Abstract
The increasing trend of hyperuricemia in urban areas of China has been noted in the past decade. However, the prevalence of hyperuricemia in rural China has not been extensively investigated. We aimed to estimate the prevalence and risk factors of hyperuricemia and the associated comorbidities in rural Northeast China. This survey was conducted from July 2012 to August 2013. In this study, a total of 11,576 residents from the rural Northeast China were randomly selected and examined. Hyperuricemia was defined as serum uric acid ≥416 μmol/l in men and ≥357 μmol/l in women. Data regarding the demographic and lifestyle characteristics and the blood biochemical indexes of these participants were collected by well-trained personnel. The prevalence of hyperuricemia was 10.9 % and was more prevalent in men than in women (15.0 vs. 7.3 %, P < 0.001). Multivariate logistic regression models revealed that besides age, hyperuricemia in men was associated with ethnic minority [OR (95 %): 0.683 (0.472,0.989)], physical activity [moderate, OR (95 %): 0.716 (0.596,0.859); high, OR (95 %): 0.527 (0.354,0.786)], current smoking [OR(95 %):1.380 (1.179,1.616)], and current drinking [OR(95 %):0.705 (0.603,0.825)], while in women was only associated with ethnic minority [OR(95 %):0.485 (0.262,0.896)]. After adjusting for possible confounders, hyperuricemia was related to different subtypes of cardiometabolic comorbidities in both gender like abdominal obesity, general obesity, hypertriglyceridemia, hypertension, hypercholesterolemia, and low HDL-C. Besides, in women only, hyperuricemia was related to diabetes and high LDL-C. Hyperuricemia was common among residents living in rural Northeast China especially among men. Ethnic minority, physical activity, current smoking, and drinking contributed to hyperuricemia in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiologic studies show that hyperuricemia has become a common metabolic disorder worldwide in recent decades [1]. According to the National Health and Nutrition Examination Survey conducted in the USA, the prevalence of hyperuricemia during 2007–2008 was 21.1 % in men and 21.6 % in women [2]. This high incidence of hyperuricemia in developed countries may be related to the epidemic occurrence of overweight and obesity, as well as the Western diet rich in purine, alcohol, meat consumption, and soft drinks [3, 4]. With a more open policy and economic development in China, westernization has became common not only in urban but also in rural areas [5]. Dietary patterns play a key role in hyperuricemia. The prevalence of hyperuricemia is increasing in some cities in China, such as Shanghai (6.79 vs. 22.52 % for men, 3.65 vs. 15.32 % for women) [6, 7]. Moreover, the prevalence of hyperuricemia in urban areas of China like Guangdong (23.43 % for men and 11.77 % for women), Beijing (25.74 % for men and 10.52 % for women), and Hainan (27.30 % for men and 15.33 % for women) are very close to levels in developed countries [2, 8, 9].
Previous studies have demonstrated that an association exists between serum uric acid levels and hypertension, chronic kidney disease (CKD), heart failure, metabolic syndrome, obesity, and cardiovascular events [10]. In China, studies have investigated the possible relationship between hyperuricemia and other metabolic comorbidities [11, 12]. However, most of the studies were conducted in urban areas of China. As far as we know, few studies have focused on determining the prevalence and epidemiological features of hyperuricemia in rural areas of China. A nationwide population-based survey conducted in 2005 found that 57.01 % of Chinese residents lived in rural areas. Therefore, we performed the present study to estimate the prevalence of hyperuricemia and its risk factors in rural Northeast China and to evaluate the independent effects of hyperuricemia on different cardiac metabolic comorbidities in a large sample size of a rural population from 2012 to 2013.
Subjects and methods
Study population
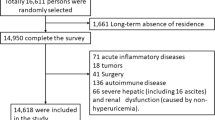
Liaoning Province is located in Northeast China. From January 2012 to August 2013, a representative sample of participants aged ≥35 years was selected to characterize the prevalence, incidence, and natural history of cardiovascular risk factors in rural areas of Liaoning Province. The study adopted a multi-stage, stratified, random-cluster sampling scheme. In the first stage, three counties (Dawa, Zhangwu, and Liaoyang County) were selected from the eastern, southern, and northern regions of Liaoning province. In the second stage, one town was randomly selected from each county (for a total of three towns). In the third stage, 8–10 rural villages from each town were randomly selected (for a total of 26 rural villages). Participants who were pregnant or had malignant tumors or mental disorders and had severe psychiatric disturbances, hepatic failure, or end-stage renal failure were excluded from the study. All the eligible permanent residents aged ≥35 years from each village were invited to attend the study (a total of 14,016 participants). Of those, 11,956 participants agreed and completed the study to give a response rate of 85.3 %. The study was approved by the Ethics Committee of China Medical University (Shenyang, China). All procedures were performed in accordance with ethical standards. Written consent was obtained from all participants after they had been informed of the objectives, benefits, medical items, and confidentiality agreement regarding their personal information. For participants who were illiterate, we obtained written informed consent from their proxies. In this report, we used only the data from participants who completed the study, which provided a final sample size of 11,576 (5359 men and 6217 women).
Data collection and measurements
Data were collected during a single visit to the clinic by cardiologists and trained nurses using a standard questionnaire in a face-to-face interview. Before the survey was performed, we invited all eligible investigators to attend an organized training session. The training included the purpose of this study, how to administer the questionnaire, the standard method of measurement, the importance of standardization, and the study procedures. A strict test was administered after this training, and only those who scored perfectly on the test were accepted as investigators in this study. During data collection, our inspectors had further instructions and support.
Data regarding the demographic characteristics, lifestyle risk factors, dietary habits, family income, and family history of chronic diseases were obtained during the interview using the standardized questionnaire. The study was guided by a central steering committee with a subcommittee for quality control. Educational level was assessed as completion of primary school or less, middle school, or high school and higher. Self-reported sleep duration (including nocturnal and nap duration) was obtained from the questionnaire. The responses were categorized into four groups: ≤7, 7–8, 8–9, and >9 h/day. Family income was classified as ≤5000, 5000–20,000, and >20,000 CNY/year.
According to American Heart Association protocol, blood pressure (BP) was measured three times at 2-min intervals after at least 5 min of rest using a standardized automatic electronic sphygmomanometer (HEM-907; Omron), which had been validated according to the British Hypertension Society protocol [13]. The participants were advised to avoid caffeinated beverages and exercise for at least 30 min before the measurement. During the measurement, the participants were seated with their arms supported at the level of the heart. The mean of three BP measurements was calculated and used in all analyses.
Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with the participants wearing light weight clothing and without shoes. Waist circumference (WC) was measured at the umbilicus using a non-elastic tape (to the nearest 0.1 cm), with the participants standing at the end of normal expiration. Body mass index (BMI) was calculated as the weight in kilograms divided by the square root of the height in meters.
Fasting blood samples were collected in the morning after at least 12 h of fasting. Blood samples were obtained from an antecubital vein into Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA). Fasting plasma glucose (FPG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), and other routine blood biochemical indexes were analyzed enzymatically using an autoanalyzer. All laboratory equipments were calibrated, and blinded duplicate samples were used for these analyses.
Definitions
Hyperuricemia was defined as serum uric acid ≥416 μmol/l in men and ≥357 μmol/l in women according to guidelines [14]. According to JNC-7 report [15], prehypertension was defined as not being on antihypertensive medication and having a systolic blood pressure (SBP) of 120–139 mmHg or diastolic blood pressure (DBP) of 80–89 mmHg. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg and/or use of antihypertensive medications. BMI were categorized into 3 groups as normal (BMI <25 kg/m2), overweight (25 ≤ BMI <30 kg/m2), and obesity (BMI ≥30 kg/m2), according to the World Health Organization (WHO) criteria [16]. Abdominal obesity was defined as WC ≥ 88 cm for females and WC ≥ 102 cm for males [17]. Dyslipidemia was defined according to the National Cholesterol Education Program-Third Adult Treatment Panel (ATP III) criteria [18]. High TC was defined as TC ≥ 6.21 mmol/L(240 mg/dL). Low HDL-C was defined as HDL-C < 1.03 mmol/L (40 mg/dL). High LDL-C was de fined as LDL-C ≥ 4.16 mmol/L (160 mg/dL). High TG was defined as ≥ 2.26 mmol/L (200 mg/dL). Diabetes mellitus was diagnosed according to the WHO criteria [19]: FPG ≥ 7 mmol/L (126 mg/dL) and/or being on treatment for diabetes.
Physical activity included occupational and leisure time physical activity. A detailed description of the methods for assessing physical activity has been presented elsewhere [20, 21]. Occupational and leisure time physical activity were merged and regrouped into the following three categories: (1) low—subjects who reported light levels of both occupational and leisure time physical activity; (2) moderate—subjects who reported moderate or high levels of either occupational or leisure-time physical activity; and (3) high—subjects who reported a moderate or high level of both occupational and leisure time physical activity.
Dietary patterns were assessed by having participants recall the foods they had eaten during the previous year. The questionnaire included questions regarding the average consumption of several food items per week. The reported consumption was quantified approximately in terms of grams per week. Vegetable consumption was assessed on the following scale: rarely = 3, <1000 g = 2, 1000–2000 g = 1, ≥2000 g = 0, and meat consumption, including red meat, fish, and poultry was assessed on the following scale: rarely = 0, <250 g = 1, 250–500 g = 2, and ≥500 g = 3. A special diet score (vegetable consumption score plus meat consumption score) was calculated for each participant (range 0–6). Higher values of the diet score indicated higher meat consumption, lower vegetable consumption, and greater adherence to a Westernized diet, while lower values indicate adherence to the Chinese diet. Similar methods for calculating a diet score can be found in the ATTICA study [22].
Statistical analysis
Descriptive statistics were calculated for all the variables, including continuous variables (reported as mean values and standard deviations) and categorical variables (reported as numbers and percentages). The differences between the hyperuricemia and non hyperuricemia groups were evaluated using the Student’s t test, ANOVA, non-parametric test, or the χ 2 test, as appropriate. Multivariate logistic regression analyses were used to identify independent factors of hyperuricemia, and odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) were calculated. All the statistical analyses were performed using SPSS version 17.0 software, and P values less than 0.05 were considered to be statistically significant.
Results
Baseline characteristics of study population
Table 1 provides the baseline characteristics of the study subjects. Current smoking and drinking were more common in men than in women. Age, weight, height, SBP, DBP, serum uric acid, serum creatinine, serum glucose, sleep duration, and dietary score levels were significantly higher for men than for women, with no significant differences in HDL cholesterol and triglycerides. Women compared to men had a lower educational level (primary school or below), were less physically active, and had a lower family income (≤5000 CNY/year), with no significant difference in ethnic composition.
Prevalence of hyperuricemia in different gender and age groups
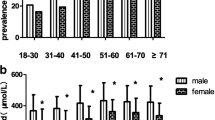
The overall prevalence of hyperuricemia was 10.9 % (data not shown) for subjects >35 years of age. The serum uric acid level was higher for men than for women (333.7 ± 83.5 vs. 255.8 ± 67.8 μmol/L, P < 0.001), and the prevalence of hyperuricemia was greater in men than in women (15.0 vs.7.3 %, P < 0.001). For men, the prevalence of hyperuricemia decreased with age (35–45 years: 18.2 %; 45–55 years: 15.3 %; 55–65 years: 12.7 %; >65 years: 14.5 %). For women, the prevalence of hyperuricemia increased with age (35–45 years: 4.0 %; 45–55 years: 5.5 %; 55–65 years: 9.4 %; >65 years: 12.6 %) (Fig. 1).
Factors associated with hyperuricemia
Table 2 shows the multivariable logistic regression analysis of risk factors related to hyperuricemia in men and women. Increasing age was associated with an increased risk of hyperuricemia in women, but a decreased risk of hyperuricemia in men. Being from an ethnic minority was associated with a lower risk of hyperuricemia compared to being ethnic Han in both genders (men: OR: 0.683, 95 %CI: 0.472 to 0.989; women: OR: 0.485, 95 %CI: 0.262 to 0.896). Current male smokers were less likely to have hyperuricemia but current female smokers were not, while current male drinkers had a higher possibility to developing hyperuricemia than did current female drinkers. Moderate or high physical activity was found to be a protective factor for hyperuricemia (for moderate OR: 0.716, 95 %CI: 0.596 to 0.859; for high OR: 0.527, 95 %CI: 0.354 to 0.786)
Hyperuricemia and cardiometabolic comorbidities
As shown in Fig. 2, the overall mean serum uric acid level was significantly higher for subjects with metabolic disorders like general obesity (319.01 ± 91.86 vs. 289.60 ± 83.87), high TC (307.08 ± 88.84 vs. 288.86 ± 83.74), high TG (332.05 ± 92.81 vs. 283.46 ± 80.62), low HDL-C (318.10 ± 90.98 vs. 287.98 ± 83.23), high LDL-C (301.56 ± 88.15 vs. 291.09 ± 84.56), impaired fasting glucose (IFG) (300.13 ± 91.26 vs. 290.93 ± 84.06), and hypertension (301.79 ± 87.78 vs. 281.52 ± 80.436), as was the prevalence of hyperuricemia. On multivariate analysis shown in Table 3, hyperuricemia was a risk factor for abdominal obesity, general obesity, hypertension, hypertriglyceridemia, diabetes, hypercholesterolemia, low HDL-C, and high LDL-C in both genders except for high LDL-C and diabetes in men (Fig. 3).
Discussion
The present study found that the prevalence of hyperuricemia in a rural Northeast Chinese population was 10.9 %. Men had a significantly higher incidence of hyperuricemia than women had (15.0 vs. 7.3 %, P < 0.001). In addition, men and women had different risk factors and different associated comorbidities. Hyperuricemia in men was associated with age, ethnicity, physical activity, and current smoking and drinking, while in women hyperuricemia was only related to age and ethnicity.
With a more open policy and economic development, dietary patterns have changed remarkably during the past decades. A previous study demonstrated that the traditional Chinese diet has become westernized. Grain consumption has been decreasing, and animal food, fried food, and fast food, which is full of purine, has been markedly increasing [22]. As a result, common health problems in China are now similar to those in Western countries, e.g., metabolic syndrome, obesity, and diabetes. The prevalence of these metabolic disorders was high in our study: abdominal obesity (3.9 % for men, 24.8 % for women), hypertension (53.9 % for men, 48.7 % for women), and low HDL-C (16.1 % for men, 18.4 % for women).
Moreover, the prevalence of hyperuricemia in urban areas of China has increased and is now very close to that in developed countries. Huang and colleagues reported that 39.18 % of men and 30.18 % of women suffer from hyperuricemia in Fujian province [23]. In addition, hyperuricemia is prevalent in many developed cities in China like Beijing (25.74 % for men, 10.52 % for women), Chongqing (57.14 % for men, 12.73 % for women), and Tibet (34.53 % for men, 13.70 % for women) [8, 24, 25]. However, most of the previous studies were carried out in urban areas of China. Few studies reported on the prevalence and epidemiological features of hyperuricemia because measuring serum uric acid was not included in the regular health examination in rural areas. A survey of a total population of 4683 rural adults in Java reported that the prevalence of hyperuricemia was 24.3 % [26]. Our study estimated that 10.5 % of rural residents had hyperuricemia, which was lower than that in the urban cities in China and rural areas outside of China. Residents who live in urban areas have quite a different life style than those of rural residents. They live a more sedentary life, with fewer physical activities, and a more westernized diet. The above may partially count for the lower prevalence of hyperuricemia in rural areas. Our findings are consistent with those of other studies that concluded that men have a higher possibility of hyperuricemia than women have [27]. This result is in line with the most recent study also conducted in Northern and Northeastern China with the same results of a higher prevalence of hyperuricemia in men than in women (men vs. women, 21 vs. 7.9 %; P < 0.0001) [28]. Previous studies have observed that women compared with men have a significantly lower serum uric concentration [8, 24, 25]. This could be due to the decreasing fractional excretion of urate by the kidneys and a substantial increase in body mass during male adolescence, contributing to an increase in uric acid levels. During early adolescence, girls weigh more than their male peers, resulting in slightly higher serum uric acid levels in girls. However, during mid-adolescence, boys tend to be heavier, with higher uric acid levels, compared to girls, which is partially associated with androgen levels that promote kidney uric acid reabsorption [29]. In addition, men in our study had a significantly higher rate of current drinking than did the women (45.4 vs. 2.9 %). Studies confirm that alcohol consumption increases the demands on purine elimination resulting in hyperuricemia [10].
Many risk factors for hyperuricemia have been described, e.g., age, gender, diet, and others. In our study, we found that men and women had different risk profiles. The hyperuricemia prevalence decreased with increasing age for men, while in women, older age seemed to result in a higher risk of hyperuricemia. This is consistent with a previous study also conducted in China [30]. The mechanism of the decreasing trend in hyperuricemia with increasing age in men is possibly due to the higher prevalence of current drinking and lack of physical activity among younger residents, while the increasing hyperuricemia prevalence with age for women may be related to menopause, causing changes in the endocrine system and estrogen levels [31]. In our study, we found that ethnic minorities had a relatively lower risk of hyperuricemia than did the Han, which was also demonstrated in other studies [32]. The relatively lower prevalence of hyperuricemia in ethnic minorities is possibly due to the low consumption of seafood and higher prevalence of current drinking [32]. Additionally, ethnic diversity may partially count for this discrepancy. Another interesting finding in our study is that current smoking was a protective factor for hyperuricemia in both genders but without statistical significance in women. This is consistent with the findings in many other studies [33]. It has been suggested that smoking might suppress serum uric acid levels via the action of the superoxides found in cigarette smoke [33]. Some studies have confirmed that an inverse association exists between smoking and abdominal obesity and general obesity, which have been proven to be risk factors for hyperuricemia [34]. We further investigated the association between current smoking and obesity and found that current smoking men had a lower prevalence of abdominal obesity (2.8 vs. 5.3 %) and general obesity (5.2 vs. 8.4 %) than noncurrent male smokers. However, this relationship did not exist among women. Our study also found that current drinking increased the risk of hyperuricemia in men. However, current drinking did not correlate with the prevalence of hyperuricemia in women in our study because only 2.9 % of the women were current drinkers. Previous epidemiological studies based mainly on cross-sectional observations or interventions have confirmed a causal relationship between alcohol consumption and hyperuricemia [35]. Furthermore, one 6-year prospective study in Japan also confirmed that habitual alcohol intake significantly contributed to the development of hyperuricemia, regardless of type of alcoholic beverage consumed [36]. We estimated that there was an inverse association between hyperuricemia and physical activity in our study. Many other studies have reached the same conclusion [37]. Some studies have demonstrated that physical activity might decrease serum uric acid excretion and accelerate purine degradation, while others have claimed that insulin sensitivity mediated the relationship between physical activity and serum uric acid [37, 38].
In the past decade, several studies have confirmed that serum uric acid is related to traditional cardiovascular risk factors, including diabetes, metabolic syndrome, hypertension, and dyslipidemia [39]. The effect of hyperuricemia on adverse cardiometabolic profiles varied in many epidemiologic studies of different ethnic groups. Our study found that hyperuricemia was a risk factor for hypertriglyceridemia, general obesity, abdominal obesity, hypertension, diabetes, hypercholesterolemia, high LDL-C, and low HDL-C. The relationship between hyperuricemia and cardiovascular diseases like hypertension, dyslipidemia, and obesity has been demonstrated by previous epidemiologic studies [40, 41]. On the one hand, a recent meta-analysis of 8 prospective cohort studies provided strong evidence that a high level of serum uric acid was independent of other risk factors, especially metabolic syndrome, for developing type 2 diabetes in middle and older aged people [42]. On the other hand, Wang SF and colleagues reported that hyperuricemia was associated with increased risk of hypertension in both males [OR(95 %CI):1.680(1.110–2.543)] and females [OR(95 %CI):1.065 (1.012–1.118)] [43]. Moreover, studies in cell culture and animal models have explored possible mechanisms that might explain the relationship. The possible mechanisms have been studied extensively, e.g., renin-angiotensin-aldosterone-dependent arteriolopathy, inhibition of neuronal nitric oxide synthase, endothelial dysfunction leading to reduced insulin-stimulated nitric oxide-induced vasodilatation, and inflammatory and oxidative changes [39, 44–46].
The present study has several limitations. First, our study was a cross-sectional study, which restricted the interpretation of the observed associations in terms of cause and effect. Longitudinal studies are required for further investigation of these findings. Second, the prevalence of hyperuricemia was based on a single assessment of blood samples, which may introduce error. In addition, although the researchers had been trained according to a standardized measurement protocol, measurements at a single visit might lead to incorrect values for the anthropometric indexes. Finally, in our study, we did not enroll residents <35 years of age. Finally, in our study, we did not evaluate insulin resistance which was in a core component in the pathophysiology of the metabolic syndrome.
In summary, our population-based study reports the latest prevalence of hyperuricemia in rural Northeast China. The prevalence of hyperuricemia among men was twice that of women. Men and women exhibited different risk profiles and had different adverse comorbidities. With economic development and the population aging, hyperuricemia will lead to increased health-care costs in rural China. It is urgent for the Chinese government to pay more attention to finding ways to halt or reduce the burden of hyperuricemia.
References
Wallace KL, Riedel AA, Joseph-Ridge N, Wortmann R (2004) Increasing prevalence of gout and hyperuricemia over 10 years among older adults in a managed care population. J Rheumatol 31:1582–1587
Zheng ML, Lai YH, He XN, Tan FW (2008) Correlation of detection of hyperuricemia with hypertension in healthy population in Guangzhou City. China Trop Med 8:512–514
Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G (2004) Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med 350:1093–1103
Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G (2004) Alcohol intake and risk of incident gout in men: a prospective study. Lancet 363:1277–1281
Batis C, Sotres-Alvarez D, Gordon-Larsen P, Mendez MA, Adair L, Popkin B (2014) Longitudinal analysis of dietary patterns in Chinese adults from 1991 to 2009. Br J Nutr 111:1441–1451
Du S, Chen S-L, Wang Y, Xu LQ (1998) The epidemiological of hyperuricemia and gout in a community population of Huangpu district in Shanghai. Chin J Rheumatol 2:75–78
Tian XC, Jiang Z-CH, Bao G-CH, Gao WG, Nan HR, Wang SH-J (2008) Analysis on prevalence and influence factors of hyperuricemia among residents in Qingdao. Chin J Pubic Health 24:360–362
Han XH, Yao XY, Fang X-SH (2008) The prevalence of hyperuricemia and abnormal of lipid and glucose in medical examination population. Shanxi Med J 37:438–439
Cao YJ, Liu Y, Li T, Yan BS, Wang ZH-ZH, Yang XL (2010) Investigation of the increasing of policemen in blood uric acid and triglyceride in Haikou. Hainan Med J 21:120–121
Gustafsson D, Unwin R (2013) The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol 14:164
Lu X, Li X, Zhao Y, Zheng Z, Guan S, Chan P (2014) Contemporary epidemiology of gout and hyperuricemia in community elderly in Beijing. Int J Rheum Dis 17:400–407
Villegas R, Xiang YB, Cai Q, Fazio S, Linton M, Li H (2010) Prevalence and determinants of hyperuricemia in middle-aged, urban Chinese men. Metab Syndr Relat Disord 8:263–270
O'Brien E, Petrie J, Littler W, de Swiet M, Padfield PL, O'Malley K (1990) The British hypertension society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J Hypertens 8:607–619
Feig DI, Johnson RJ (2003) Hyperuricemia in childhood primary hypertension. Hypertension 42:247–252
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr (2003) The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289:2560–2572
Hypertension control. Report of a WHO Expert Committee (1996) World Health Organ Tech Rep 862:1–83
WHO/FAO (2003) Expert consultation on diet, nutrition and the prevention of chronic diseases, report of the joint WHO/FAO expert consultation. World Health Organization, Geneva
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285:2486–2497
World H, Wang CN, Liang Z, Wei P, Liu P, Yu JX, Zhang DM (2002) Changes in dietary patterns and certain nutrition-related diseases in urban and rural residents of Jiangsu Province, China, during the 1990s. Biomed Environ Sci 15:271–276
Health Organization and International Diabetes Fedaration (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. World Health Organization, Geneva
Hu G, Tuomilehto J, Silventoinen K, Barengo N, Jousilahti P (2004) Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J 25:2212–2219
Panagiotakos DB, Pitsavos C, Chrysohoou C, Risvas G, Kontogianni MD, Zampelas A (2004) Epidemiology of overweight and obesity in a Greek adult population: the ATTICA study. Obes Res 12:1914–1920
Huang HB, Jin HW, Chen ZH-SH (2009) The analysis of hyperuricemia about medical examination population in Xiamen. Henan J Prev Med 20:345–347
Chen YQ (2008) The prevalence of hyperuricemia and related factors which is from urban population in Chongqing. J Third Mil Med Univ 30:1013–1014
Yang C, Liu G-ZH, Liu YX, Feng GJ (2009) Hyperuricemia in Tibetans undergoing health examination in Linzhi District in Tiebet. Mil Med J South China 23:10–12
Darmawan J, Valkenburg HA, Muirden KD, Wigley RD (1992) The epidemiology of gout and hyperuricemia in a rural population of Java. J Rheumatol 19:1595–1599
Lohsoonthorn V, Dhanamun B, Williams MA (2006) Prevalence of hyperuricemia and its relationship with metabolic syndrome in Thai adults receiving annual health exams. Arch Med Res 37:883–889
Qiu L, Cheng XQ, Wu J, Liu JT, Xu T, Ding HT, Liu YH, Ge ZM, Wang YJ, Han HJ, Liu J, Zhu GJ (2013) Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health 13:664
Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY (2000) Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 24:485–491
Liu L, Lou S, Xu K, Meng Z, Zhang Q, Song K (2013) Relationship between lifestyle choices and hyperuricemia in Chinese men and women. Clin Rheumatol 32:233–239
Antón FM, García Puig J, Ramos T, González P, Ordás J (1986) Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism 35:343–348
You L, Liu A, Wuyun G, Wu H, Wang P (2014) Prevalence of hyperuricemia and the relationship between serum uric acid and metabolic syndrome in the Asian Mongolian area. J Atheroscler Thromb 21:355–365
Munan L, Kelly A, PetitClerc C (1976) Population serum urate levels and their correlates. The Sherbrooke regional study. Am J Epidemiol 103:369–382
Janghorbani M, Amini M, Willett WC, Mehdi Gouya M, Delavari A, Alikhani S (2007) First nationwide survey of prevalence of overweight, underweight, and abdominal obesity in Iranian adults. Obesity 15:2797–2808
Qu YL, Yu L, Li ZM, Kong LH, Xiao TC, Li M (2013) Epidemiological status and risk factors of hyperuricemia in rural area of the Three Gorges. Zhonghua Xin Xue Guan Bing Za Zhi 4:427–431, Chinese
Nakamura K, Sakurai M, Miura K, Morikawa Y, Yoshita K, Ishizaki M (2012) Alcohol intake and the risk of hyperuricaemia: a 6-year prospective study in Japanese men. Nutr Metab Cardiovasc Dis 22:989–996
Wannamethee SG, Shaper AG, Alberti KG (2000) Physical activity, metabolic factors, and the incidence of coronary heart disease and type 2 diabetes. Arch Intern Med 160:2108–2116
Ka T, Yamamoto T, Moriwaki Y, Kaya M, Tsujita J, Takahashi S (2003) Effect of exercise and beer on the plasma concentration and urinary excretion of purine bases. J Rheumatol 30:1036–1042
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821
Chelyabinsk M, Ambroszkiewicz J, Gajewska J, Laskowska-Klita T, Leibschang J (2007) The effect of tobacco smoking during pregnancy on concentration of uric acid in matchedmaternal cord pairs. Przegl Lek 64:667–670
Kosugi T, Nakagawa T, Kamath D, Johnson RJ (2009) Uric acid and hypertension: an age-related relationship? J Hum Hypertens 23:75–76
Lv Q, Meng XF, He FF, Chen S, Su H, Xiong J (2013) High serum uric acid and increased risk of type 2 diabetes: a systemic review and meta-analysis of prospective cohort studies. PLoS One 8:e56864
Wang SF, Shu L, Wang S, Wang XQ, Mu M, Hu CQ (2014) Gender difference in the association of hyperuricemia with hypertension in a middle-aged Chinese population. Blood Press 6:1–6
Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL (2001) Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38:1101–1106
Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q (2002) Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Ren Physiol 282:F991–F997
Kanellis J, Kang DH (2005) Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol 25:39–42
Acknowledgments
This study was supported by grants from the "Twelfth Five-Year" project funds (National Science and Technology Support Program of China, Grant # 2012BAJ18B02) and the Social Development Research Program of Liaoning Province (2013225089).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, S., Yang, H., Guo, X. et al. Prevalence of hyperuricemia and its correlates in rural Northeast Chinese population: from lifestyle risk factors to metabolic comorbidities. Clin Rheumatol 35, 1207–1215 (2016). https://doi.org/10.1007/s10067-015-3051-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3051-6