. Similar to SLE, the possible underlying mechanisms include accelerated atherosclerosis, degenerative changes in the aortic wall, and vasculitis.
Multiple factors may be involved in the development of accelerated atherosclerosis in RA patients, including the clustering of traditional CV risk factors and inflammatory-mediated factors. The presence of traditional risk factors for vascular disease such as smoking, hypertension, diabetes, and hyperlipidemia are extremely important in RA patients, but RA itself is also directly ascribed to the higher risk of CV disorders in these patients [1, 2].
Accelerated atherosclerosis and aneurysm development may be influenced by RA-related systemic inflammation and immune dysregulation through several mechanisms. These mechanisms include endothelial dysfunction, activation of immune cells by raised CRP levels, and alteration of inflammatory-related markers including lipoproteins, ox-LDLs, nitric oxide (NO), TNF-α, RANKL, CD40L, IL-18, MMP-9, and MCP-1 [28–35].
Another potential mechanism of AA development in RA patients is progressive elastic fiber degeneration in the aortic wall that results in decreased elasticity of the aorta and increased aortic stiffness. In this regard, it has been reported that RA is associated with increased aortic stiffness in both genders [36]. Also, aortic stiffness in RA patients was associated with prolonged disease duration and higher extra-articular disease severity and CRP levels, reflecting that ongoing inflammation may be responsible for the arterial stiffening in addition to older age, smoking, visceral obesity, and high triglycerides [36].
Several reports have shown that arterial wall inflammation is an integral part of the systemic inflammatory process of RA. In an interesting study, Vizzardi et al. [37] measured aortic elastic properties by echocardiographic-derived thoracic aortic diameters; they observed that anti-TNF-α treatment after 12 months significantly modified the elastic properties of the aorta. Increased arterial stiffness as assessed by aortic pulse-wave velocity has been reported in RA patients with active disease whereas anti-TNF-α therapy was shown to reduce this stiffness to levels comparable with those measured in healthy individuals [38]. These findings have been also been corroborated by observed increased aortic (18)F-fluorodeoxyglucose uptake in patients with RA who have stable cardiovascular disease; similarly anti-TNF-α therapy reduced the extent of inflammation, a parameter that was found to be in concordance with the aortic stiffness [39].
Therefore, it is plausible that AA formation may occur secondary to rheumatoid vasculitis, but this is considered to be uncommon. Usually, rheumatoid vasculitis involves small- and medium-sized vessels and occurs in 1–5 % of RA patients, especially in those with severe deforming RA and high titers of rheumatoid factor [40].
The present study has several limitations. We had no data on the severity and the duration of RA as well as information regarding the treatment of RA patients. Due to the large number of samples and the structure of the CHS database, it was impossible to validate each case. Patients with several congenital diseases predisposing for AA and aortic dissection such as Turner syndrome, aortic coarctation, bicuspid aortic valve, Marfan syndrome, and Ehler-Danlos syndrome were not excluded from the study but they were present in both the study group and the control group.
In summary, our study has demonstrated the existence of increased prevalence of AA in patients with RA in comparison with the general population. Future large randomized studies are important to address and define the prevalence of AA in patients with RA and the relationship between RA and cardiovascular- and disease-related risk factors.
Reference
Hollan I, Meroni PL, Ahearn JM, Cohen Tervaert JW, Curran S, Goodyear CS, et al. (2013) Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 12(10):1004–1015
Houri LE, Watad A, Whitby A, Tiosano S, Comaneshter D, Cohen AD, et al. (2016) Coexistence of ischemic heart disease and rheumatoid arthritis patients—a case control study. Autoimmun Rev 15(4):393–396
Prete M, Racanelli V, Digiglio L, Vacca A, Dammacco F, Perosa F (2011) Extra-articular manifestations of rheumatoid arthritis: an update. Autoimmun Rev 11(2):123–131
Baghdadi LR, Woodman RJ, Shanahan EM, Mangoni AA (2015) The impact of traditional cardiovascular risk factors on cardiovascular outcomes in patients with rheumatoid arthritis: a systematic review and meta-analysis. PLoS One 10(2):e0117952
Pope JE (2016) Rheumatoid arthritis: TNF inhibitors and cardiovascular risk management in RA. Nat Rev Rheumatol 12(6):317–318
Gravallese EM, Corson JM, Coblyn JS, Pinkus GS, Weinblatt ME (1989) Rheumatoid aortitis: a rarely recognized but clinically significant entity. Medicine (Baltimore) 68(2):95–106
Guedes C, Bianchi-Fior P, Cormier B, Barthelemy B, Rat AC, Boissier MC (2001) Cardiac manifestations of rheumatoid arthritis: a case-control transesophageal echocardiography study in 30 patients. Arthritis Rheum 45(2):129–135
Isselbacher EM (2005) Thoracic and abdominal aortic aneurysms. Circulation 111(6):816–828
Kaneko S, Yamashita H, Sugimori Y, Takahashi Y, Kaneko H, Kano T, et al. (2014) Rheumatoid arthritis-associated aortitis: a case report and literature review. Springerplus 3:509
Katz-Agranov N, Khattri S, Zandman-Goddard G (2015) The role of intravenous immunoglobulins in the treatment of rheumatoid arthritis. Autoimmun Rev 14(8):651–658
Mimura K, Sueishi K, Tanaka K, Kinjo M (1989) Aneurysm in the sequestrated lung and aortitis associated with the malignant rheumatoid arthritis. Pathol Res Pract 185(3):381–385
Reimer KA, Rodgers RF, Oyasu R. Rheumatoid arthritis with rheumatoid heart disease and granulomatous aortitis. JAMA 1976; 235(23 k0):2510–2512.
Guy A, Tiosano S, Comaneshter D, Tekes-Manova D, Shovman O, Cohen AD, et al. (2016) Aortic aneurysm association with SLE—a case-control study. Lupus 25(9):959–963
Dahan S, Shor DB, Comaneshter D, Tekes-Manova D, Shovman O, Amital H, et al. (2016) All disease begins in the gut: Celiac disease co-existence with SLE. Autoimmun Rev 15(8):848–853
Farhi A, Cohen AD, Shovman O, Comaneshter D, Amital H, Amital D (2016) Bipolar disorder associated with rheumatoid arthritis: a case-control study. J Affect Disord 189:287–289
Watad A, Mahroum N, Whitby A, Gertel S, Comaneshter D, Cohen AD, et al. (2016) Hypothyroidism among SLE patients: case-control study. Autoimmun Rev 15(5):484–486
Watad A, Cohen AD, Comaneshter D, Tekes-Manova D, Amital H (2016) Hyperthyroidism association with SLE, lessons from real-life data—a case-control study. Autoimmunity 49(1):17–20
Panchal RR (2014) An established entity in rheumatoid arthritis? Rheumatology Rheumatology 53(suppl 1):i102
Modi P, Suvarna K, Cooper GJ (2000) Images in cardiology. Descending thoracic aortic aneurysm in rheumatoid arthritis. Heart 84(2):122
Schattner A, Gotler J (2013) Precordial pain in a patient with rheumatoid arthritis. Mayo Clin Proc 88(9):e103–e104
Evans JM, O’Fallon WM, Hunder GG (1995) Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 122(7):502–507
Kieffer E, Chiche L, Bertal A, Koskas F, Bahnini A, Bla TO, et al. (2004) Descending thoracic and thoracoabdominal aortic aneurysm in patients with Takayasu’s disease. Ann Vasc Surg 18(5):505–513
Gasparovic H, Djuric Z, Bosnic D, Petricevic M, Brida M, Dotlic S, et al. (2011) Aortic root vasculitis associated with Cogan’s syndrome. Ann Thorac Surg 92(1):340–341
Jacobs CE, March RJ, Hunt PJ, Rivera AG, Cavanagh S, McCarthy WJ (2013) Repair of a complex thoracic aneurysm from relapsing polychondritis. Vasc Endovasc Surg 47(5):387–389
Takagi H, Mori Y, Umeda Y, Fukumoto Y, Kato Y, Shimokawa K, et al. (2003) Abdominal aortic aneurysm with arteritis in ankylosing spondylitis. J Vasc Surg 38(3):613–616
Kurata A, Kawakami T, Sato J, Sakamoto A, Muramatsu T, Nakabayashi K (2011) Aortic aneurysms in systemic lupus erythematosus: a meta-analysis of 35 cases in the literature and two different pathogeneses. Cardiovasc Pathol 20(1):e1–e7
Wang SH, Chang YS, Liu CJ, Lai CC, Chen TJ, Chen WS (2014) Incidence and risk analysis of aortic aneurysm and aortic dissection among patients with systemic lupus erythematosus: a nationwide population-based study in Taiwan. Lupus 23(7):665–671
Di MP, Ferrazzi P, Libre L, Mendolicchio L, Quaglia I, De MM, et al. (2009) Intima-media thickness evolution after treatment with infliximab in patients with rheumatoid arthritis. Int J Gen Med 2:141–144
Dinarello CA (2007) Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol 27(1):98–114
Garcia-Bermudez M, Gonzalez-Juanatey C, Lopez-Mejias R, Teruel M, Corrales A, Miranda-Filloy JA, et al. (2012) Study of association of CD40-CD154 gene polymorphisms with disease susceptibility and cardiovascular risk in Spanish rheumatoid arthritis patients. PLoS One 7(11):e49214
Kao AH, Krishnaswami S, Cunningham A, Edmundowicz D, Morel PA, Kuller LH, et al. (2008) Subclinical coronary artery calcification and relationship to disease duration in women with rheumatoid arthritis. J Rheumatol 35(1):61–69
Keeling SO, Landewe R, van der Heijde D, Bathon J, Boers M, Garnero P, et al. (2007) Testing of the preliminary OMERACT validation criteria for a biomarker to be regarded as reflecting structural damage endpoints in rheumatoid arthritis clinical trials: the example of C-reactive protein. J Rheumatol 34(3):623–633
Profumo E, Di FM, Buttari B, Masella R, Filesi C, Tosti ME, et al. (2012) Biomarkers of subclinical atherosclerosis in patients with autoimmune disorders. Mediat Inflamm 2012:503942
Sodergren A, Karp K, Boman K, Eriksson C, Lundstrom E, Smedby T, et al. (2010) Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther 12(4):R158
Yiu KH, Wang S, Mok MY, Ooi GC, Khong PL, Lau CP, et al. (2010) Role of circulating endothelial progenitor cells in patients with rheumatoid arthritis with coronary calcification. J Rheumatol 37(3):529–535
Sliem H, Nasr G (2010) Change of the aortic elasticity in rheumatoid arthritis: relationship to associated cardiovascular risk factors. J Cardiovasc Dis Res 1(3):110–115
Vizzardi E, Cavazzana I, Sciatti E, Bonadei I, D’Aloia A, Tincani A, et al. (2014) Evaluation of ascending aorta wall in rheumatoid arthritis by tissue and strain Doppler imaging during anti-tumor necrosis factor-alpha therapy. Clin Cardiol 37(12):738–743
Maki-Petaja KM, Hall FC, Booth AD, Wallace SM (2006) Yasmin, Bearcroft PW et al. rheumatoid arthritis is associated with increased aortic pulse-wave velocity, which is reduced by anti-tumor necrosis factor-alpha therapy. Circulation 114(11):1185–1192
Maki-Petaja KM, Elkhawad M, Cheriyan J, Joshi FR, Ostor AJ, Hall FC, et al. (2012) Anti-tumor necrosis factor-alpha therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation 126(21):2473–2480
Genta MS, Genta RM, Gabay C (2006) Systemic rheumatoid vasculitis: a review. Semin Arthritis Rheum 36(2):88–98
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Ora Shovman and Shmuel Tiosano are the two first authors who shared equal contribution.
Rights and permissions
About this article
Cite this article
Shovman, O., Tiosano, S., Comaneshter, D. et al. Aortic aneurysm associated with rheumatoid arthritis: a population-based cross-sectional study. Clin Rheumatol 35, 2657–2661 (2016). https://doi.org/10.1007/s10067-016-3372-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3372-0