Abstract
Anti-neutrophil cytoplasmic antibodies (ANCA) play an important role in the pathogenesis of ANCA-associated vasculitis. The lack of ANCA antibodies may indicate a variation in clinical presentation and outcomes of this disease. We identified 74 adult patients between 1995 and 2009 with the diagnosis of pauci-immune glomerulonephritis. Demographics, histological features, and treatment outcomes were compared between ANCA-positive and ANCA-negative patients. These factors were correlated with renal function at presentation and follow-up. Of the 74 patients, 57 were ANCA-positive, and 17 were ANCA-negative. Demographics and mean Birmingham Vasculitis Activity Score were similar between ANCA-negative and ANCA-positive patients at presentation. Renal function was significantly worse at presentation in the ANCA-negative patients (eGFR 16.59 vs. 31.89 ml/min/1.73 m2, p = 0.03). Patients in the ANCA-negative group had a significantly higher interstitial fibrosis score compared to the ANCA-positive group (2.1 vs.1.6, p = 0.04). The median time to remission was shorter in the ANCA-negative patients (51 vs. 78 days, p = 0.01). Long-term renal function and 1-year patient and renal survival were similar between ANCA-negative and ANCA-positive patients. Baseline eGFR, percentage of normal glomeruli, glomerular sclerosis, and tubulointerstitial scarring predicted eGFR at 1 year in both groups similarly. This is the first historical review of American patients with pauci-immune glomerulonephritis, comparing patients with ANCA-negative and ANCA-positive serology. Although ANCA-negative patients present with lower eGFR and more interstitial fibrosis, 1-year and long-term outcomes in both groups are similar.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pauci-immune glomerulonephritis (GN), secondary to granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and renal limited vasculitis (RLV) account for 80 % of rapidly progressive glomerulonephritis and result in significant morbidity and mortality. Renal involvement is characterized by focal necrotizing and crescentic glomerulonephritis on light microscopy with minimal immunoglobulin staining on immunofluorescence [1–3].
The presence of anti-neutrophil cytoplasmic antibodies (ANCA) has been associated with small-vessel vasculitis. In addition to being a serologic marker, ANCA plays a pathogenic role in both in vitro and in vivo models [4]. However, 10–20 % of patients with pauci-immune GN do not have circulating ANCA. Given the small proportion of patients with ANCA-negative pauci-immune GN, few studies describe this group’s clinical and pathologic features.
Cohorts in the UK, China, Taiwan, and France have been studied, but data on American patients is limited [5–8]. The purpose of this study is to further characterize patients with ANCA-negative vasculitis and pauci-immune GN based on our experience at the Johns Hopkins Bayview Vasculitis Center and compare the clinical characteristics, histologic findings, and treatment outcomes to those with ANCA-positive vasculitis.
Material and methods
Study population
The study population included adult patients with biopsy-proven pauci-immune crescentic glomerulonephritis from 1995 to 2009. “Pauci-immune” was defined as the intensity of glomerular immunoglobulin staining by direct immunofluorescence assay in renal sections as negative to 1+, from a staining scale of 0 to 4+. All patients had ANCA serologies measured. Patients were classified as GPA or MPA per the Chapel Hill Consensus Conference definitions [9]. Patients were followed until ESRD, transplant, transfer to another facility, end of the study period, or death. The study protocol was approved by the Institutional Review Board and respected Human and Animal Rights.
Data acquisition
Clinical data
Data regarding patient demographics at the time of diagnosis, new versus established diagnosis, Birmingham Vasculitis Activity Score/Wegener’s granulomatosis (BVAS/WG) at the time of diagnosis calculated by review of the medical record at the time of presentation, time to remission, occurrence of disease relapse, need for renal replacement therapy at the time of diagnosis and at last follow-up, details of induction and maintenance immunosuppression, and major adverse events were collected [10]. Remission was defined as the first medical record entry where the patient had a BVAS/WG score of 0, without symptoms for the preceding 30 days. Relapse was defined by a BVAS/WG score greater than 1.
Laboratory data
Serum creatinine at the time of diagnosis, 6, 12, and 24 months were recorded. Renal function was measured using the CKD-EPI formula [11]. When available, 24-h quantification of proteinuria or urine protein to creatinine ratio was documented. Erythrocyte sedimentation rate (ESR, upper limit of normal 30 mm/h), C-reactive protein level, titers of c-ANCA, p-ANCA, anti-PR3 ELISA, and anti-MPO ELISA at the time of diagnosis were recorded. Results of testing for ANA, serum complements, and anti-GBM antibody when available were recorded. ANCA testing was done by standard indirect immunofluorescence assay on ethanol-fixed neutrophils. A 1:10 dilution is used by our immunology lab. Anti-PR3 and MPO testing was done by direct ELISA with commercially available kits. An ANCA titer of less than 10 was considered to be negative for both c-ANCA and p-ANCA. Anti- PR3 and MPO levels of less than 10 units were deemed negative. In 15 of 17 patients, the ANCA was confirmed to be persistently negative.
Histologic data
Paraffin-embedded biopsies were stained with hematoxylin and eosin (H&E), trichrome, silver, and Periodic acid-Schiff (PAS). Immunofluorescence (IF) and electron microscopy (EM) were performed on all patient samples included within the study. The classic direct IF technique using antibodies against IgA, IgG, IgM, C3, C1q, fibrin, albumin, and kappa and lambda light chains was employed. The staining intensity of each antibody was scored on a scale of 0 to 4+. If the staining intensity was less than 1+, the diagnosis of pauci-immune GN was confirmed. All biopsies were reviewed by one blinded pathologist, and renal lesions representative of disease activity and chronicity were scored using a standardized protocol for ANCA-associated systemic vasculitis as reported elsewhere [12]. Each glomerulus was scored separately for the presence or absence of fibrinoid necrosis, crescents (cellular-segmental, cellular-circumferential, or fibrous), and global sclerosis. The fraction of glomeruli revealing these lesions was expressed as the percentage of the total number of glomeruli in the biopsy. The percentage of normal glomeruli was scored as well. Interstitial and tubular lesions were scored semi-quantitatively based on the percentage of the tubulointerstitium that was affected such as: interstitial infiltrates (0 %; 1 to 20 %; 21 to 50 %; and >50 %), interstitial fibrosis (0 %; 1 to 50 %; and >50 %), and tubular atrophy (0 %; 1 to 50 %; and >50 %). Arteries and arterioles were evaluated for the presence of vasculitis, arterial hyalinosis, or sclerosis.
Statistical analyses
All groups were stratified based on ANCA positivity and, if positive, further defined by cytoplasmic versus peri-nuclear staining patterns. To compare differences between two groups, appropriate statistical tests were conducted considering the normality and distribution of data. For continuous variables, means and standard deviations were calculated, and a t test was conducted for normally distributed data. For data with a skewed distribution, median and interquartile ranges were calculated and a non-parametric test (two sample Wilcoxon rank-sum test or Mann-Whitney test) was conducted. For categorical variables, Pearson’s Chi-square test and Fisher’s exact test were conducted given the distribution of data in each cell. Linear regression analysis was conducted, and regression coefficients were assessed to ascertain the relationships between continuous outcome variables and continuous independent variables of interest. To measure the difference in relationship between continuous outcome and continuous independent variables of interest between the two ANCA groups, we regressed the outcome variable on the interaction between the ANCA group and the independent variable of interest. Also, the proportion of the variance in the dependent variable predicted from the independent variable was reported by the coefficient of determination (R 2). All data was analyzed using Stata SE vs 10.1 college station Texas.
Results
Seventy-four patients with biopsy-proven pauci-immune glomerulonephritis and clinical follow-up were included in this study. The demographics are listed in Tables 1 and 2. There were no significant differences in median age at presentation, gender, and race between groups. More ANCA-negative patients had a new diagnosis at the time of first kidney biopsy compared to ANCA-positive patients (100 vs. 68 %, p < 0.01).
All 74 patients underwent ANCA testing by both IF assay and antigen-specific ELISA. Seventeen were ANCA-negative, and 57 were ANCA-positive (26 c-ANCA and anti-PR3 positive, 31 p-ANCA and anti-MPO positive). Among the ANCA-positive patients, 22 patients were classified as MPA, and 35 were classified as GPA. Among the ANCA-negative patients, five had renal limited vasculitis (presumably MPA using a broader definition since this group is not specifically recognized by the CHCC definitions), nine had MPA, and three had GPA.
At the time of diagnosis, ANCA-negative patients had less paranasal involvement (12 vs. 39 %, p = 0.04) and lower CKD-EPI eGFR (16.59 vs. 31.89 ml/min/1.73 m2, p = 0.03) than ANCA-positive patients. There was no difference in the prevalence of pleurisy, nodules, or cavitary lesions or BVAS/WG score between groups. There was also no significant difference in the percentage of patients requiring dialysis at presentation between groups (Table 3).
Histologically, ANCA-negative patients had a greater degree of interstitial fibrosis than ANCA-positive patients (fibrosis score of 2.06 vs. 1.63, p = 0.04). There were otherwise no significant differences in the percentage of normal glomeruli, sclerosed glomeruli, cellular crescents, or interstitial inflammation.
With regard to treatment, 16 of the 17 ANCA-negative patients were treated with immunosuppressive therapy, and one patient did not receive any treatment for unclear reasons. Three patients were treated with steroids only, and 13 were treated with combination of cytotoxic therapy and steroids (10 with cyclophosphamide, 1 with mycophenolate mofetil, 1 with azathioprine, and 1 with rituximab). Among the 57 ANCA-positive patients, no patients were treated with steroids alone, and 55 patients were treated with a combination of steroids and cytotoxic therapy (45 with cyclophosphamide, 4 with mycophenolate mofetil, 3 with rituximab, 1 with methotrexate, 1 with cyclosporine, 1 with intravenous immunoglobulin). Two patients were not treated with immunosuppressive therapy, of whom one patient was dialysis-dependent at presentation and the other patient expired due to alveolar hemorrhage. There was a significant difference in the use of steroids alone (p = 0.01) and steroids with cytotoxic therapy (p = 0.02) between ANCA-negative and ANCA-positive patients.
Severe adverse events in the ANCA-negative patients included one CMV infection, 2 episodes of pneumonia, 1 episode of gross hematuria, and 3 leukopenic events compared to one loss of fertility, 1 episode of gross hematuria, 1 episode of pneumonia, and 13 episodes of leukopenia among the ANCA-positive patients. There were no differences in the incidence of severe adverse events between ANCA-negative and ANCA-positive patients.
Approximately 94 % of ANCA-negative and 96 % of ANCA-positive patients achieved remission. Median time to remission was significantly less in the ANCA-negative group vs. the ANCA-positive group (51 vs. 78 days, p = 0.01), but there were no differences in the number of relapses or time to first relapse between groups (Table 3).
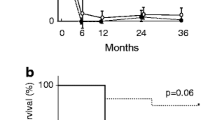
The mean CKD-EPI eGFR at 6, 12, and 24 months was similar between groups (Table 3). The CKD-EPI eGFR at 1 year correlated with baseline CKD-EPI eGFR (regression coefficient 0.88, R 2 = 0.32) and percentage normal glomeruli (regression coefficient 0.31, R 2 = 0.36) in both groups. However, the association between CKD-EPI eGFR at 1 year and baseline CKD-EPI eGFR was not significantly different between ANCA-negative and ANCA-positive groups (p = 0.77, Fig. 1). There was a borderline significant difference in association between CKD-EPI eGFR at 1 year and percentage of glomeruli when comparing ANCA-negative and ANCA-positive groups (p = 0.05, Fig. 2). Additionally, the degree of tubulointerstitial scarring was inversely associated with the CKD-EPI eGFR in both groups (regression coefficient −0.58, R 2 = 0.07).
Seven (41 %) of the ANCA-negative patients compared to 18 (32 %) of the ANCA-positive patients developed ESRD. Of these, two ANCA-negative patients and four ANCA-positive patients underwent renal transplant. Post transplant, none of the ANCA-negative patients relapsed, but two of the ANCA-positive patients experienced relapses (1 extra-renal and 1 with renal/extra-renal involvement) due to non-compliance with their anti-rejection medications. Two deaths occurred in the ANCA-negative group and ANCA-positive group, respectively, within the first year of diagnosis. One death was due to cardiac arrest in both ANCA-negative and ANCA-positive groups. The other death in the ANCA-positive group was attributed to active vasculitis.
Discussion
This is the first descriptive study of American patients with ANCA-negative and ANCA-positive pauci-immune crescentic glomerulonephritis where demographics, clinical and histological features at presentation, treatment response, and predictors of renal outcome were compared. Twenty-three percent of our patients with pauci-immune GN were ANCA-negative. ANCA-negative patients had less paranasal involvement, a lower eGFR, and a higher degree of interstitial fibrosis at presentation. Our study shows otherwise similar demographics, presentation, and renal outcomes in patients with ANCA-negative pauci-immune glomerulonephritis compared to patients with ANCA-positive pauci-immune glomerulonephritis. We also demonstrate that CKD-EPI eGFR at 1 year correlated with baseline CKD-EPI eGFR, percentage normal glomeruli, and had a strong graded negative correlation with percentage of tubulointerstitial scarring for both groups.
The proportion of ANCA-negative patients in our cohort is similar to what has been reported by Hedger et al., but it is less than what was seen in studies by Chen et al. and Hung et al. [5–7]. The mean age of ANCA-negative patients at diagnosis was also similar to patients described by Hedger et al., but older than patients studied in the two Asian cohorts. These differences may be related genetic or environmental factors.
Our patient population shared some clinical and serologic findings as those in prior studies. ANCA-negative patients had less sinus involvement compared to ANCA-positive patients, and this was also seen in studies by Hedger et al. and Chen et al. However, our cohort of ANCA-negative patients had more respiratory failure compared to ANCA-positive patients (but did not reach statistical significance) with similar rates of pleurisy, pulmonary nodules, and cavitary lesions between the two groups. This confirms that ANCA-negative disease is not renal limited, and ANCA negativity may be related to a delay in diagnosis leading to higher rates of respiratory failure at presentation.
Histologically, our findings were dissimilar from those reported by other studies. We found a higher percentage of normal glomeruli and lower percentage of cellular crescents in ANCA-negative patients compared to ANCA-negative patients described by Chen, Hedger, and Eisenberger et al. [5, 6, 8]. This difference may explain why a smaller proportion of ANCA-negative patients in our study required hemodialysis at diagnosis compared to their Chinese and European counterparts [5, 6]. The variation in histopathologic findings between studies may also reflect differing selection criteria for renal biopsy, potential for sampling error within a biopsy, and subjective judgment differences between pathologists describing biopsy findings.
Thirty percent of ANCA-positive patients had an established diagnosis prior to first renal biopsy, as compared to none of the ANCA-negative patients. This may be due to the more frequent sinus and ocular involvement seen in the c-ANCA-positive patient group and may explain the relative difficulty of establishing a diagnosis in patients with negative serology.
ANCA-negative patients received significantly different immunosuppressive therapies compared to ANCA-positive patients. We postulate that this may be related to a higher degree of tubulointerstitial scarring seen on initial biopsy suggesting greater disease chronicity compared to ANCA-positive patients. This may have resulted in less aggressive treatment with cytotoxic therapy. Despite differences in treatment, time to remission was shorter in the ANCA-negative patients, but the proportion of patients achieving remission in both groups was similar. These disparities may be explained by a different underlying pathophysiology in ANCA-negative disease and suggests that patients with ANCA-negative serologies may require a unique treatment strategy compared to ANCA-positive patients.
Previous studies in ANCA-positive vasculitis have shown that age, serum creatinine at presentation, percentage of normal glomeruli, and extent of glomerulosclerosis are predictive of renal function at 12 and 18 months [12–15]. In our cohort, CKD-EPI eGFR at 1 year correlated with baseline CKD-EPI eGFR in both groups. This is similar to the findings by Eisenberger et al. who showed a positive correlation between initial serum creatinine and renal outcome in ANCA-negative patients [8]. In this study, CKD-EPI eGFR at 1 year in the ANCA-negative patients correlated with percentage normal glomeruli.
Long-term renal outcome was best predicted by percentage of tubulointerstitial scarring, where more scarring correlated with worse renal outcomes. This data is novel for ANCA-negative patients, although well established for ANCA-positive patients and patients with other glomerulonephritides [12]. Other predictors, including baseline renal function, percentage glomerular sclerosis, and percentage normal glomeruli also showed a strong correlation with renal outcome at 1 year, but there were no differences between ANCA-negative and ANCA-positive patients in this regard, which was expected.
Rates of death and end-stage kidney disease were similar in all groups and to the same magnitude as other groups [5, 6]. Long-term renal survival is poor, and mortality rates are high irrespective of ANCA positivity.
Our study has limitations its historical nature and reliance on clinical notes for data collection. Additionally, ANCA testing was not standardized. Our study is limited by the small patient numbers; we lack the statistical power to make solid conclusions based on multivariate analysis. Given the rarity of this condition, multi-center collaboration and centralized electronic medical record systems are required to pool the numbers necessary to make concrete implications. Despite these limitations, our study is the first cohort of its size to describe outcomes of ANCA-negative vs. ANCA-positive vasculitis patients in the USA. Thus, our findings are likely more generalizable to patients in our country compared to other cohorts. We report predictive factors for long-term renal outcomes and remission data, novel for this disease.
It is unclear what the underlying etiologic autoimmune agent is in ANCA-negative vasculitis. Antiendothelial cell antibodies (AECA) have been detected in small-vessel vasculitis including GPA and MPA, and multiple mechanisms including activation of endothelial cells, increased leukocyte adhesion, and direct endothelial cell cytotoxicity have been proposed with regard to their pathogenicity [16, 17]. However, the correlation between the presence of AECA and disease activity has been inconsistent, and the specific targets of these antibodies are unknown [17–19]. Sebastian et al. examined the association between active GPA and the presence of AECA and found that titers were not highly prevalent among their cohort with GPA and did not correlate with degree of disease activity [18]. In contrast, Gobel et al. found that higher AECA titers were present in patients with active disease and cite other studies with similar findings [19]. They also found elevated AECA titers in patients with ANCA-negative GPA with active disease. Although AECA have been detected in GPA and MPA, it is possible that these antibodies may develop secondarily in the setting of vasculitis and may not have a substantive role in the underlying pathogenesis of either ANCA-negative or ANCA-positive vasculitis.
Antibodies targeting lysosomal membrane protein 2 (LAMP-2) cause pauci-immune glomerulonephritis in rats and activate neutrophils and endothelial cells in vitro [20]. Kain et al. detected LAMP-2 autoantibodies in most of their patients diagnosed with pauci-immune focal necrotizing glomerulonephritis. Interestingly, these autoantibodies cross react to a protein found on bacterial cell walls, suggesting that a preceding infection with resultant molecular mimicry may play a role in producing these potentially pathogenic antibodies. In a subsequent study, 104 patients with ANCA-negative serologies were tested for the presence of LAMP-2 autoantibodies, and 28.8 % had detectable levels above background, but titers were low and not statistically significantly different than what was seen in their control group [21]. Despite these conflicting reports, more recent evidence suggests a stronger correlation between LAMP-2 antibodies and ANCA-negative status in pauci-immune glomerulonephritis. The degree of glycosylation of the LAMP-2 epitope in the study by Roth et al. was higher than that used by Kain et al. and may have resulted in these disparate results [22]. Therefore, the role of LAMP-2 autoantibodies in the pathogenesis of pauci-immune glomerulonephritis requires further investigation.
ANCA detection may be masked in patients with circulating fragments of ceruloplasmin, rendering patients ANCA “negative.” One study found that purified immunoglobulins from ANCA-negative patients reacted with pathogenic MPO antigens, but their sera did not. A fragment of ceruloplasmin was found to bind to the pathogenic epitope of MPO which reduced anti-MPO autoantibody detection by 30–50 % [23]. This and other circulating proteins may be responsible for decreased recognition of ANCA.
Additional investigation is needed to delineate these disease processes. ANCA-negative vasculitis may represent a variant of small-vessel vasculitis, and this could explain the differences seen clinically and histopathologically at presentation. Further understanding of the pathogenesis of ANCA-negative vasculitis may aid in development of promising treatments that decrease renal morbidity and mortality.
Conclusion
In our historical review, ANCA-negative patients had less paranasal sinus involvement, lower estimated eGFR, and higher degrees of interstitial fibrosis at presentation compared to ANCA-positive patients. Despite these differences, both groups endured similar rates of ESRD at 1 year. Although therapies varied, responses were comparable. ANCA likely plays an important role in the pathogenesis of this disease. The ANCA-negative serology in this group may represent difficulties in detecting low levels of antibody with the currently available assays or might point to other antigen targets leading to alternate antibody-mediated injury that are yet to be elucidated.
References
Falk RJ, Jennette JC (1988) Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med 318(25):1651–1657
Booth AD, Almond MK, Burns A et al (2003) Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 41(4):776–784
Jennette JC, Falk RJ (1997) Small-vessel vasculitis. N Engl J Med 337(21):1512–1523
Jennette JC, Falk RJ, Gasim AH (2011) Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens 20(3):263–270
Hedger N, Stevens J, Drey N, Walker S, Roderick P (2000) Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in wessex, UK: a 10-year retrospective study. Nephrol Dial Transplant 15(10):1593–1599
Chen M, Yu F, Wang SX, Zou WZ, Zhao MH, Wang HY (2007) Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol 18(2):599–605
Hung PH, Chiu YL, Lin WC et al (2006) Poor renal outcome of antineutrophil cytoplasmic antibody negative pauci-immune glomerulonephritis in Taiwanese. J Formos Med Assoc 105(10):804–812
Eisenberger U, Fakhouri F, Vanhille P et al (2005) ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant 20(7):1392–1399
Jennette JC, Falk RJ, Andrassy K et al (1994) Nomenclature of systemic vasculitides. proposal of an international consensus conference. Arthritis Rheum 37(2):187–192
Stone JH, Hoffman GS, Merkel PA et al (2001) A disease-specific activity index for Wegener’s granulomatosis: modification of the birmingham vasculitis activity score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 44(4):912–920
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Bajema IM, Hagen EC, Hermans J et al (1999) Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 56(5):1751–1758
Hauer HA, Bajema IM, Van Houwelingen HC et al (2002) Determinants of outcome in ANCA-associated glomerulonephritis: a prospective clinico-histopathological analysis of 96 patients. Kidney Int 62(5):1732–1742
de Lind van Wijngaarden RA, Hauer HA, Wolterbeek R et al (2006) Clinical and histologic determinants of renal outcome in ANCA-associated vasculitis: a prospective analysis of 100 patients with severe renal involvement. J Am Soc Nephrol 17(8):2264–2274
Day CJ, Howie AJ, Nightingale P et al (2010) Prediction of ESRD in pauci-immune necrotizing glomerulonephritis: quantitative histomorphometric assessment and serum creatinine. Am J Kidney Dis 55(2):250–258
Carvalho D, Savage CO, Isenberg D, Pearson JD (1999) IgG anti-endothelial cell autoantibodies from patients with systemic lupus erythematosus or systemic vasculitis stimulate the release of two endothelial cell-derived mediators, which enhance adhesion molecule expression and leukocyte adhesion in an autocrine manner. Arthritis Rheum 42(4):631–640
Guilpain P, Mouthon L (2008) Antiendothelial cells autoantibodies in vasculitis-associated systemic diseases. Clin Rev Allergy Immunol 35(1–2):59–65
Sebastian JK, Mahr AD, Ahmed SS et al (2007) Antiendothelial cell antibodies in patients with Wegener’s granulomatosis: prevalence and correlation with disease activity and manifestations. J Rheumatol 34(5):1027–1031
Gobel U, Eichhorn J, Kettritz R et al (1996) Disease activity and autoantibodies to endothelial cells in patients with Wegener’s granulomatosis. Am J Kidney Dis 28(2):186–194
Kain R, Exner M, Brandes R et al (2008) Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 14(10):1088–1096
Roth AJ, Brown MC, Smith RN et al (2012) Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis. J Am Soc Nephrol 23(3):545–555
Kain R, Tadema H, McKinney EF et al (2012) High prevalence of autoantibodies to hLAMP-2 in anti-neutrophil cytoplasmic antibody-associated vasculitis. J Am Soc Nephrol 23(3):556–566
Roth AJ, Ooi JD, Hess JJ et al (2013) Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 123(4):1773–1783
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Duvuru Geetha, MD has served as a consultant for Genentech.
Shivani Shah, MD, John Havill, MD, and M. Hafizur Rahman, MD have no conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Shah, S., Havill, J., Rahman, M.H. et al. A historical study of American patients with anti-neutrophil cytoplasmic antibody negative pauci-immune glomerulonephritis. Clin Rheumatol 35, 953–960 (2016). https://doi.org/10.1007/s10067-015-3086-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3086-8