Abstract
SLE is a systemic autoimmune disease with high prevalence of hypertension. Around 40–75 % of SLE patients develop nephritis, a major cause of hypertension and mortality. Angiotensin-converting enzyme (ACE) maintains the blood pressure and blood volume homeostasis. An insertion/deletion (I/D) polymorphism in intron 16 of ACE gene was reported to influence the development of hypertension, nephritis, and cardiovascular diseases in different ethnic populations. Despite compelling evidence for the high prevalence of hypertension in individuals with SLE, underlying factors for its development are not well studied. With this background, we analyzed the influence of ACE insertion/deletion polymorphism on susceptibility to SLE, development of nephritis and hypertension, other clinical features and autoantibody phenotype in South Indian SLE patients. Three hundred patients with SLE and 460 age and sex similar ethnicity matched individuals were included as patients and healthy controls, respectively. The ACE gene insertion/deletion polymorphism was analyzed by PCR. Insertion (I) and deletion (D) alleles were observed to be equally distributed among patients (57 and 43 %) and controls (59 and 41 %), respectively. The mutant (D) allele did not confer significant risk for SLE (II vs. ID: p = 0.4, OR 1.15, 95 % CI 0.8–1.6; II vs. DD: p = 0.34, OR 1.22, 95 % CI 0.8–1.85). There was no association of the ACE genotype or the allele with development of lupus nephritis (II vs. ID: p = 0.19, OR 1.41, 95 % CI 0.84–2.36; II vs. DD: p = 0.41, OR 0.74, 95 % CI 0.38–1.41) or hypertension (II vs. ID: p = 0.85, OR 0.9, 95 % CI 0.43–1.8; II vs. DD: p = 0.66, OR 1.217, 95 % CI 0.5–2.8). The presence of mutant allele (D) was not found to influence any clinical features or autoantibody phenotype. The insertion/deletion polymorphism of the ACE gene is not a genetic risk factor for SLE and does not influence development of hypertension or lupus nephritis in South Indian Tamils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SLE is a systemic autoimmune disorder characterized by the presence of autoantibodies against nuclear antigens. Twin studies revealed the importance of genetic factors in the pathogenesis of SLE [1]. About one in six SLE patients manifests the signs of kidney involvement at the time of diagnosis, and 40–75 % of patients develop nephritis during the course of their illness [2].
Hypertension is common in SLE but its origin has been obscure. Though renal glomerular damage and renal vascular endothelial dysfunction are likely contributors, hypertension occurs also in those without renal involvement. To explain this, other possible mechanisms have been evoked. The renin-angiotensin-aldosterone system (RAAS) regulates the blood pressure. Angiotensin-converting enzyme (ACE), a key component of the RAAS, plays a crucial role in blood pressure and blood volume homeostasis. The gene encoding ACE spans a 21-kb region encompassing 25 introns and 26 exons on chromosome 17 (17q23) [3]. Cambien et al. reported that the ACE biosynthesis and the circulatory ACE levels were genetically determined [4]. Later, Rigat et al. confirmed that a 287-bp insertion/deletion polymorphism in the intron 16 of ACE gene affects the circulatory levels of ACE. Individuals harboring the homozygous deletion (DD) mutation were reported to have twice the circulatory levels of ACE than those harboring heterozygous (ID) and homozygous insertion (II) genotypes [5]. Many studies across different ethnics have shown that the ACE insertion/deletion polymorphism may influence the susceptibility to hypertension, atherosclerosis, coronary heart disease, stroke, diabetic nephropathy, and Alzheimer’s disease [6]. In addition to the lifestyle activities, genetic factors were also reported to be associated with the development of hypertension and a prevalence rate of 74 % was reported in SLE patients [7]. Hence, this study was carried out to analyze the role of ACE insertion/deletion polymorphism with development of hypertension and nephritis in SLE patients.
Materials and methods
Three hundred patients fulfilling the modified 1997 American College of Rheumatology (ACR) criteria for SLE [8] under treatment and follow-up at the Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India, and 460 age, sex, and ethnicity-matched subjects were enrolled for this study after informed written consent. The institutional ethics committee approved the study protocol.
SLE disease activity index (SLEDAI) was used to assess and grade the disease activity [9]. SLE patients with active urinary sediment and 24-h urine protein excretion of more than 500 mg were subjected to renal biopsy. Based on histopathology, lupus nephritis was graded according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 classification criteria [10]. The seventh report of the Joint National Committee (JNC7) on prevention, detection, evaluation, and treatment of high blood pressure criteria were followed to diagnose and grade the hypertension in patients [11]. In addition, the development of cardiovascular, renal, and hemodynamic dysfunction during the course of therapy was monitored and recorded.
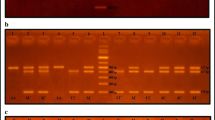
The genomic DNA from whole venous blood samples (5 ml) collected from the study subjects was extracted by salting out procedure. The procedure involves selective lysis of RBCs. The isolated WBCs were subjected to chemical, enzymatic, and osmotic lysis using sodium dodecyl sulphate, proteinase K, and sodium chloride, respectively. From the WBC lysate, phenol and chloroform were used to segregate the DNA into the aqueous phase which then was precipitated in absolute ethanol. The precipitated DNA was then dissolved in 0.1 M Tris-EDTA buffer and checked for its quality and concentration [12]. The DNA was diluted to contain 50 ng/μl and used for genotyping. The ACE insertion/deletion polymorphism was analyzed by an established PCR-based procedure [5]. The antinuclear autoantibody (ANA) status was qualitatively determined using a commercial immunoblot kit (Euroimmun, Marburg, Germany), and the levels of anti-dsDNA, antiphospholipid autoantibodies were measured using commercially available ELISA kits (Biosystems SA, Barcelona, Spain), following the manufacturer’s instructions. The complement levels were measured at the onset and during active phase of the disease by a nephelometry-based procedure (Dade Behring, Erlangen, Germany).
The allele and genotype frequencies were determined by direct counting. The difference between the observed and expected frequencies was evaluated by a χ 2 method to check if the Hardy-Weinberg equilibrium was respected. Data analysis was performed using SPSS, V.17. Odds ratio and confidence intervals were calculated by multiple logistic regression analysis. Kruskal-Wallis test was used to compare the mean C3 and C4 levels within the case group. A p value <0.05 was considered significant.
Results
All the study subjects enrolled in this study belonged to the South Indian Tamil ethnic population. Of the 300 SLE patients included in the study, 279 (93 %) were females and 21 (7 %) were males with a male to female ratio of 1:13. The control group (n = 460) consisted of 420 (91 %) females and 40 (9 %) males with a male:female ratio of 1:11. The mean age of the cases and controls were 27.9 ± 9.6 years and 33.1 ± 12.7 years, respectively. The mean age of SLE onset was 25.5 ± 8.4 years. The average SLEDAI score at the time of enrolment in the study was 14.61 ± 7.92, suggesting an active disease.
Mucocutaneous lesions (82 %) and arthritis (70 %) were the most common clinical features observed followed by hematological manifestations (49 %) in the form of lymphopenia (30 %), thrombocytopenia (23 %), autoimmune hemolytic anemia (20 %), and neutropenia (7 %). A total of 131 (44 %) patients developed lupus nephritis. Their mean 24-h urine protein excretion was 1076 ± 84.5 mg/dl, and the mean renal SLEDAI was 7.9 ± 3.72. The studies of renal histopathology revealed class IV nephritis (DPGN) in 93 (71 %), class II nephritis (MPGN) in 20 (15 %), class III nephritis (FPGN) in 12 (9 %), and class V nephritis (MN) in 6 (5 %) patients. The clinical and autoantibody profiles of the patients are given in Table 1.
Analysis of the ACE gene insertion/deletion polymorphism revealed that both alleles were present at comparable frequencies in patients (57 % I vs. 43 % D) and controls (59 % I vs. 41 % D) and were in Hardy-Weinberg equilibrium and did not confer significant risk to develop SLE (II vs. ID p = 0.4, OR 1.15, 95 % CI 0.8–1.6; II vs. DD p = 0.34, OR 1.22, 95 % CI 0.8–1.85), (II vs. ID + DD p = 0.31, OR 1.17, 95 % CI 0.9–1.6) (Table 2). Further, no genetic influence of ACE insertion/deletion polymorphism on clinical manifestations, autoantibody status, overall disease activity, 24-h urine protein excretion, and serum complement levels were noted (Supplementary Tables 1, 2 and Table 3).
During the course of the study, 47 patients (16 %) developed persistent hypertension with a mean systolic and diastolic pressure of 148.7 ± 1.5 and 97.6 ± 0.6 mmHg respectively. Of these, 36 (77 %) had deranged renal functions. Their renal histopathology revealed class II nephritis in 3, class III in 4, class IV in 28, and class V in 1 patient. Here again, no association between ACE polymorphism and development of persistent hypertension in SLE (Table 4) or progression towards more severe histopathological classes of nephritis was observed (Supplementary Table 3).
Discussion
Lupus nephritis is a fulminant form of the disease causing irreversible renal damage. It is associated with poor prognosis and may warrant renal transplantation at later stages [13]. We observed a high frequency of lupus nephritis in our patients (44 %) much higher than that reported for European Caucasians (10 %) but lesser than that observed in Afro-Caribbean SLE patients (58 %) [13, 14]. An earlier study reported similar frequency in Indian SLE patients [15].
Several studies have reported a higher incidence of hypertension in female SLE patients. In an age-matched women without SLE, the prevalence of hypertension was 2.7 % in the age group 20–34 years and 14 % in the age group between 35 and 44 years. Though the contribution of lupus nephritis to hypertension in SLE is evident, the relationship is not that straightforward, as it alone could not explain all the cases [7].
ACE, a member of RAAS system, is a key enzyme associated with hemodynamic and electrolyte homeostasis. Alterations in this system have been consistently implicated in the pathogenesis of essential hypertension and development of renal complications [16]. We hypothesized that the genetic variations in ACE gene may also contribute to hypertension in south Indian Tamil SLE patients who exhibit particularly high incidence of hypertension. Our study, as other studies involving SLE patients from Brazil [17] and Israel [18], failed to reveal any role of ACE insertion/deletion polymorphism in SLE-associated hypertension, albeit the frequency of deletion allele (D) was uniformly higher in the above-mentioned populations including ours and African Americans (range 50–55 %) [14] as compared to Caucasians (35–40 %) [13].
Our data are in disagreement with what had been previously reported in the literature. Sato et al. reported genetic association of ACE insertion allele (I) with disease activity, development of class IV lupus nephritis, high antidsDNA titer, and reduced CH50 activity [9, 17]. They also pointed out that the ACE deletion allele conferred protection against lupus nephritis by delaying its development. Contrary to the above report, Sprovieri et al. showed that in Brazilian SLE patients with nephritis, the deletion allele was associated with progression to CRF [17]. Even more discrepant results were reported by Prkacin et al. from Croatia who found that the ACE insertion allele was associated with lower proteinuria and creatinine levels and thereby conferred protection against development of lupus nephritis. They also associated the ACE insertion allele with faster disease remission as compared to ACE DD genotype [19]. Further Akai et al. have reported that the ACE gene insertion/deletion polymorphism influenced the renal disease activity in Japanese SLE patients [20]. In support to the above reports, Zhou et al. in their meta-analysis found that the polymorphic ACE gene deletion allele was a genetic risk factor for SLE and development of lupus nephritis [21]. However, our findings are in agreement with the reports by Topete-Reyes et al., who suggested that the ACE insertion/deletion polymorphism neither influenced the incidence of lupus nephritis nor the different histopathological classes of lupus nephritis in Mexican mestizos [22].
Among other related complications, Pullman et al. reported significant association between the ACE deletion allele and higher incidence of vascular damage. They suggested that the prospective screening of the ACE insertion/deletion polymorphism might help to predict vascular damage in SLE [23]. Here again, we did not observe any association between the ACE insertion/deletion polymorphism and development of vascular lesions such as vasculitis or Raynaud’s phenomenon in our South Indian Tamil SLE patients.
The first study on the role of ACE polymorphism on the autoantibody status was reported by Uhm et al. They reported that the ACE insertion allele influenced the production of antiSm autoantibody, again on disagreement with our present data [24]. Overall, the significant difference between our present results and those of others concerning the contribution of ACE locus to various features of SLE could be due to differences in population genetics or due to environmental factors. But the striking observation is with the results of a study in a population ethnically identical to our study population, where the ACE homozygous deletion genotype (DD) was reported to confer male gender-specific risk to develop essential hypertension in a non-SLE context. Indeed, this observation encouraged us to explore this aspect in the context of SLE [3]. The absence of such association observed in our study may be attributable to the predominance of female patients in our SLE cohort. However, we cannot rule out the role of ACE gene polymorphism in SLE given the limitation of our study in that we have not explored other functionally relevant polymorphisms in this gene.
Conclusion
The insertion/deletion polymorphism at intron 16 of the ACE gene seems not to be a genetic risk factor for development of hypertension or lupus nephritis in South Indian Tamil SLE patients.
Abbreviations
- ACE:
-
Angiotensin-converting enzyme
- SLE:
-
Systemic lupus erythematosus
- ACR:
-
American College of Rheumatology
- LN:
-
Lupus nephritis
- SEM:
-
Standard error of mean
- SD:
-
Standard deviation
References
Wakeland EK, Liu K, Graham RR, Behrens TW (2001) Delineating the genetic basis of SLE. Immunity 15:397–408
Canetta PA, Bomback AS, Radhakrishnan J (2011) Treating lupus in the kidney: where are we now, and where are we going? Discov Med 12:341–349
Ramu P, Umamaheswaran G, Shewade DG, Swaminathan RP, Dutta TK, Balachander J, Adithan C (2011) Candidate gene polymorphisms of renin angiotensin system and essential hypertension in south Indian Tamilian population. Int J Hum Genet 11:31–40
Cambien F, Alhenc-Gelas F, Herbeth B, Andre JL, Rakotovao R, Gonzales MF, Allegrini J, Bloch C (1988) Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy Study. Am J Hum Genet 43:774–780
Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F (1990) An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 86:1343–1346
Tabatabaei FAS, Oostra BA, Isaacs AM, Van Duijn CM, Witteman JCM (2006) ACE polymorphisms. Circ Res 98:1123–1133
Ryan MJ (2009) The pathophysiology of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol 296:R1258–R1267
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 35:630–640
Markowitz GS, D’Agati VD (2007) The ISN/RPS 2003 classification of lupus nephritis: an assessment at 3 years. Kidney Int 71:491–495
Chobanian AV, George LB, Henry RB et al (2003) The Seventh Report of the Joint National Committee on report prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 289:2560–2571
Miller SA, Dykes DD, Polesky HF (1988) Simple salting out procedure for extracting DNA from human nucleated cells. Nuc Acids Res 16:1215
Patel M, Clarke AM, Bruce IN, Symmons DP (2006) The prevalence and incidence of biopsy-proven lupus nephritis in the UK: evidence of an ethnic gradient. Arthritis Rheum 54:2963–2969
Tassiulas IO, Aksentijevich I, Salmon JE, Kim Y, Yarboro CH, Vaughan EM et al (1998) Angiotensin I converting enzyme gene polymorphisms in systemic lupus erythematosus: decreased prevalence of DD genotype in African American patients. Clin Nephrol 50:8–13
Malaviya AN, Chandrasekaran AN, Kumar A, Shamar PN (1997) Systemic lupus erythematosus in India. Lupus 6:690–700
Remuzzi G, Perico N, Macia M, And Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int 2005;68 9 (Suppl 99): pp. S57–S6
Sprovieri SR, Sens YA (2005) Polymorphisms of the renin-angiotensin system genes in Brazilian patients with lupus nephropathy. Lupus 14:356–362
Molad Y, Gal E, Magal N, Sulkes J et al (2000) Renal outcome and vascular morbidity in systemic lupus erythematosus (SLE): lack of association with the angiotensin-converting enzyme gene polymorphism. Semin Arthritis Rheum 30:132–137
Prkacin I, Novak B, Sertić J, Mrzljak A (2001) Angiotensin-converting enzyme gene polymorphism in patients with systemic lupus. Acta Med Croatica 55:73–76
Akai Y, Sato H, Iwano M, Kurumatani N, Kurioka H, Kubo A et al (1999) Association of an insertion polymorphism of angiotensin-converting enzyme gene with the activity of lupus nephritis. Clin Nephrol 51:141–146
Zhou TB, Liu YG, Lin N, Qin YH, Huang K, Shao MB, Peng DD (2012) Relationship between angiotensin-converting enzyme insertion/deletion gene polymorphism and systemic lupus erythematosus/lupus nephritis: a systematic review and metaanalysis. J Rheumatol 39:686–693
Topete-Reyes JF, SotoVargas J, Morán Moguel MC, Dávalos-Rodríguez (2013) Insertion/deletion polymorphism of the angiotensin-converting enzyme gene in lupus nephritis among Mexicans. Immunopharmacol Immunotoxicol 35:174–180
Pullmann RJ, Lukac J, Skerenova M, Rovensky J, Hybenova J, Melus V et al (1999) Association between systemic lupus erythematosus and insertion/deletion polymorphism of the angiotensin converting enzyme (ACE) gene. Clin Exp Rheumatol 17:593–596
Uhm WS, Lee HS, Chung YH, Kim TH et al (2002) Angiotensin-converting enzyme gene polymorphism and vascular manifestations in Korean patients with SLE. Lupus 11:227–233
Acknowledgments
This research was funded by the Department of Science and Technology, Govt. of India, New Delhi (Grant No. SR/SO/HS-67/2004 dated 03.08.2007).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 33 kb)
Rights and permissions
About this article
Cite this article
Negi, V.S., Devaraju, P. & Gulati, R. Angiotensin-converting enzyme (ACE) gene insertion/deletion polymorphism is not a risk factor for hypertension in SLE nephritis. Clin Rheumatol 34, 1545–1549 (2015). https://doi.org/10.1007/s10067-015-2954-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-2954-6