Abstract
Auto-antibodies against aminoacyl-tRNA-synthetases (anti-ARS Abs) represent the hallmark of the anti-synthetase syndrome that is defined as the clinical association of fever, Raynaud’s phenomenon, myositis, interstitial lung disease (ILD), arthritis and mechanic’s hands. Recently, differences in clinical features depending on specific anti-ARS Abs have been reported. We describe three cases of anti-EJ (anti-glycyl) antibody-positive patients presenting with ILD as a common feature, but with heterogeneous histopathological and radiographic patterns and with different responses to treatment. Relapsing-remittent fever, refractory muscle involvement and seronegative arthritis were also striking clinical manifestations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aminoacyl-tRNA-synthetase autoantibodies (anti-ARS Abs) target the enzymes that catalyze the binding of aminoacids to the corresponding tRNAs. To date, eight ARS Abs have been identified, named anti-Jo-1 (anti-hystidyl), PL-12 (alanyl), PL-7 (threonyl), EJ (glycyl), OJ (isoleucyl), KS (asparginyl), Ha (tyrosyl) and Zo (phenylalanyl) [1]. Among myositis-specific antibodies (MSAs), anti-ARS Abs are detected in 25–35 % of patients with idiopathic inflammatory myopathies (IIM). Anti-Jo1 antibodies are the most common, found in 15–30 % of patients with IIM and in up to 90 % of those with interstitial lung disease (ILD) [2]. Autoantibodies targeting other ARS are less common, each with prevalence below 5 % [3]. Although all the anti-ARS Abs represent the hallmark of the anti-synthetase syndrome (ASS), a clinical combination of fever, Raynaud’s phenomenon (RP), myositis, ILD, non-erosive arthritis and mechanics’ hands [4], several reports have associated different clinical features with distinct anti-ARS Abs and with different prognoses. In a cohort of 166 adult Japanese patients with anti-ARS Abs, myositis was closely associated with anti-Jo-1, anti-EJ and anti-PL7, while ILD correlated with all anti-ARS Abs [5]. Heliotrope rash and Gottron’s sign were frequently observed in patients with anti-Jo-1, anti-EJ, anti-PL7 and anti-PL12. The frequency of fever varied among anti-ARS-based subgroups but without statistical significance. RP was more frequently observed in patients with anti-PL12 and anti-PL7 than in patients with other anti-ARS Abs. Polyarthritis was most common in patients with anti-Jo-1 and infrequently observed in patients with anti-OJ. Mechanic’s hands were present in all anti-ARS Ab-based subgroups, but the frequency was highest in patients with anti-Jo-1 antibodies [5]. Lung and joint involvement is often the prominent feature of patients with anti-ARS antibodies while myositis may remain on a subclinical level or be absent at the onset, especially in the non-anti-Jo-1-positive group (anti PL-12, KS, OJ) [6–8]. Anti-ARS Abs are more rarely detected in patients with dermatomyositis (DM) or juvenile DM (JDM) than in those with polymyositis (PM) [9], and they can also be associated with necrotizing myopathy (anti-PL-12) [10]. Anti-ARS Abs have been detected in the pre-clinical phase before clinical symptoms of ILD or myositis and can predict clinical outcomes with a high risk to develop ILD. The strong association between anti-ARS antibodies and ILD, as well as the observation that ILD is often present as an early manifestation of disease has led to speculations of an immunological phase of the disease taking place in the lung as an early manifestation of disease [3].
According to the literature, anti-EJ-Ab is the least frequent of anti-ARS antibodies and has mainly been reported in DM patients with ILD [11]. The most common lung involvement is non-specific interstitial pneumonia (NSIP), but here we describe three cases of patients testing positive for anti-EJ Abs with uncommon histopathological or radiographic ILD patterns and other distinct clinical features.
Case 1
In 1995, a 21-year-old non-smoking Caucasian woman without remarkable previous medical history was admitted to the Division of Infectious Diseases because of remittent fever with chills, proximal muscle weakness, RP and weight loss. Diagnostic tests for infections were negative, and the fever was resistant to broad spectrum antibiotics. Screening for cancer was negative. Anti-nuclear antibodies (ANA), antibodies against extractable nuclear antigens (ENA) and double-strand DNA were absent. Her blood tests revealed high erythrocyte sedimentation rate (ESR) 60 mm/1°h (reference value <20), elevated serum levels of lactate-dehydrogenase (LDH) 820 U/L (reference value 84–246) and creatine-kinase (CK) 4210 U/L (reference value 26–192). Serum levels of aspartate and alanine aminotransferases (AST, ALT) were four times the upper normal limit. The electromyogram (EMG) showed polyphasic potentials and early recruitment of motor units. A muscle biopsy showed only myofibrillar rarefactions without inflammatory infiltrates. Polymyositis (PM) was suspected and the patient was initially treated with methylprednisolone (MPN) at the dosage of 60 mg/day (1.5 mg/kg). The fever promptly resolved but relapsed as soon as the dosage of MPN was tapered below 20 mg/day. The combination with several different immunosuppressive drugs (azathioprine 2 mg/kg/day, cyclophosphamide 2 mg/kg/day, cyclosporine 2 mg/kg/day, methotrexate 15 mg/week, etanercept 50 mg/week, intravenous immunoglobulins 1 mg/kg/day for two days) was ineffective. Over 7 years the patient experienced several flares always characterized by remittent fever and increase in muscle enzymes. A single episode of large joint arthritis occurred, and the search for rheumatoid factor (RF) and anti-cyclic-citrullinated peptide (anti-CCP) Abs was negative. In 2000, she started complaining of dyspnoea and dysphagia. High-resolution computed tomography (HRCT) showed localized intra-lobular septal thickening, and pulmonary function tests (PFTs) demonstrated reduced forced vital capacity (FVC 60 %) and carbon monoxide diffusion capacity (DLCO/VA 25 %). In 2002, a second muscle biopsy revealed muscle fibres with fibre size variation and inflammatory cell infiltrates with CD-20-positive cells. The latter prompted us to speculate that B cell-targeting therapy might be effective and the patient underwent 11 weekly plasmapheresis treatments in combination with 4 weekly rituximab (RTX) infusions (375 mg/m2 i.v. weekly for four consecutively weeks). She quickly improved and treatment was switched to MPN (4 mg/daily) and azathioprine (AZA, 2 mg/kg/daily) as maintenance therapy. Hereafter, no new flares occurred up to now while on treatment with low doses of MPN (4 mg/daily) and AZA (100 mg/daily). In 2013, we had the opportunity to test a new immunoblotting assay for MSAs, and anti-EJ Abs were detected. Currently, the patient is afebrile and has a slight reduction in proximal muscle strength (Manual Muscle Test 8-MMT8: 72/80). Muscle enzymes, ESR, and CRP are in the normal range. RP has disappeared and the PFTs values have improved (FVC 94 %, DLCO/VA 70 %).
Case 2
A 36-year-old non-smoking Caucasian woman without remarkable previous medical history presented in 2000 with 9-month history of asthenia, alopecia, dry cough and dyspnea on exertion. Diagnostic tests for infections were negative. Clinical examination revealed arthritis of wrists, proximal interphalangeal joints, and ankles bilaterally and severe symmetrical, proximal muscle weakness (MMT8 49/80). Skin examination was normal. Cardiorespiratory examination revealed basal crackles on lung auscultation. Laboratory investigations demonstrated high ESR (60 mm/1°h, reference value <20), high CRP (34 mg/L, reference value <5) and increased white blood cell count (WBC) 15.2 × 10 (9)/ L), AST (1.52 μkat/L, reference value <0.61), ALT (1.12 μkat/L, reference value <0.76) and CK (54.7, reference value 0.6–3.5 μkat/L). ANA, anti-ENA, anti-ds-DNA, RF and anti-CCP were absent. The EMG showed polyphasic potentials and low amplitude of motor units. In her muscle biopsy degenerative and regenerative muscle fibres, as well as perivascular mononuclear cell infiltrates were observed. HCRT revealed a NSIP pattern with diffuse ground-glass opacities and traction bronchiectasis. By PFTs, a reduction in FVC (73 %) and DLCO/VA (52 %) was recorded. The diagnosis of PM with associated ILD was made and the patient started treatment with 1 mg/kg prednisone, then tapered, in combination with azathioprine (2 mg/kg). Despite of normalization of muscle enzymes, ESR, CRP and remission of arthritis, she experienced several flares characterized by remitting fever, dyspnea and dry cough. Over 13 years of follow-up, there was a worsening of PFT values and progression of pulmonary fibrosis. A combination of azathioprine with cyclosporine (3 mg/kg) was started and due to ineffectiveness, the therapy was switched to mycophenolate mofetil (MMF) 2 g/day. Despite this there was a further worsening of her ILD with appearing of wide honeycombing areas and dropping of FVC to 49 % and DLCO/VA to 40 %. In 2014, for the first time, the no anti-Jo-1 ARS Abs were screened for, and patients was found to be positive for anti-EJ Abs consistent with the diagnosis of ASS. RTX was added to the treatment with MMF. This is still ongoing.
Case 3
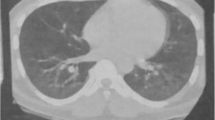
In 2014, a 56-year-old non-smoking Caucasian woman without remarkable previous medical history presented with acute dyspnea on exertion without other symptoms. Clinical examination revealed basic bilateral pulmonary crackles. Chest X-ray demonstrated bronchopneumonia with multifocal airspace consolidation. Antibiotic therapy was promptly started, but the patient worsened with dyspnea. HRCT showed patchy peripheral airspace consolidation including foci of central ground-glass opacification, suggestive of cryptogenic organising pneumonia (COP). An open lung biopsy was performed, showing acute fibrinous and organizing pneumonia (AFOP), a histopathological pattern characterized by patchy OP accompanied in some areas by alveolar fibrin with minimal lymphocytic interstitial inflammation. No arthritis, muscle weakness or skin involvement was present (MMT-8 80/80). Laboratory investigations demonstrated increased ESR, 50 mm/h (reference value <20), and CRP, 20 mg/L (reference value <5). WBC and muscle enzymes were within the normal range. ANA, anti-ENA, anti-dsDNA, RF and anti-CCP Abs were absent, but anti-EJ antibodies were detected. The EMG did not reveal any changes suggestive of myositis. PFTs showed a reduction in FVC (73 %) and DLCO (44 %). The diagnosis of anti-EJ positive ASS was made and the patient began treatment with prednisone 25 mg/day, with tapering, and MMF (2 g/day). During the 16-month follow-up, ESR, CRP and muscle enzymes have been in the normal range. Of note, FVC increased to 94 % and DLCO to 63 % and HRCT showed only residual ground glass areas. Currently, the patient is on MMF (2 g/day) and low dose of prednisone (2.5 mg/day), being completely asymptomatic.
Discussion
We report three cases of patients with anti-EJ positive ASS, characterized by ILD as a common clinical feature. In anti-EJ-positive patients, the pulmonary involvement usually occurs as an early manifestation like in our cases 2 and 3 [12]. On the contrary, patient 1 presented with dyspnea and cough 5 years after the onset of myositis, as observed in approximately 7–33 % of ASS patients, emphasizing the need for careful long-term follow-up of patients with anti-ARS Abs [13]. The phenotype of lung involvement in patients with ASS is heterogeneous. Both non-specific interstitial pneumonia (NSIP) and usual interstitial pneumonia (UIP) have been described in association with anti-EJ Abs. However, ground glass opacities and traction bronchiectasis seem to occur more frequently than honeycombing areas [14]. NSIP pattern was found on HRCT in patients 1 and 2, but ILD course and response to treatment varied between the two patients; thus, patient 1 had a mild chronic course with improvement of PFT values on glucocorticoids and conventional immunosuppressive drug treatment, while patient 2 had severe and progressive course, resistant to treatment and evolving to UIP pattern on HRCT; thus, the molecular mechanisms may still be different despite the same histopathological phenotype. In contrast, patient 3 had acute onset ILD, and thoracoscopic biopsy revealed a new histopathological interstitial lung pattern defined as fibrinous and organizing pneumonia (AFOP), which has been recently described in association with connective tissue disease [15]. Also, Sauter and Butnor reported a case of ASS with anti-EJ who presented with AFOP, expanding the spectrum of pulmonary histopathological manifestations of ASS [16]. Our patient 3 improved significantly on glucocorticoids and MMF.
All three patients had a reduction in DLCO at onset, suggesting that a decrease in DLCO is an early abnormality of ILD in this phenotype. Furthermore, it may indicate that DLCO is the most sensitive test to detect impairment of pulmonary function in patients with ASS as previously suggested [13]. The low DLCO may indicate a possible involvement of micro-vessels of the lungs. This is further supported by previous findings of pulmonary capillaritis and early endothelial activation in lung biopsies from patients with ASS [17, 18]. None of the three patients had rapidly progressive ILD as observed in presence of antibodies directed against the melanoma differentiation associated gene 5 (MDA5) [19]. Anti-MDA5 is associated with rapidly progressive lung disease and poor survival often associated with the so-called amyopathic dermatomyositis [19, 20].
Although ILD remains the main clinical manifestation and the major cause of death in anti-EJ patients [5], our case series suggest that other features may be associated with these antibodies. Patients 1 and 2 had recurrent flares of remitting fever together with muscle weakness. Relapsing-remitting fever has already been described in almost 20 % of patients with ASS [21]. Fever does not seem to be a specific feature of anti-ARS Abs, as a large study comparing 95 IIM patients positive and 152 negative for anti-ARS Abs did not demonstrate an association between fever and ASS [5]. Response to immunosuppressive treatment varies in patients with anti-EJ antibodies. In one report, six anti-EJ positive patients required more aggressive treatment because of refractory myositis than anti-EJ-negative patients [22]. Our first patient had recurrent fever and weakness responding to high dose of prednisone, whereas muscle strength of patient 2 did not improve despite immunosuppressive treatment. Unlike the previously reported cases with anti-EJ Abs in association with DM and amyopathic DM patients [5, 11], our patients did not exhibit skin involvement. Joint involvement was present in two out of three patients, but neither anti-CCP Abs nor RF were detected. Interestingly, 3 cases of anti-EJ syndrome and simultaneously presence of anti-CCP Abs have been published: these patients later developed rheumatoid arthritis (RA) [23]. Arthralgia was reported as the main pattern of joint manifestation in ASS [24], while anti-CCP negative arthritis was present in two of our patients. A similar join involvement was reported from a study on 225 anti-Jo-1-positive patients [25].
Patient 1 and 2 had significant delayed diagnosis, which may have contributed to the poor response to treatment [26]. One of the factors affecting this delay was the absence of ANA, anti-ENA, and anti-ds-DNA on common ELISA-screening. A take home message from these cases is that ANA-negative patients may test positive for auto-Abs directed to cytoplasmic antigens and MSAs are predominantly directed against cytoplasmic epitopes [27]. Additionally, anti-ARS Abs are not rare among idiopathic interstitial pneumonias (IIPs) as demonstrated by a retrospective study performed in 198 cases with IIPs. In this cohort, 13 patients (6.6 %) were positive for anti-ARS Abs and anti-EJ was the most prevalent (3 %) [14].
In summary, we present three anti-EJ positive ASS patients with heterogeneous lung involvement. The first case had mild chronic ILD, as the HRCT did not progress over time and the PFTs findings improved. The second case had a refractory ILD. The third case with a lung biopsy proven AFOP in the setting of ASS syndrome expands the pulmonary histopathological spectrum of this syndrome. In addition, recurrent-relapsing fever, arthritis, and myositis refractory to conventional treatment may be part of the clinical phenotype related to anti-EJ Abs. Although further studies are needed to better define the clinical features and effective treatments of anti-EJ syndrome, identifying anti-ARS Abs in patients with ILD or myositis may be useful to predict the clinical course and response to treatment. Finally, searching for MSAs should always be performed in patients who are diagnosed with idiopathic ILD even if suggestive extra-pulmonary manifestations are lacking.
References
Gunawardena H, Betteridge ZE, McHugh NJ (2009) Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology 48:607–612. doi:10.1093/rheumatology/kep078
Connors GR, Christopher-Stine L, Oddis CV, Danoff SK (2010) Interstitial lung disease associated with the idiopathic inflammatory miopathies: what progress has been made in the past 35 years? Chest 138(6):1464–1474. doi:10.1378/chest.10-0180
Mahler M, Miller FW, Fritzler MJ (2014) Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev 13(4–5):367–371. doi:10.1016/j.autrev.2014.01.022, Review. PubMed PMID: 24424190; PubMed Central PMCID: PMC3970575
Targoff IN (1992) Autoantibodies in polymyositis. Rheum Dis Clin N Am 18:455–482
Hamaguchi Y, Fujimoto M, Matsushita T, Kaji K, Komura K, Hasegawa M et al (2013) Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 8(4):e60442. doi:10.1371/journal.pone.0060442
Hirakata M, Suwa A, Takada T, Sato S, Nagai S, Genth E et al (2007) Clinical and immunogenetic features of patients with autoantibodies to asparaginyl-transfer RNA synthetase. Arthritis Rheum 56:1295–1303. doi:10.1002/art.22506
Sato S, Kuwana M, Hirakata M (2007) Clinical characteristics of Japanese patients with anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibodies. Rheumatology 46:842–845. doi:10.1093/rheumatology/kel435
Targoff IN, Arnett FC (1990) Clinical manifestations in patients with antibody to PL-12 antigen (alanyl-tRNA synthetase). Am J Med 88:241–251. doi:10.1016/0002-9343(90)90149-8
Ghirardello A, Bassi N, Palma L, Borella E, Domeneghetti M, Punzi L et al (2013) Autoantibodies in polymyositis and dermatomyositis. Curr Rheumatol Rep 15:335. doi:10.1007/s11926-013-0335-1
Mehndiratta P, Mehta S, Manjila SV, Kammer GM, Cohen ML, Preston DC (2012) Isolated necrotizing myopathy associated with ANTI-PL12 antibody. Muscle Nerve 46:282–286. doi:10.1002/mus.23383
Targoff IN, Trieu EP, Plotz PH, Miller FW (1992) Antibodies to glycyl-transfer RNA synthetase in patients with myositis and interstitial lung disease. Arthritis Rheum 35(7):821–830
Schneider F, Yousem SA, Bi D, Gibson KF, Oddis CV, Aggarwal R (2014) Pulmonary pathologic manifestations of anti-glycyl-tRNA synthetase (anti-EJ)-related inflammatory myopathy. J Clin Pathol 67:678–683. doi:10.1136/jclinpath-2014-202367
Koreeda Y, Higashimoto I, Yamamoto M, Takahashi M, Kaji K, Fujimoto M et al (2010) Clinical and pathological findings of interstitial lung disease patients with anti-aminoacyl- tRNA synthetase autoantibodies. Intern Med 49(5):361–369
Watanabe K, Handa T, Tanizawa K, Hosono Y, Taguchi Y, Satoshi N et al (2011) Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med 105(8):1238–1247. doi:10.1016/j.rmed.2011.03.022
Hernandez-Prera J, Beasley MB (2013) Novel patterns of interstitial lung disease. Diagn Histopathol 19:276–281
Sauter JL, Butnor KJ (2014) Expanding the spectrum of pulmonary histopathological manifestations of anti-synthetase syndrome: anti-EJ-associated acute fibrinous and organizing pneumonia. Histopathology 65(4):581–582. doi:10.1111/his.12420
Schwarz MI, Sutarik JM, Nick JA, Leff JA, Emlen JW, Tuder RM (1995) Pulmonary capillaritis and diffuse alveolar hemorrhage. A primary manifestation of polymyositis. Am J Respir Crit Care Med 151(6):2037–2040
Helmers Barbasso S, Englund P, Engstrom M, Ahlin E, Fathi M et al (2009) Sera from anti-JO1-positive patients with polymyositis and interstitial lung disease induce expression of intercellular adhesion molecule 1 in human lung endothelial cells. Arthritis Rheum 60(8):2524–2530. doi:10.1002/art.24683
Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R (2015) Anti-MDA5 is associated with rapidly progressive lung disease and poor survival in U.S. patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken). doi:10.1002/acr.22728
Koga T, Fujikawa K, Horai Y, Okada A, Kawashiri SY, Iwamoto N et al (2012) The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology (Oxford) 51(7):1278–1284. doi:10.1093/rheumatology/ker518
Chatterjee S, Prayson R, Farver C (2013) Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 80:655–666. doi:10.3949/ccjm.80a.12171
de Souza FHC, Cruellas MGP, Levy-Neto M, Shinjo SK (2013) Anti-synthetase syndrome: anti-PL-7, anti-PL-12 and anti-EJ. Rev Bras Reumatol 53:352–357
Tomioka H, Kaneko M, Kogata Y, Katsuyama E, Ishikawa S, Fujii T (2012) A case of interstitial lung disease with anti-EJ and anti-CCP antibodies preceding rheumatoid arthritis. Respir Investig 50:66–69. doi:10.1016/j.resinv.2012.04.003
Hervier B, Devilliers H, Stanciu R, Meyer A, Uzunhan Y, Masseau A et al (2012) Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase autoantibody specificity. Autoimmun Rev 12(2):210–217. doi:10.1016/j.autrev.2012.06.006
Cavagna L, Nuño L, Scirè CA, Govoni M, Javier Lopez Longo F, Franceschini F et al (2015) Clinical spectrum time course in anti-JO1 positive antisynthetase syndrome: results from an international retrospective multicenter study. Medicine (Baltimore) 4(32):1144. doi:10.1097/MD.0000000000001144
Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP et al (2014) Patients with non-Jo1 anti-tRNA synthetase autoantibodies have worse survival than Jo1 positive patients. Ann Rheum Dis 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
Song JS, Hwang J, Cha H-S, Jeong B-H, Suh GY, Chung MP et al (2015) Significance of myositis autoantibody in patients with idiopathic interstitial lung disease. Yonsei Med J 56(3):676–683. doi:10.3349/ymj.2015.56.3.676
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Margherita Giannini and Antonella Notarnicola contributed equally to this work.
Rights and permissions
About this article
Cite this article
Giannini, M., Notarnicola, A., Dastmalchi, M. et al. Heterogeneous clinical spectrum of interstitial lung disease in patients with anti-EJ anti-synthetase syndrome: a case series. Clin Rheumatol 35, 2363–2367 (2016). https://doi.org/10.1007/s10067-016-3258-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3258-1