Abstract
We report the case of a 35-year-old male, who was diagnosed with systemic lupus erythematosus (SLE) in 2010 based on the presence of articular, serous, renal, immune, and hematologic involvement. He also had secondary antiphospholipid syndrome (APS). He was treated with prednisone 10 mg per day, hydroxychloroquine 200 mg per day, methotrexate 12.5 mg per week, leflunomide 20 mg per day, and oral anticoagulation previous to the present event. He presented to emergency room with a 7 day disease duration characterized by pain in the left thigh, which increased with physical activity, resulting in claudication; he also had malaise and fever. The X-ray films showed periostitis of the lower half of the left femur with bone marrow narrowing; the scintigraphy showed marked increased uptake in the middle and distal thirds of the left femur, and magnetic resonance imaging (MRI) showed thickening and hyperintensity of the cortex of the diaphysis and distal epiphysis of the femur and endosteal irregularity. Empirical treatment was started with vancomycin for 3 weeks. Femur biopsy and cultures were performed, isolating Salmonella spp. group “D” Vi (−); treatment with cotrimoxazole and ceftazidime for 4 weeks followed by doxycycline and cotrimoxazole for 4 months were given with a favorable functional outcome. This is an unusual case of a young adult with Garre’s sclerosing osteomyelitis associated to SLE and caused by salmonella. The literature is reviewed and the clinical conditions predisposing to this infection are discussed, particularly in patients with SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Garre’s sclerosing osteomyelitis also known as chronic non-suppurative sclerosing osteomyelitis is a specific type of osteomyelitis that particularly affects children and adolescents [1]. It has been reported in association with several chronic autoimmune diseases, including inflammatory bowel disease, granulomatosis with polyangiitis, psoriasis, and Takayasu arteritis [2]. It affects principally the mandible [3], but it can occur in any bone [2] and it generally originates from a low virulence infection [1].
Infectious complications that increase morbi-mortality in patients with systemic lupus erythematosus (SLE) are frequent and well-recognized. Goldblatt et al. reported 25.4 % of deaths due to infection [4]. SLE is a frequent underlying condition of bacteremia by salmonella [5]. Extra-intestinal focal infections caused by salmonella described in SLE are pneumonia, meningitis, urinary tract infections, pericarditis, skin abscesses, septic arthritis, and—as in our case—osteomyelitis [6].
Despite being associated with autoimmune diseases, Garre’s sclerosing osteomyelitis is a rare entity with only a few cases related to SLE published in the literature this far. A recent observation in our institution prompted this report.
Case report
We report the case of a 35-year-old male who early in 2010 and within the span of about a month was diagnosed with APS and SLE. Early in January, he presented with an acute pulmonary embolism (PE) with anti-cardiolipin and anti-beta-2 glycoprotein positivity and in February with joint (knee synovitis), serosal (pleural effusion), hematological (lymphopenia), and renal involvement (24-h proteinuria 1328 mg/dl, a kidney biopsy showing Class II lupus nephritis) with positive ANA (1:640—homogenous pattern), anti-dsDNA and anti-histone antibodies. He was put on warfarin and high dose prednisone with gradual tapering to 10 mg. In May 2010, hydroxychloroquine and methotrexate were added to the therapeutic regimen. Azathioprine 150 mg/day was started instead of methotrexate and later stopped in 2012 based on elevated serum transaminases; subsequently cyclosporine 50 mg/day was started, and gradually increased to 150 mg/day. In March 2013, the patient was receiving prednisone 10 mg/day, hydroxychloroquine 200 mg/day, methotrexate 12.5 mg/week, leflunomide 20 mg/day, and oral anticoagulation.
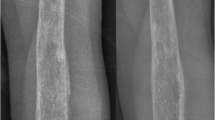
In March 2014, the patient presented to our emergency room with a 1-week history of claudication and severe pain in his left thigh exacerbated by palpation and physical activity, malaise, and fever (39 °C). White blood cell count (WBC) was 6000/mm3 (absolute neutrophil count (ANC) 4800, absolute lymphocyte count (ALC) 720 and absolute monocyte count (AMC) 72, C-reactive protein (CRP): 58.2 mg/L (Reference range, <1 mg/L), and erythrocyte sedimentation rate (ESR): 34 mm/h). An antero-posterior X-ray film of the left femur showed cortical thickening and periosteal reaction along the diaphysis (Fig. 1). Empirical initial therapy with vancomycin for three weeks was started on March 6th; the patient had a torpid hospital stay with persistent fever and pain in his left thigh, leucocytosis with neutrophilia and left shift. Scintigraphy revealed increased flow and tracer uptake—in the middle and distal third of the left thigh soft tissues; the delayed phase showed increased uptake in the same area. Additionally, a multi-slice helical computed tomography (CT) showed increased density of the intramedullary bone, endosteal reaction, and narrowing of the medullary cavity in the left femur (Fig. 2).
Subsequently a magnetic resonance imaging (MRI) of both thighs was performed and showed hypodensity on T1-weighted images from the distal femoral diaphysis to the epiphysis, significant multilayered periosteal reaction in the middle and distal third of the diaphyseal cortex, and irregular endosteal border with hyposignal (Fig. 3); the STIR images showed periosteal reaction and soft tissue edema (Fig. 4).
Three weeks after initiation of vancomycin, a surgical bone biopsy was performed and showed osteoblastic activity in the cortical tissue and mixed inflammatory infiltrate in the stroma; in the medullary tissue, changes suggestive of chronic and acute osteomyelitis were found with devitalized tissue and apposition of new bone.
Salmonella spp. Group D Vi negative was isolated from the medullary cavity. The patient was then treated with ceftazidime and cotrimoxazole for 4 weeks; subsequently doxycycline and cotrimoxazole were prescribed for four additional months with the patient experiencing a full recovery.
Discussion
Sclerosing osteomyelitis is a specific type of chronic osteomyelitis described by Carl Garre in 1893 [1] as a focal gross thickening of periosteum with peripheral reactive bone formation resulting from infection, henceforth Garre’s sclerosing osteomyelitis [7]. This entity is seen in children, adolescents, and young adults [8]. In young people, the persistence of considerable activity of osteoblastic cells in the periosteum is responsible for causing a condensation of cortical bone rather than an osteolytic process. It usually affects the mandible [1], but it has been reported in several bones like femur, tibia, humerus, and others [9].
Osteomyelitis in SLE is unusual; however, it should be noted that salmonella, an uncommon pathogen for osteomyelitis in normal hosts, has repeatedly been reported to be a causative agent in these patients [10]. In their report Lim et al. remarked that most salmonella infections occur during periods of active SLE (83 % SLEDAI ≥ 4), and that the mainly serogroups isolated were B and D [6]. Wu et al. reported coexistent underlying systemic disease, chronic renal disease, and intensive immunosuppressive therapy as predisposing factors for osteomyelitis [10]. Furthermore, Salmonella bacteremia is commonly associated with SLE [5]; for example, in a survey of patients with gram-negative bacteremia conducted in a New York hospital 30 were due to salmonella species, lupus patients accounted for 6 out of these 30 cases, but only for 13 out of 2388 non-salmonella gram-negative bacteremias, suggesting that SLE patients are at increased risk for salmonella sepsis [11].
In general, severe infections contribute to morbidity and mortality of SLE patients. Around 50 % of SLE patients will have one or more events during their lifetime [12] and will be responsible for approximately 25 % of all deaths [13]. For example, in the GLADEL cohort, the largest Latin-American lupus cohort, infections were the first cause of death [14]. Risk factors for infection are disease activity, associated comorbidities, the use glucocorticoids with doses over 7.5 mg/day [5], and genetic factors like complement deficiencies—lower concentrations of C3—[12], allelic variants of Fc gamma receptor—like Fc gamma RIIA-H131 that also increased the risk for lupus nephritis—[15], and amplified expression of colony-stimulating factor-1 (CSF-1) which also correlated with the onset and recurrence of lupus nephritis [16]. Additionally, the presence of the silent polymorphism at position 707 (707C > T) (rs1126616) in the case of osteopontin is associated with opportunistic infections [17] and the mannose-binding lectin 2 (MBL2) O/O genotype with an increased incidence of gram-positive infections [18]. Lymphopenia is also a strong predictor factor for infections in SLE [19].
In short, patients with SLE have a higher risk of infection than the general population and—in some studies, salmonella has been the most frequently isolated gram-negative organism recovered from their blood. Salmonella osteomyelitis is not unusual in SLE patients, but Garre’s sclerosing osteomyelitis certainly is. It is important to exclude infections in SLE patients, because they can masquerade as exacerbation of the disease and if they go undetected could contribute to significant morbidity and early mortality in these patients.
References
Suma R, Vinay C, Shashikanth MC, Subba Reddy VV (2007) Garre’s sclerosing osteomyelitis. J Indian Soc Pedod Prev Dent 25(Suppl):S30–S33
Franco-Jimenez S, Romero-Aguilar JF, Bervel-Clemente S, Martinez-Vaquez M, Alvarez-Benito N, Grande-Gutierrez P, Maldonado-Yanza RG (2013) Garre’s chronic sclerosing osteomyelitis with sacral involvement in a child. Rev Esp Cir Ortop Traumatol 57(2):145–149. doi:10.1016/j.recot.2012.11.003
de Moraes FB, Motta TM, Severin AA, de Alencar Faria D, de Oliveira Cesar F, de Souza Carneiro S (2014) Garre’s sclerosing osteomyelitis: case report. Rev Bras Ortop 49(4):401–404. doi:10.1016/j.rboe.2014.04.010
Goldblatt F, Chambers S, Rahman A, Isenberg DA (2009) Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus 18(8):682–689. doi:10.1177/0961203308101019
Danza A, Ruiz-Irastorza G (2013) Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus 22(12):1286–1294. doi:10.1177/0961203313493032
Lim E, Koh WH, Loh SF, Lam MS, Howe HS (2001) Non-thyphoidal salmonellosis in patients with systemic lupus erythematosus. A study of fifty patients and a review of the literature. Lupus 10(2):87–92
Schwartz S, Pham H (1981) Garre’s osteomyelitis: a case report. Pediatr Dent 3(3):283–286
Eswar N (2001) Garre’s osteomyelitis: a case report. J Indian Soc Pedod Prev Dent 19(4):157–159
Wood RE, Nortje CJ, Grotepass F, Schmidt S, Harris AM (1988) Periostitis ossificans versus Garre’s osteomyelitis. Part I. What did Garre really say? Oral Surg Oral Med Oral Pathol 65(6):773–777
Wu KC, Yao TC, Yeh KW, Huang JL (2004) Osteomyelitis in patients with systemic lupus erythematosus. J Rheumatol 31(7):1340–1343
Abramson S, Kramer SB, Radin A, Holzman R (1985) Salmonella bacteremia in systemic lupus erythematosus. Eight-year experience at a municipal hospital. Arthritis Rheum 28(1):75–79
Zandman-Goddard G, Shoenfeld Y (2003) SLE and infections. Clin Rev Allergy Immunol 25(1):29–40. doi:10.1385/CRIAI:25:1:29
Kamen DL (2009) How can we reduce the risk of serious infection for patients with systemic lupus erythematosus? Arthritis Res Ther 11(5):129
Pons-Estel BA, Catoggio LJ, Cardiel MH, Soriano ER, Gentiletti S, Villa AR, Abadi I, Caeiro F, Alvarellos A, Alarcon-Segovia D (2004) The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 83(1):1–17. doi:10.1097/01.md.0000104742.42401.e2
Salmon JE, Millard S, Schachter LA, Arnett FC, Ginzler EM, Gourley MF, Ramsey-Goldman R, Peterson MG, Kimberly RP (1996) Fc gamma RIIA alleles are heritable risk factors for lupus nephritis in African Americans. J Clin Invest 97(5):1348–1354. doi:10.1172/JCI118552
Menke J, Amann K, Cavagna L, Blettner M, Weinmann A, Schwarting A, Kelley VR (2015) Colony-stimulating factor-1: a potential biomarker for lupus nephritis. J Am Soc Nephrol 26(2):379–389. doi:10.1681/ASN.2013121356
Forton AC, Petri MA, Goldman D, Sullivan KE (2002) An osteopontin (SPP1) polymorphism is associated with systemic lupus erythematosus. Hum Mutat 19(4):459. doi:10.1002/humu.9025
Pradhan V, Surve P, Rajadhyaksha A, Rajendran V, Patwardhan M, Umare V, Ghosh K, Nadkarni A (2015) Mannose binding lectin (MBL) 2 gene polymorphism & its association with clinical manifestations in systemic lupus erythematosus (SLE) patients from western India. Indian J Med Res 141(2):199–204
Ng WL, Chu CM, Wu AK, Cheng VC, Yuen KY (2006) Lymphopenia at presentation is associated with increased risk of infections in patients with systemic lupus erythematosus. QJM 99(1):37–47. doi:10.1093/qjmed/hci155
Acknowledgments
We are grateful to Graciela S. Alarcón, MD, MPH for providing expert assistance in the review of this manuscript.
Contributorship statement
All authors were involved in drafting or revising this article critically for important intellectual content, and all authors approved the final version to be published.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Elera-Fitzcarrald, C., Alfaro-Lozano, J.L. & Pastor-Asurza, C.A. Garre’s sclerosing osteomyelitis caused by salmonella group D in a patient with systemic lupus erythematosus: an unusual complication. Clin Rheumatol 34, 2155–2158 (2015). https://doi.org/10.1007/s10067-015-3092-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3092-x