Abstract
We analyzed the longitudinal changes in gray-scale ultrasonography (GSUS) and power Doppler ultrasonography (PDUS) parameters and correlated them with clinical, functional, and radiologic outcomes in patients with newly diagnosed rheumatoid arthritis (RA). GSUS and PDUS examinations, 44-joint disease activity score (DAS44) calculations, measurements of erythrocyte sedimentation rate, and C-reactive protein levels were performed in 68 RA patients at baseline and after 1, 3, 6, 9, and 12 months. Metacarpophalangeal joints, wrist, elbow, knee, ankle, metatarsophalangeal joints, and wrist and ankle tendons were examined by GSUS and PDUS. The laboratory and clinical findings began to decrease significantly at 1 month (P < 0.05). Improvement of the ultrasonography (US) variables began at 3 months. After 6 months, all of the joint synovitis scores, except those of the knee, elbow, and ankle joints, showed a statistically significant reduction compared to baseline scores (P < 0.001). DAS44 scores were lower in the very early RA group at 12 months compared to those whose symptom duration was greater than 3 months of RA (respectively, 1.53 ± 0.34; 1.80 ± 0.38; z = −2501, P = 0.012). The total modified Sharp scores at 12 months correlated with total PDUS synovitis scores at 12 months (r = 0.354, P = 0.003). Regression of US synovitis at large joints such as the knee, elbow, and ankle tended to be delayed compared to that at small joints. PD synovitis that is persistent despite disease-modifying anti-rheumatic drug therapy may cause radiographic bone erosions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessment of disease activity in rheumatoid arthritis (RA) is important for monitoring treatment efficiency and predicting the disease outcome. Thus, a thorough clinical examination and sensitive imaging methods are required to monitor the disease process. Clinical remission is a realistic therapeutic goal in patients with RA [1].
In particular, gray-scale ultrasonography (GSUS) to detect synovitis and power Doppler (PD) signals at multiple joint levels are valid disease activity markers in RA that are modified after effective therapy [2]. PD serves as a useful adjunct to gray-scale imaging, in that it is more sensitive for the detection of early disease, and can more accurately differentiate between chronic and acute disease in the thickened synovium [3]. There are few studies on the predictive value of longitudinal ultrasonography (US) joint assessment in the areas of disease activity, functional status, and ultrasonographic progression in early RA [4, 5]. We analyzed the longitudinal changes in GSUS and PD ultrasonography (PDUS) parameters to correlate them with the clinical, functional, and radiologic outcomes in patients with newly diagnosed RA, who had started treatment with disease-modifying anti-rheumatic drugs (DMARDs) and low-dose corticosteroids.
Methods
This prospective study included 72 newly diagnosed RA patients according to the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) RA 2010 classification criteria between January 2012 and September 2013. Patients were recruited from the outpatient rheumatology clinics at the same center and consecutively included the ACR/EULAR 2010 RA classification criteria feature evaluation of (a) joint involvement, (b) rheumatoid factor (RF) and anti-citrullinated protein antibody (anti-cyclic citrullinated peptide (CCP)) levels, (c) acute phase reactant levels, and (d) duration of symptoms [6]. The study protocol was approved by our local ethics committee, and informed consent was obtained from all patients. Patients with cancer, those with any hematological abnormality, those who were pregnant or in the recent post-partum period (6 months), and those who had previously taken low-dose corticosteroids or synthetic DMARDs were excluded.
Gender, age, DMARD type, body mass index (BMI), the time elapsed from when the first clinical symptoms developed, the number of tender and swollen joints, and the patient’s assessment of disease activity (0–10 scale) were noted. Initially, patients received synthetic DMARDs or corticosteroids (<10 mg/day). If the clinical response (improvement of the 44-joint disease activity score [DAS44] <0.6) was inadequate at 3 months, another synthetic or biologic DMARD was added to the therapy. Disease activity was assessed by calculating the DAS44 value for each patient at each visit [7].
DAS44 assessment includes the following joints: sternoclavicular, acromioclavicular, shoulder, elbow, wrist, metacarpophalangeal (MCP), proximal interphalangeal (PIP), knee, ankle, and metatarsophalangeal (MTP). A DAS44 value <1.6 indicates remission, between 1.6 and 2.4 indicates low disease activity, between 2.4 and 3.7 indicates moderate disease activity, and >3.7 indicates high disease activity [7]. To evaluate quality of life, we used the Rheumatoid Arthritis Quality of Life Questionnaire (RAQoL) (consisting of 30 questions) [8].
Laboratory assessment included measurement of erythrocyte sedimentation rate (ESR) and assay of the levels of C-reactive protein (CRP), rheumatoid factor (RF), and anti-cyclic citrullinated peptide (anti-CCP) antibody. The ESR was measured immediately after blood collection using a Greiner SRS 20/II instrument (Vacuette Greiner, Austria). RF and CRP levels were measured by nephelometric methods, using an IMAGE 800 analyzer (Beckman Coulter Inc., USA). Anti-CCP antibodies were measured via enzyme-linked immunosorbent assay (ELISA), and a result was considered positive if the level was above a cutoff of five arbitrary units (as suggested by Abbott ARCHITECT i1000SR).
US
Hand, wrist, elbow, knee, ankle, and toe joints were evaluated via GSUS and PDUS. Fourteen joint regions (thus, 28 in both extremities) were evaluated. These included the first through to the fifth MCP joints, the radiocarpal, ulnocarpal, and intercarpal compartments of the wrist; the humeroradial and humeroulnar compartments of the elbow; the suprapatellar and medial and lateral parapatellar recesses of the knee; the tibiotalar, talonavicular, and posterior subtalar compartments of the ankle; and the first through to the fifth MTP joints. The MCP and MTP joints were scanned at palmar/plantar and dorsal sites; wrist joints were scanned at dorsal sites. All of the patients were examined by a trained ultrasonographer with 3 years of experience (US1), after which examinations were repeated by another trained ultrasonographer with 1 year of experience (US2); both clinicians had a rheumatology background. US examinations were repeated at each visit (baseline, 1, 3, 6, 9, and 12 months). The settings for the GSUS and PDUS were the same for all patients. US examinations were completed in 30 min, and all of the images were stored. A US platform featuring a 5–13-MHz linear array transducer was employed to this end (LOGIQ P5; General Electric, New York, NY).
Synovitis was classified on gray-scale images using a semiquantitative scoring method. We considered only synovial proliferation as a sign of synovitis (not synovial effusion). The approach features use of a 0–3 scale, in which 0 corresponds to no synovitis, 1 to mild synovitis, 2 to moderate synovitis, and 3 to severe synovitis [9]. Grade 1 synovitis may occur in normal populations, and for this reason, patients of grades 2 and 3 (only) were considered to have abnormal synovitis [9]. Semiquantitative scoring method was evaluated for each of 28 joints, and total synovial scores were calculated by summing grade 2 and 3 synovitis. The range of total GSUS synovitis score was 0–84. Also, GSUS synovitis score was calculated for each joint. Mean value of each joint was calculated by using the formula.
The maximal area of augmentation on PDUS was recorded using a previously described semiquantitative technique featuring use of a 0–3 scale, in which 0 corresponds to normal/minimal vascularity, 1 to mild hyperemia (single vessel signal), 2 to moderate hyperemia (confluent vessels), and 3 to marked hyperemia (vessel signals in >50 % of the joint area) [10]. Semiquantitative scoring method was evaluated for each of 28 joints, and total synovial scores were calculated by summing each semiquantitative grade. The range of total PDUS synovitis score was 0–84. Also, PDUS synovitis score was calculated for each joint. Mean value of each joint was calculated by using the formula.
The PD signal was used to differentiate patients with active synovitis and tenosynovitis from those with persistent synovitis and tenosynovitis (Figs. 1 and 2).
Tenosynovitis was recorded in the extensor digitorum carpi, the extensor carpi ulnaris, in each of the five flexor digitorum tendons of the hand, the tibialis posterior, and the peroneus tendons of the ankle (thus a total of 18 tendons in both extremities). A four-grade semiquantitative scoring system (i.e., grade 0, normal; grade 1, minimal; grade 2, moderate; grade 3, severe) was used to score tenosynovitis revealed on GSUS [11]. Semiquantitative scoring method was evaluated for each of 18 tendon regions, and total tenosynovitis scores were calculated by summing each semiquantitative grade. The range of total GSUS tenosynovitis score was 0–54. Also, GSUS tenosynovitis score was calculated for each tendon. Mean value of each tendon was calculated by using the formula.
A four-grade semiquantitative scoring system (i.e., grade 0, no Doppler signal; grade 1, minimal signal; grade 2, moderate signal; grade 3, severe signal) was used to score pathological peritendinous PD signals within the synovial sheath [11]. Semiquantitative scoring method was evaluated for each of 18 tendon regions, and total PD tenosynovitis scores were calculated by summing each semiquantitative grade. The range of total PDUS tenosynovitis score was 0–54. Also, PDUS tenosynovitis score was calculated for each tendon. Mean value of each tendon was calculated by using the formula.
Radiographic assessment
Posteroanterior radiographs of the patients’ wrists and anteroposterior radiographs of the patients’ feet were obtained at baseline and at the 12-month follow-up. The radiographs were read by an independent radiologist, who was blinded regarding the clinical, laboratory, and PDUS findings. Radiologic damage was assessed according to the van der Heijde-modified Sharp method [12]. This method measures erosions (score range 0–280) and joint space narrowing (score range 0–168) in 44 different joints and provides a sum score ranging from 0 to 448.
Statistical analysis
The SPSS software (IBM SPSS statistics version 20.0) was used for statistical analysis. Quantitative variables (clinical, laboratory, and US parameters) are given as means with SDs and ranges. Upon univariate analysis, the Mann-Whitney U test was used to compare continuous variables and the chi-square test to compare categorical variables. Spearman’s correlation coefficients between radiographic and sonographic findings were calculated. To compare clinical and ultrasonographic parameters with radiographic progression, changes in each parameter during the study were evaluated by calculating time-integrated values throughout the year using the area under the curve (AUC) method [27]. A P value less than 0.05 was considered to indicate statistical significance. The course of the clinical, laboratory, and US variables was obtained by calculating time-integrated values using the AUC method. Unweighted kappa statistics were used to calculate interobserver agreements for diagnosis of synovitis, tenosynovitis, PD synovitis, and PD tenosynovitis. Interobserver agreement was performed for all patients in baseline visit and at the end of follow-up.
Results
Baseline clinical and laboratory findings
We included 72 newly diagnosed RA patients aged 18–78 years. Available data from four patients with incomplete follow-up were excluded from the study. The principal demographic and clinical features are summarized in Table 1. In total, 78 % were RF-positive and 85 % were anti-CCP-positive. The average ESR and CRP values for all of the patients were 42.47 mm/h and 30.98 mg/dL at baseline, respectively (ESR 0–20 mm/h, CRP 0–5 mg/dL, normal range).
Of a total of 1904 examined joint regions, 13.2 % (n = 253) of all examined joints had tender joints and 10.5 % (n = 201) had swollen joints. On clinical examination, tenderness was predominantly observed in the wrist, MCP2, and ankle joints (25.2, 17.5, and 10.5 %, respectively); swelling was predominantly observed in the wrist, MCP2, and ankle joints (21.5, 16.5, and 9.5 %, respectively).
At inclusion, all patients were administered methotrexate (10–15 mg/week) and low-dose prednisone or methylprednisolone. After the 12-month follow-up, 43 (63.3 %) patients were taking one DMARD and 21 (30.9 %) were taking two or more synthetic DMARDs. Three patients (4.4 %) had started therapy with adalimumab, and one (1.4 %) had started therapy with golimumab. All of the patients who started tumor necrosis factor (TNF) blocker therapy were established RA. One patient had pulmonary involvement of RA at 1-year follow-up.
According to the DAS44 score, 3.0 % (n = 2) of patients exhibited low-level disease activity, 76.4 % (n = 52) exhibited moderate disease activity, and 20.6 % (n = 14) exhibited high-level disease activity at baseline. According to the DAS44 score, 48.5 % (n = 33) of patients were in remission (DAS44 < 1.6), 48.5 % (n = 33) exhibited low-level disease activity, and 3.0 % (n = 2) exhibited moderate disease activity at the 12-month follow-up.
Baseline ultrasonography findings
A total of 1904 joints were examined by US in all of the RA patients. Of these joints, GSUS synovitis was detected in 17.0 % (n = 324) of joints and PDUS synovitis was detected in 13.7 % (n = 261) of joints. GSUS synovitis was predominantly observed in the wrist, MCP2, and ankle joints (31.12, 21.8, and 12.5 %, respectively); PDUS synovitis was predominantly observed in the wrist, MCP2, and ankle joints (26.7, 18.5, and 10.7 %, respectively). A total of 1224 tendons from all of the RA patients were examined by US. Of these tendons, GSUS tenosynovitis was detected in 8.4 % (n = 138) of tendons and PDUS tenosynovitis was detected in 6.8 % (n = 111) of tendons. GSUS tenosynovitis was observed in the extensor carpi ulnaris, second flexor digitorum, and tibialis posterior tendon (29.4, 18.3, and 9.5 %, respectively); PDUS tenosynovitis was predominantly observed in the extensor carpi ulnaris, second flexor digitorum, and tibialis posterior tendon (25.7, 15.4, and 9.5 %, respectively).
Baseline radiographic findings
Baseline and 12-month radiographs were available for 54 patients. The total mean (±standard deviation [SD]) modified Sharp score was 15.03 ± 4.31 at the study entry. The erosion score mean ± SD was from 8.54 ± 2.34 at the study entry. The joint space narrowing (JSN) score mean ± SD was 7. 03 ± 3.45 at the study entry.
Laboratory, ultrasonographic, and radiographic course
The mean ESR and CRP values decreased significantly from baseline to 1 month and from 1 to 3 months. Laboratory values tended to be stable at other visits. The VAS and DAS44 values decreased significantly throughout the follow-up period.
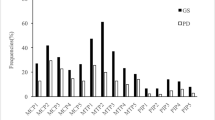
Improvement of US variables began at 3 months. The course of clinical, laboratory, and US variables throughout the study is shown in Table 2. Wrist GSUS synovitis and PDUS synovitis, as well as wrist GSUS tenosynovitis and PDUS tenosynovitis scores, showed a statistically significant decrease from baseline to 3 months. At the 6-month follow-up, all of the joint synovitis scores, except those of the knee, elbow, and ankle joints, showed a statistically significant reduction compared to those at baseline. Finally, the ankle, knee, and elbow synovitis scores were decreased at the 9-month follow-up (Fig. 3a–e).
Changes in the mean values of all joints at GSUS and PDUS throughout the follow-up period in 68 rheumatoid arthritis patients. a Wrist, MCP, and MTP joints at GSUS. b Wrist, MCP, and MTP joints at PDUS. c Knee, ankle, and elbow joints at GSUS. d Knee, ankle, and elbow joints at PDUS. e Extensor carpi ulnaris, extensor digitorum carpi, flexor digitorum, tibialis posterior, and peroneus tendons at gray-scale ultrasonography. *p < 0.05 versus the baseline, by pairwise comparison; **p < 0.01 versus the baseline, by pairwise comparison; ***p < 0.001 versus the baseline, by pairwise comparison. PDUS power Doppler ultrasonography, GSUS gray-scale ultrasonography, MCP metacarpophalangeal, MTP metatarsophalangeal
The total mean ± SD modified Sharp scores increased from 15.03 ± 4.31 at the study entry to 23.31 ± 5.39 at 12 months. The erosion score mean ± SD increased from 8.54 ± 2.34 at the study entry to 16.54 ± 4.32 at 12 months. The JSN score mean ± SD increased from 7.03 ± 3.45 at the study entry to 9.35 ± 3.19 at 12 months.
Correlation between radiography or ultrasonography and clinical variables
Patients whose symptom duration was less than 3 months were classified as having very early RA. DAS44 scores were lower in the very early RA group at 12 months than in those whose symptom duration was longer than 3 months (1.53 ± 0.34 and 1.80 ± 0.38, respectively; z = −2.501, P = 0.012).
The correlations between the time-integrated values of the clinical and US parameters and the outcome variables are displayed in Table 3. The time-integrated values of total GSUS synovitis scores and total PDUS synovitis score demonstrated stronger significant correlations with the progressions in radiographic erosion score, JSN scores, and total radiographic scores. There was not a significant correlation between the time-integrated values of total tenosynovitis and total PD tenosynovitis scores and the progressions in radiographic scores. The time-integrated values of the US parameters demonstrated a significant correlation with the DAS44 after 1 year.
Interobserver reliability of ultrasonography
The extent of interobserver agreement was 87, 91, 77, and 92 % for the presence/absence of GSUS synovitis, PDUS synovitis, GSUS tenosynovitis, and PDUS tenosynovitis with κ values of 0.70, 0.72, 0.64, and 0.78, respectively.
Discussion
We found a significant improvement in the clinical and laboratory indices earlier than GSUS and PDUS parameters throughout the present study. We showed that the improvement of large-joint US synovitis such as that of the knee, elbow, and ankle tended to be delayed compared to small-joint US synovitis. This issue was the main focus of our study. Radiographic worsening was observed in patients with late diagnosis with a persistent PD signal. Therefore, the treatment regimen should be planned for the prevention of persistent PD synovitis.
The primary objective of our study was to analyze the longitudinal changes for rigorous US examination. Although we found many longitudinal studies in the literature that evaluated the joint inflammatory activity of the wrist and finger joints in patients with RA by GSUS and PDUS, studies on large joints such as the knee, elbow, and ankle are scarce. Most of the follow-up studies were mainly performed on the wrist and finger joints of RA patients treated with TNF blockers. DAS28 scores were used to evaluate disease activity in those studies. In one of those studies, improvement of PD signals was observed from 2 to 6 weeks after TNF blocker treatment had begun at the finger joints [13].
Iagnocco et al. [14] also found significant improvement of US synovitis in the finger, wrist, and knee joints 3 months after the start of TNF blocker treatment. This study did not evaluate the 1-month follow-up. Naredo et al. [15] suggested that improvement of GSUS and PDUS synovitis started 1 month after TNF blocker treatment. However, this study did not include ultrasonographic assessment of each joint follow-up, and the patients included in the study had long-standing RA. Hartung et al. [16] only evaluated the ultrasonographic assessment of large joints such as the elbow, hip, knee, and shoulder at the 1-year follow-up and concluded that improvement of GSUS and PDUS synovitis scores at large joints began in the third month. The patients received synthetic DMARDs and/or biologic DMARDs in the present study and had established RA; the results were not compared to joints such as MTP and ankle joints. Backhaus et al. [17] studied the effect of various therapies on ultrasonographic erosions. The patients, switched from one biologic to another, exhibited a significant decline in erosions after 12 months, while the erosion scores in the other groups were stable. The present study included the wrist, MCP 2 and 3, PIP 2 and 3, and MTP 2 and 5 joints. A limitation of this study was that large joints were not considered for ultrasonographic examination.
In the current study, patients were newly diagnosed with RA, and involvement of large joints showed a delayed ultrasonographic response to treatment as observed at the early stage of the disease. Synthetic DMARD and low-dose corticosteroid therapy were administered to the patients as first-line treatment, followed by TNF blocker treatment according to the patients’ clinical and laboratory response. We applied TNF blocker treatment in only four patients. It took 3 months to receive an ultrasonographic response from the patients with synthetic DMARD and low-dose corticosteroid therapy. On the other hand, regression of large-joint US synovitis appeared at the ninth month. Considering the design of our study, we believe that our data are more amenable regarding daily practice and will provide guidance to rheumatologists who perform ultrasonographic follow-up of patients. We suggest that US synovitis of large joints is delayed because suppression of inflammation is more difficult in these joints and more aggressive immunosuppression is needed. Kanazawa et al. [18] proposed that the prevalence of large-joint involvement might be higher since they are subjected to higher mechanical loading than smaller joints and are, therefore, more susceptible to inflammation. In the current study, a decline in inflammatory markers was observed starting during the first month. During the follow-up, laboratory markers were stable after the third month. Consistent with our results, other studies found an improvement of laboratory findings 1 month after therapy [15, 16].
In the present study, we demonstrated that high baseline PD scores were not closely associated with radiographic worsening; however, persistence of the PD signal led to radiographic deterioration. The current results are in agreement with those of previous studies [19, 20]. In addition, lower radiographic damage scores in very early RA cases are indicators of the importance of early diagnosis and treatment. Ultrasonographic follow-up of tenosynovitis distinguishes the present work from those of previous studies. The results of this study showed that tenosynovitis of the wrist and ankle joints responds to standard DMARD and low-dose corticosteroid therapy within 3 months. One of the advantages of our study is its convenience for daily practice, application of DMARD therapy right after initial RA diagnosis, and the clinical and ultrasonographic follow-up. The follow-up of frequently involved large joints, as well as those of small joints, is an additional advantage. Moreover, the third advantage is that our study cohort was composed of newly diagnosed, previously untreated RA patients. Our study had some limitations. Particularly, we cannot explain all of our observations, possibly because although interobserver reliability was good to excellent, patients were evaluated by different clinicians at different times and intrareader reliabilities for PDUS synovitis and GSUS synovitis scoring could not be calculated. The prevalence of PDUS findings was relatively low compared to that in other studies. This may be caused by the relatively high pulse repetition frequency (1.2 kHz). Also, the machine, which is middle-size ultrasound equipment, may not be performing well for PDUS examination.
In conclusion, our data encourage clinicians to use US to determine synovitis in newly diagnosed RA patients at the follow-up, as well as during clinical and laboratory evaluations. Considering the US findings, persistent synovitis with a PD signal despite DMARD therapy may cause radiographic bone erosions. US findings are supplementary to standard clinical evaluation in multiple joint assessment. However, clinicians must be careful concerning the persistence of large-joint US synovitis.
References
Montecucco C (2006) Remission, a therapeutic goal in inflammatory arthropathies? Clinical data from adalimumab studies. Drugs 66:1783–1795
Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A (2005) Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann Rheum Dis 64:375–381
Brown AK (2009) Using ultrasonography to facilitate best practice in diagnosis and management of RA. Nat Rev Rheumatol 5(12):698–706
Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P (2007) Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum 57:116–124
Backhaus M, Ohrndorf S, Kellner H, Strunk J, Backhaus TM, Hartung W, Sattler H, Albrecht K, Kaufmann J, Becker K, Sörensen H, Meier L, Burmester GR, Schmidt WA (2009) Evaluation of a novel 7 joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum 61(9):1194–1201
Neogi T, Aletaha D, Silman AJ, Naden RL, Felson DT, Aggarwal R, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Khanna D, Kvien TK, Laing T, Liao K, Mease P, Ménard HA, Moreland LW, Nair R, Pincus T, Ringold S, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G, American College of Rheumatology, European League Against Rheumatism (2010) The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: phase 2 methodological report. Arthritis Rheum 62:2582–2591
Prevoo ML, van Gestel AM, van T Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL (1996) Remission in prospective study of patients with rheumatoid arthritis, American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol 35(11):1101–1105
Tijhuis GJ, de Jong Z, Zwinderman AH, Zuijderduin WM, Jansen LM, Hazes JM, Vliet Vlieland TP (2001) The validity of the Rheumatoid Arthritis Quality of Life (RAQoL) questionnaire. Rheumatology (Oxford) 40(10):1112–1119
Witt M, Mueller F, Nigg A, Reindl C, Leipe J, Proft F, Stein N, Hammitzsch A, Mayer S, Dechant C, Schulze-Koops H, Grunke M (2013) Relevance of grade 1 gray-scale ultrasound findings in wrists and small joints to the assessment of subclinical synovitis in rheumatoid arthritis. Arthritis Rheum 65:1694–1701
Szkudlarek M, Court-Payen M, Strandberg C, Klarlund M, Klausen T, Ostergaard M (2001) Power Doppler ultrasonography for assessment of synovitis in the metacarpophalangeal joints of patients with rheumatoid arthritis: a comparison with dynamic magnetic resonance imaging. Arthritis Rheum 44:2018–2023
Naredo E, D’Agostino MA, Wakefield RJ, Möller I, Balint PV, Filippucci E, Iagnocco A, Karim Z, Terslev L, Bong DA, Garrido J, Martínez-Hernández D, Bruyn GA, OMERACT Ultrasound Task Force (2013) Reliability of a consensus-based ultrasound score for tenosynovitis in rheumatoid arthritis. Ann Rheum Dis 72:1328–1334
Sokka T (2008) Radiographic scoring in rheumatoid arthritis: a short introduction to the methods. Bull NYU Hosp Jt Dis 66(2):166–168
Kamishima T, Sagawa A, Tanimura K, Shimizu M, Matsuhashi M, Shinohara M, Hagiwara H, Henmi M, Narita A, Terae S, Shirato H (2010) Semi-quantitative analysis of rheumatoid finger joint synovitis using power Doppler ultrasonography: when to perform follow-up study after treatment consisting mainly of antitumor necrosis factor alpha agent. Skeletal Radiol 39(5):457–465
Iagnocco A, Filippucci E, Perella C, Ceccarelli F, Cassarà E, Alessandri C, Sabatini E, Grassi W, Valesini G (2008) Clinical and ultrasonographic monitoring of response to adalimumab treatment in rheumatoid arthritis. J Rheumatol 35:35–40.10
Naredo E, Möller I, Cruz A, Carmona L, Garrido J (2008) Power Doppler ultrasonographic monitoring of response to anti-tumor necrosis factor therapy in patients with rheumatoid arthritis. Arthritis Rheum 58:2248–2256
Hartung W, Kellner H, Strunk J, Sattler H, Schmidt WA, Ehrenstein B, Fleck M, Backhaus M (2012) Development and evaluation of novel ultrasound score for large joints in rheumatoid arthritis: one year of experience in daily clinical practice. Arthritis Care Res (Hoboken) 64(5):675–682
Backhaus TM, Ohrndorf S, Kellner H, Strunk J, Hartung W, Sattler H, Iking-Konert C, Burmester GR, Schmidt WA, Backhaus M (2013) The US7 score is sensitive to change in large cohort of patients with rheumatoid arthritis over 12 months of therapy. Ann Rheum Dis 72(7):1163–1169
Kanazawa T, Nishino J, Tohma S, Tanaka S (2013) Analysis of the affected joints in rheumatoid arthritis patients in large Japanese cohort. Mod Rheumatol 23(1):44–49
Tian J, Chen J, Li F, Xie X, Du J, Mao N, Gao J (2013) Grey scale and power Doppler ultrasonographic assessment of bone erosion and disease activity in early rheumatoid arthritis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 38(12):1270–1274
Funck-Brentano T, Gandjbakhch F, Etchepare F, Jousse-Joulin S, Miquel A, Cyteval C, Lukas C, Tobón GJ, Saraux A, Boumier P, Goupille P, Bourgeois P, Fautrel B (2013) Prediction of radiographic damage in early arthritis by sonographic erosions and power Doppler signal: a longitudinal observational study. Arthritis Care Res (Hoboken) 65(6):896–902
Acknowledgments
We thank the following physiatrist for inclusion and follow-up of their patients: Nedim Kaban, Ekrem Süleyman, and Mustafa Serdar Sağ.
Disclosure
None.
Authors’ contributions
All authors contributed to acquisition, analysis, and interpretation of the data and drafting of the manuscript.
Ethics approval
The study protocol was approved by Sakarya University local ethics committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harman, H., Tekeoğlu, İ., Takçı, S. et al. Improvement of large-joint ultrasonographic synovitis is delayed in patients with newly diagnosed rheumatoid arthritis: results of a 12-month clinical and ultrasonographic follow-up study of a local cohort. Clin Rheumatol 34, 1367–1374 (2015). https://doi.org/10.1007/s10067-015-2926-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-2926-x