Abstract
Acute gouty arthritis (AGA) is an auto-inflammatory disease characterized by resolving spontaneously, which suggests that negative feedback loops control inflammatory and immunological responses to monosodium urate (MSU) crystals. By now, the molecular mechanism for spontaneous resolution of acute GA remains unclear; this study was undertaken to evaluate whether IL-37 is involved in spontaneous resolution of AGA. A total of 45 acute GA (AGA),29 non-acute GA (NAGA) male patients and 82 male health control (HC) were involved in this study, we measured IL-7 expression in the peripheral blood mononuclear cells (PBMCs), together with levels of IL-1β, IL-6, IL-10, TNF-α and TGF-β1 in the serum. Further, we either inhibited IL-37 expression in human PBMCs with siRNA or over-expressed the cytokine in human macrophages. Pro-inflammatory cytokine IL-1β, IL-6, and TNF-α expressions were significantly higher in the AGA group than in the NAGA or HC group (P < 0.05, respectively). However, anti-inflammatory IL-37, TGF-β1, and IL-10 were greater in the NAGA group than in the AGA and HC groups (P < 0.05, respectively). Expression of IL-37 in MSU crystal-treated macrophages inhibited the expression of pro-inflammatory cytokines, whereas the abundance of these cytokines increased with silencing of endogenous IL-37 in human blood cells. However, anti-inflammatory TGF-β1 and IL-10 expressions in these supernatants were unaffected by over-expression or knockdown of IL-37. Our study indicates that IL-37 is an important anti-inflammatory cytokine in AGA by suppressing the production of pro-inflammatory cytokines. Thus, IL-37 may provide a novel research target for the pathogenesis and therapy of GA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gout is an inflammatory disease induced by the over-production of uric acid in the blood and the crystallization of monosodium urate (MSU) in articular and periarticular tissues [1]. The epidemiological evidence suggests that both the incidence and prevalence of gout are on a striking rise [2], and the incidence is 1.14 and 1.4 % in the Shandong coastal cities of Eastern China and in the eastern counties, respectively [3, 4]. Although the accurate pathogenic mechanism of gout is still unknown, much evidence suggests that genetic factors, environmental aspects, and immune disorders may be associated with its development [5]. Attacks of gout are initiated by the deposition of MSU crystals in the joints, and MSU crystals are extensively recognized as endogenuos “danger signal” by elements of the innate immune system [6, 7].
An interesting feature of the acute inflammatory response in gout is self-limiting after 7–10 days [8]. A variety of reasons are thought to be involved in the molecular mechanism of spontaneous remission of inflammation, including coating of MSU crystals with inhibitory proteins [9], breaking the balance of pro-inflammatory and anti-inflammatory molecules within inflamed joints[10–14]. High level of transforming growth factor β1 (TGF-β1) has been reported in the synovial fluid (SF) of patients with acute gout arthritis [15], and in the rat air pouch model of gout, it has been shown that TGF-β1 significantly inhibits cellular recruitment [10]. It has been observed that cytokine-inducible SH2-containing protein (CIS) and suppressors of cytokine signaling (SOCS3) were up-regulated in GA synovial fluid mononuclear cells (SFMCs); CIS over-expression accelerated the binding of MSU crystal-induced STAT3 to the TGF-β1 promoter, which activated the TGF-β1 transcription [16]. More recently, Anna Scanu et al. reported that cytokine levels in SF may change depending on the different stages of acute gout, highlighting the role of TGF-β1 in the resolution of gout arthritis [17].
IL-37, a new member of the IL-1 family, contains 11 structurally related members sharing a β-barrel motif [18–20]. IL-37 has five splice variants: IL-37a to IL-37e; IL-37b (NM014439.3) is the largest cytokine member and is encoded by five of the six exons spanning the IL-37 gene, of which exon 1 encodes the putative caspase-1-processing site [21, 22]. The IL-37b precursor is cleaved by caspase-1 into mature form, demanding the mature form to translocate actively into the cell nucleus [18, 22]. IL-37-specific mRNA has been detected in a variety of tissues, including the lymph node, thymus, bone marrow, placenta, lung, and testis. IL-37 protein production in peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) was shown to be up-regulated when stimulated by TLR ligands, as well as several pro-inflammatory cytokines [21, 23].
In human peripheral blood mononuclear cells (PBMCs), suppression endogenous IL-37 leads to increased expression of LPS- as well as IL-1β-induced cytokines [18]. Mice transgenic for full-length human IL-37 (IL-37tg) are preserved from LPS-induced systemic inflammation [18], chemical colitis [24], metabolic syndrome [25], and acute myocardial infarction [26]. Previous studies also have found that TGF-β1 can effectively induce the production of IL-37 [18]. Recently, it has been reported that IL-37 was highly up-regulated in the serum and PBMCs of patients with systemic lupus erythematosus (SLE) [27] and in the synovial tissues and plasma of patients with RA [18, 28]. Ye et al. revealed that recombinant human (rh) IL-37 inhibited the production of pro-inflammatory IL-1β and IL-6 in PBMCs from patients with systemic lupus erythematosus in vitro [27]. Based on the data described above, it is postulated that IL-37 may play a prominent role in the pathogenesis of GA by curbing pro-inflammatory cytokines.
In this study, we investigated IL-37 expression levels in the peripheral blood mononuclear cells (PBMCs) and major inflammatory cytokines of serum in GA patients and further analyzed function of endogenous IL-37 in MSU-mediated inflammatory responses. Finally, this study was undertaken to evaluate whether over-expression IL-37 in macrophages can inhibited the expression of MSU crystal-induced inflammatory cytokines. To our knowledge, this is the first study to show the anti-inflammatory property of IL-37 in MSU-mediated inflammatory responses.
Methods
Patients
A cross-sectional study was carried out at the Department of Rheumatology and Immunology, the Affiliated Hospital of North Sichuan Medical College from June 2008 to July 2011. Seventy-four consecutive male patients with primary GA were joined into the study and fulfilled the classification criteria proposed by the American College of Rheumatology (ACR). All the GA patients had no history of cancer, hematopathy, nephropathy, infection, or other autoimmune diseases. Patients were subsequently divided into an acute GA (AGA) group (including 45 patients with acute attack) and a non-acute GA (NAGA) group (including 29 patients) based on whether patients are presenting onset of symptoms or not. According to Zhang et al [28], after resolution of acute gouty arthritis, in general, there are no obvious sequelae. There are some skin calms in the affected region, desquamation, and prickle. After initial onset, most patients will recur within 1 to 2 years. With the progress of patients’ condition, attack will gradually increase and duration of symptoms will extend. Asymptomatic inter-mission period will shorten. When the GA patients stay in the asymptomatic inter-mission period, and they are considered as NAGA.
All patients were not receiving any systemic anti-inflammatory treatment or drugs to control the production and elimination of uric acid during the previous months, and their hypouricaemic treatment had been stable in the past 2 months. Eighty-two aged men in the healthy control (HC) group who experienced regular physical examination at the Affiliated Hospital of North Sichuan Medical College between June 2009 and July 2013 were included as the healthy control in this study. They were healthy according to the data of examination, without any history of systemic inflammatory disease. Blood samples from all participants were obtained and collected into sterile, anticoagulant-coated tubes and immediately transported to the laboratory for detecting protein expression and inflammation cytokines production. This study was approved by the Affiliated Hospital of North Sichuan Medical College and with informed consent by all participants.
Plasmid construction
Human IL-37 (NM 014439) cDNA open reading frame was cloned into pcDNA3.1 vector and was sequenced to exclude mutations; the vector also contained a green fluorescence protein (GFP)-coding sequence.
Preparation of MSU crystal
MSU crystals were prepared as described by Scanu et al. [29]. Crystal sizes were verified by polarizing light microscopy. Endo-toxin levels in MSU crystal preparations, as detected by Limulus amoebocyte cell lysate assay (Sigma-Aldrich).
Isolation of PBMCs, cell culture, and MSU stimulation
Peripheral venous blood samples were collected in heparin-containing tubes. PBMCs were isolated by density-gradient centrifugation using Ficoll-Paque Plus solution (Amersham Biosciences, Uppsala, Sweden). Freshly isolated PBMCs were suspended in a complete medium, consisting of RPMI1640, 2 mM L-glutamine, 100 units/ml of penicillin, and 100 μg/ml of streptomycin, supplemented with 10 % FBS (Gibco BRL, Grand Island, NY, USA) and then plated on 6-cm dishes at a density of 5 × 105 cells/ml. After MSU crystals were added directly to the culture plates, cells were cultured in completed media for the indicated times at 37 °C in a 5 % CO2-humidified incubator.
Transfection
PBMCs were transfected with IL-37-specific RNA interference (siIL-37) versus non-specific siRNA (negative control); THP-1 cells were transfected with pcDNA3.1-IL-37b plasmid or pcDNA3.1 vector. Transfection was performed by using Lipofectamine® LTX Reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. THP-1 cells, after transfected for 24 h, were treated with phorbol 12-myristate 13-acetate/ionomycin (PMA; 100 ng/ml) for 3 h to detect the levels of inflammatory cytokines. This treatment increases the phagocytic properties of the cells and induces a constitutive production of pro-inflammatory cytokines [30]. After PMA stimulation, cells were washed and then stimulated with MSU(100 μg/ml) for 24 h, as described. The transfection efficiency of PBMCs and THP-1 cells are respectively about 35 and 40 %.
Western blot analysis
Il-37 protein expression was measured using western blotting in 15 patients with AGA, NAGA, and HC, respectively. PBMCs were treated with 10 % SDS lysis buffer to analyze the protein expression of IL-37. After removing insoluble debris by centrifugation, supernatants were collected. Protein concentration was quantified by Bradford protein assay according to manufacturer’s instruction (Bio-Rad Laboratories). For each sample, 10 μg proteins were separated by 12 % SDS polyacrylamide gel. After transferring onto PVDF membranes, non-specific binding was blocked with 5 % non-fat dry milk for 1 h at room temperature before incubation IL-37 primary mouse monoclonal antibodies at 4°C overnight (anti-IL-37:1:500 dilution, Abcam, England). After washing extensively with TBS-T buffer (10 mM Tris-HCL (pH 7.4), 100 mM NaCl, and 0.1 % Tween-20), the membranes were incubated with the anti-mouse HRP-conjugated secondary antibodies in a 1:10,000 dilution. After exposing the membrane with the ECL substrate (Amersham, Biosciences Europe, Freiburg, Germany) for 5 min, chemiluminescence was detected by Fuji Medical X-Ray films.
ELISA
Detection of IL-1β, IL-6, IL-10, TNF-α, and TGF-β1 were accomplished using the Neobioscience kit (Shenzhen, China) and following the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean (±SEM) or median (range) and analyzed by Graphpad Prism V.5.00 software (GraphPad Software, San Diego CA, USA). Comparisons between groups were made using nonparametric Mann-Whitney U test. And correlation analysis between variables was also performed with Spearman’s rank correlation test. P values under 0.05 were considered statistically significant.
Results
The clinical and laboratory data of subjects were summarized in Table 1.
In our study, gout cases were matched for age to control individuals (P > 0.05). Significant differences were observed among the AGA, NAGA, and HC groups with respect to the levels of UA and BMI (P < 0.05, respectively). The concentration of UA was much higher in the AGA patients than that in the NAGA patients or healthy control subjects (P < 0.01, respectively), and also, higher level of UA was found in the NAGA group compared with the health control group (P < 0.01, respectively). BMI were much higher in the AGA and NAGA patients than those in the healthy control (P < 0.05, respectively).
Comparison of white blood cell (WBC) count, IL-1β, IL-6, IL-10, TNF-α, and TGF-β1 expressions levels among the AGA, NAGA, and HC groups
The number of (WBC) was significantly increased in the AGA patients compared to the NAGA patients and healthy control (Fig. 1a). It has been reported that inflammatory molecules IL-1β, TNF-α, and TGF-β1 were significantly elevated in the SF of GA patients [16]. We investigated IL-1β, IL-10, IL-6, TNF-α, and TGF-β1 production in peripheral blood of GA patients. As shown in the Fig. 1b–d, significant differences in cytokine levels were observed among the AGA, NAGA, and HC groups. IL-1β, IL-6, and TNF-α levels in serum were significantly increased in the AGA group compared to the NAGA and HC groups (AGA > NAGA > HC). But TGF-β1 and IL-10 levels in serum were much higher in the NAGA group than in the AGA and HC groups (NAGA > AGA > HC) (Fig. 1e, f).
Comparison of WBC count, IL-1β, TNF-α, IL-10, IL-6, and TGF-β1 levels of serum among the AGA, NAGA, and HC groups. a Number of white blood cells (WBC). b–d Pro-inflammatory cytokine IL-1β, TNF-α, and IL-6 levels of serum were much higher in the AGA group than in the NAGA and HC groups and higher levels in the NAGA than in the HC group (P < 0.05). e, f IL-10 and TGF-β1 levels of serum were much higher in the NAGA group than in the AGA and HC groups and higher in the AGA group than in the HC group
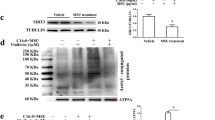
IL-37 expression level elevated in the GA patients
IL-37 is inducible in the peripheral blood mononuclear cells. TGF-β1 can effectively induce endogenous IL-37, which acts as an anti-inflammatory cytokine with intracellular as well as extra-cellular functionality [18]. This study indicated that TGF-β1 was up-regulated in the PBMCs of GA patients, so we measured the expression level of IL-37 through western blotting in the PBMCs. The data showed that the protein level of IL-37 in the NAGA group was much higher than that in the AGA and HC groups (NAGA > AGA > HC) (Fig. 2a, b).
Regulation and function of endogenous IL-37 in MSU-mediated inflammatory responses
Previous studies have shown that IL-37 is inducible in the peripheral blood mononuclear cells (PBMCs) [18]. The function of endogenous IL-37 in the MSU crystal-stimulated inflammatory response is unknown. First, we want to know whether MSU crystals can stimulate the expression of IL-37 in the PBMCs. PBMCs from six donors were treated with different concentrations (0, 50, 100, and 200 μg/ml) of MSU crystals for 24 h and IL-37 protein level reached the maximum value treatment with 100 μg/ml MSU crystals (Fig. 3a, b). Furthermore, to assess the function of endogenous IL-37, PBMCs from six donors were transfected with either non-specific siRNA (negative control) or siRNA against IL-37 (siIL-37). IL-37 protein level in the MSU crystal-stimulated PBMCs was significantly reduced by siIL-37 (Fig. 3c, d). In addition, cell treated with siIL-37 resulted in significant increases in the production of IL-1β, IL-6, and TNF-α. However, anti-inflammatory IL-10 and TGF-β1 levels were unaffected by siIL-37 treatment in these same supernatants (Fig. 3e–i).
Production and silencing of endogenous IL-37 in human PBMCs. a, b PBMCs from HCs were treated with different concentrations of MSU crystals for 24 h. IL-37 levels induced by MSU crystals were measured by western blotting analysis, and the density was normalized to the expression of β-actin (*P < 0.05 versus the control group). c, d PBMCs were transfected with 100 nM of siIL-37 or non-specific siRNA. One of five similar bolts is shown. e–i PBMCs were transfected with either 100 nM siIL-37 or non-specific siRNA and stimulated with 100 μg/ml MSU crystals. After 24 h, in pairs of siIL-37/non-specific siRNA from five donors IL-1β, IL-6, IL-10, TNF-α, and TGF-β1 production were assessed by ELISA. The results represent the mean ± SD of five independent experiments. NS no significance
Inhibition effect of MSU crystal-induced inflammatory responses in IL-37b transfected THP-1 cells
In human macrophages, THP-1 cells were transfected with human IL-37 plasmid which can decrease inflammatory cytokine production [18], and MSU crystals are known to induce inflammatory responses in the GA. So we investigated whether IL-37 could inhibit the MSU crystal-induced inflammatory response. Using a human macrophage THP-1 cell line, THP-1 cells were transfected with either pcDNA3.1-IL-37b or pcDNA3.1 vector for different time courses (24, 48, and 72 h). The forced expression of IL-37 was confirmed by western blotting in each group (Fig. 4a–c). The kinetics study demonstrated that, at 24-h post-transfection, IL-37 was highly expressed; this extended to the 48-h time point. At 72 h, IL-37 expression had decreased compared with that observed at 24 and 48 h, offering a relative stable condition to study the roles of IL-37 in the THP-1 cells. So THP-1 cells were transfected with pcDNA3.1-IL-37b plasmid for 24 h and subsequently were treated with PMA for 3 h. After PMA stimulation, cells were treated with 100 μg/mL of MSU crystals for 24 h. The production of IL-1β, IL-6, IL-10, and TNF-α in the culture supernatants was measured through ELISA. Pronounced reduction in the expression of IL-1β, IL-6, and TNF-α was observed, but there were no significant difference in IL-10 and TGF-β1 levels (Fig. 4d–h).
Effect of MSU crystal-induced IL-37b on cytokine production in the THP-1 cells. The control macrophages or macrophages transfected with pcDNA3.1-IL-37b or pcDNA3.1 vector for 24 h later were stimulated with MSU crystals for 24 h. a The expression of IL-37 in vitro was detected by western blotting. Molecular weight of IL-37 was ∼34 kDa. b, c IL-37 expression at different time points after transfection and the density was normalized to the expression of β-actin. d–h The expression levels of IL-1β, IL6, IL-10, TNF-α, and TGF-β1 were represented after over-expression IL-37. The results represent the mean ± SD of six independent experiments. NS no sigficance
Discussion
Previous studies have shown that high levels of cytokines such as IL-1β, IL-6, IL-10, TNF-α, and TGF-β1 have been detected in the SF of GA patients [17]. In our present study, the levels of IL-1β, IL-6, IL-10, and TNF-α were significantly elevated in the PBMCs of GA patients than that in the HC group. The levels of anti-inflammatory IL-10 and TGF-β1 were higher in the NAGA group than in the AGA group, so it is in accordance with SF of GA patients [17]. Interestingly, as another fundamental inhibitor of innate immunity, the expression of IL-37 was higher in the NAGA group than in the AGA group and HC group (NAGA > AGA > HC).. What are the functions of this fundamental innate immunity inhibitor IL-37 [18] in the GA patients? To answer this question, we investigated the function of endogenous IL-37. Firstly, MSU crystals were used to stimulate PBMCs from donors and detected the elevation of IL-37 protein level (Fig. 3a, b). Furthermore, knockdown IL-37 in the PBMCs can increase the levels of MSU crystal-stimulated inflammatory cytokines, such as IL-1β, IL-6, and TNF-α (Fig. 3c–h). However, over-expression IL-37 in the THP-1 cell could decrease the production of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α (Fig. 4d–g). Whether by suppression or over-expression of IL-37, the production of anti-inflammatory IL-10 and TGF-β1 were almost unaffected (Figs. 3i and 4h). Previous studies indicated that IL-37 could be up-regulated by IL-1β, IL-10, and TNF-α [18, 23], suggesting these cytokines work as positive feedback loops for up-regulation of IL-37 expression. Therefore, it is rational to account for the higher expression levels of IL-37 in the NAGA group than in the AGA group. It has been shown that IL-37 can suppress the production of pro-inflammatory IL-1β, IL-6, and TNF-α in the PBMCs [18]. Together with these data, the down regulation of pro-inflammatory cytokines in our experiments suggests that IL-37 may play a significant role in the control of inflammatory response in the GA.
TGF-β1 is an immune regulator which plays an important role in immune system by control several aspects of inflammatory responses. Both in vitro and in vivo studies have proved that TGF-β1 worked as a potent anti-inflammatory cytokine in MSU crystal-induced inflammation [10, 31]. Previous studies have documented that high level of TGF-β1 has been detected in the SF of patients with acute gout arthritis [16, 17]. This study also indicated that anti-inflammatory IL-37 and TGF-β1 were up-regulated greatly in the PBMCs of GA patients. Smad proteins are the main intracellular signaling effectors of TGF-β and are phosphorylated by TGF-β receptors upon binding the ligand. Following phosphorylation, Smad3 translocates to the nucleus and affects gene transcription. IL-37 also translocation to the nucleus acts via an intracellular mechanism. It has been reported that IL-37b co-localized with phosphor-Smad3, and Smad3 is required for IL-37 activity [18]. Therefore, it is possible that IL-37 interacts with Smad3 which is effector of TGF-β signal pathway. Such functional complex might reduce the levels of pro-inflammatory IL-1β, IL-6, and TNF-α in the MSU crystal-stimulated inflammatory responses. This data implies that the up-regulation of IL-37 in the GA patients may be the result of inflammation induction anti-inflammation.
In summary, our study showed, for the first time, to our knowledge, that the expression of IL-37 is elevated in the GA patients. Meanwhile, it is likely that there are some negative feedback mechanisms to suppress excessive inflammation in the GA patients. Furthermore, we demonstrated that IL-37 was highly effective in reducing the levels of IL-1β, IL-6, and TNF-α in THP-1 cells treated with MSU crystals. These observations implicate that IL-37 may play a key role in the inhibition of pathogenesis of GA. Future study is needed to elucidate the regulatory mechanisms of IL-37-mediated immune reaction in the GA patients.
References
Martinon F, Glimcher LH (2006) Gout: new insights into an old disease. J Clin Invest 116:2073–2075
Weaver AL (2008) Epidemiology of gout. Cleve Clin J Med 75:S9–S12
Miao Z, Li C, Chen Y, Zhao S, Wang Y, Wang Z et al (2008) Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol 35:1859–1864
Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T et al (2008) Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis 67:960–966
Taniguchi A, Kammatani N (2008) Control of renal uric acid excretion and gout. Curr Opin Rheumatol 20:192–197
Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425:516–521
Ghaemi-Oskouie F, Shi Y (2011) The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep 13:160–166
Cronstein BN, Terkeltaub R (2006) The inflammatory process of gout and its treatment. Arthritis Res Ther 8:S1–S3
Ortiz-Bravo E, Sieck MS, Schumacher HR Jr (1993) Changes in the proteins coating monosodium urate crystals during active and subsiding inflammation. Immunogold studies of synovial fluid from patients with gout and of fluid obtained using the rat subcutaneous air pouch model. Arthritis Rheum 36:1274–1285
Lioté F, Prudhommeaux F, Schiltz C, Champy R, Herbelin A, Ortiz-Bravo E et al (1996) Inhibition and prevention of monosodium urate monohydrate crystal-induced acute inflammation in vivo by transforming growth factor beta1. Arthritis Rheum 39:1192–1198
Falasca GF, Ramachandrula A, Kelley KA, O’Connor CR, Reginato AJ (1993) Superoxide anion production and phagocytosis of crystals by cultured endothelial cells. Arthritis Rheum 36:105–116
Di Giovine FS, Malawista SE, Nuki G, Duff GW (1987) Interleukin 1 (IL-1) as a mediator of crystal arthritis: stimulation of T cell and synovial fi broblast mitogenesis by urate crystalinduced IL-1. J Immunol 138(10):3213–3218
Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M (1989) Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest 83:585–592
Guerne PA, Terkeltaub R, Zuraw B, Lotz M (1989) Inflammatory microcrystals stimulate interleukin-6 production and secretion by human monocytes and synoviocytes. Arthritis Rheum 32:1443–1452
Fava R, Olsen N, Keski-Oja J, Moses H, Pincus T (1989) Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med 169:291–296
Chen YH, Hsieh SC, Chen WY, Li KJ, Wu CH, Wu PC et al (2011) Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFβ1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Ann Rheum Dis 70:1655–1663
Anna S, Francesca O, Roberta R, Frallonardo P, Dayer JM, Punzi L (2012) Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor β1 in the resolution phase. Ann Rheum Dis 71:621–624
Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA (2010) IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol 11:1014–1022
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117:3720–3732
Boraschi D, Lucchesi D, Hainzl S, Leitner M, Maier E, Mangelberger D et al (2011) IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw 22:127–147
Kumar S, Hanning CR, Brigham-Burke MR, Rieman DJ, Lehr R, Khandekar S et al (2002) Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine 18:61–71
Sharma S, Kulk N, Nold MF, Graf R, Kim SH, Reinhardt D et al (2008) The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol 180:5477–5482
Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA (2004) Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J 381:503–510
McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB et al (2011) Interleukin 37 expression protects mice from colitis. PNAS 108(40):16711–16716
Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ et al (2014) IL-37 protects against obesity-induced inflammation and insulin resistance. Nat Commun 5:4711
Moretti S, Bozza S, Oikonomou V, Renga G, Casagrande A, Lannitti RG et al (2014) IL-37 inhibits inflammasome activation and disease severity in murine aspergillosis. Plos Pathog 10(11), e1004462
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y et al (2014) IL-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: its correlation with disease activity. J Transl Med 12:69
Zhao PW, Jiang WG, Wang L (2014) Plasma levels of IL-37 and correlation with TNF-a, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One 9, e95346
Scanu A, Oliviero F, Gruaz L, Sfriso P, Pozzuoli A, Frezzato F et al (2010) High-density lipoproteins down-regulate CCL2 production in human fibroblast like synoviocytes stimulated by urate crystals. Arthritis Res Ther 12:R23
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
Yagnik DR, Evans BJ, Florey O, Mason JC, Landis RC, Haskard DO (2004) Macrophage release of transforming growth factor beta1 during resolution of monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 50:2273–2280
Acknowledgments
This work was supported by grants to M. Z. (No. 81301599) from the National Natural Science Foundation of China; Sichuan Province Science and Technology Support Project (2011SZZ012), Scientific Research Fund of the Sichuan Province Education Department(12ZA047) Research Development Plan of North Sichuan Medical College (CBY11-A-ZP02 and CBY12-A-ZD09).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Zeng, M., Dang, W., Chen, B. et al. IL-37 inhibits the production of pro-inflammatory cytokines in MSU crystal-induced inflammatory response. Clin Rheumatol 35, 2251–2258 (2016). https://doi.org/10.1007/s10067-015-3109-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3109-5