Abstract
The aims of the current study are to describe gadolinium-enhanced MRI features of an acute flare of established gouty arthritis in different joints and to examine a possible association between serum uric acid and MRI signs indicative of ongoing inflammation and/or structural joint damage as well as association with disease characteristics and laboratory findings. Twenty-seven male patients with established chronic gout agreed to participate, mean age 47.6 years, and mean disease duration in months 43.2 (±31.8). For all patients, detailed demographic, disease characteristics, and laboratory findings were obtained and correlated with MRI findings. In 27 patients with established gout, a total of 50 MRI studies were performed of the following joints: feet joints (n = 23), ankles (n = 18), knees (n = 5), and hand and wrist joints (n = 4). MRI revealed capsular thickening in 19 patients, bone marrow edema (BME) in 15, soft tissue edema (STE) in 20, joint effusion in 21, bone erosions in 17, cartilaginous erosions in 4, and tenosynovitis in 9 cases. In 17 cases, tophaceous lesions were found. Post contrast MRI showed synovial thickening in seven cases. Positive correlations were observed between serum uric acid levels and the following MRI findings: capsular thickening (r = 0.552, p = 0.003), BME (r = 0.668, p ≤ 0.0001), STE (r = 0.559, p = 0.002), and tenosynovitis (r = 0.513, p = 0.006). Using MRI in chronic gout, important features can be detected like BME, minute cartilaginous erosions, and hypertrophic synovial inflammation in post contrast MR images. Serum uric acid (SUA) was positively correlated with capsular thickening, BME, STE, and tenosynovitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gouty arthritis is an inflammatory condition associated with debilitating clinical symptoms, functional impairments, and a substantial impact on quality of life. This condition is initially triggered by the deposition of monosodium urate (MSU) crystals into the joint space. This causes an inflammatory cascade resulting in the secretion of several proinflammatory cytokines and neutrophil recruitment into the joint [1]. Gout is considered the most common cause of arthritis in the elderly due to an increase in risk factors such as renal diseases, metabolic syndrome, and a diet rich in purines [2]. In cases of gout with a low synovial fluid (SF), leukocyte count, and atypical clinical presentation, such as in intercritical periods, the load of MSU crystals is frequently low, and thus, methods to improve the crystal detection may be beneficial [3].
As is the case with other inflammatory arthritides, gout will often lead to significant destructive skeletal changes if left untreated. Radiographic changes are only detectable after many years of disease. A large percentage of patients with gout and normal plain radiographs have occult destructive arthropathy that is only detected by advanced imaging such as MRI and/or ultrasound (US). However, MRI appears to be much more sensitive than US at detecting these findings [4].
Moreover, computed tomography (CT) has superior capability over both plain radiography and MRI to detect bone erosions in gout [5, 6]. MRI has the advantage over CT to image both tophus and bone erosion and has provided new insights into mechanisms of joint damage in chronic gout. MRI allows early detection of tophi and bone erosion in patients with gout [7]. Synovial involvement may also be appropriately evaluated with contrast-enhanced MRI technique [8]. MRI is commonly used in autoimmune inflammatory arthritis to define disease activity and damage, but its role in gout remains unclear.
The aims of the current study are to describe gadolinium-enhanced MRI features of an acute flare of established gouty arthritis in different joints and to examine a possible association between serum uric acid and MRI signs indicative of ongoing inflammation and/or structural joint damage as well as association with disease characteristics and laboratory findings. These findings may be relevant for the treating physician.
Patients and methods
Patients
Consecutive new patients with untreated gout fulfilling at least 6 of the 12 ACR diagnostic criteria for gout were included [9]. Twenty-seven male patients agreed to participate. There were no refusals. All patients performed the MRI study during an acute flare, on top of established disease, either due to stopping urate lowering therapy or due to lack of adherence to take therapy on regular basis. For all patients, detailed demographic and clinical characteristics were obtained.
Laboratory investigations
In all patients, laboratory investigations were performed including complete blood count, erythrocyte sedimentation rate (ESR) first hour, C-reactive protein (CRP), serum creatinine, serum uric acid (SUA) levels, simple urine analysis, and 24 h urinary uric acid.
MRI protocols
All MRI studies were performed using General Electric 1.5 Tesla MR Unit. MR studies for hand joints and both wrists were performed in prone or supine position, with the arms semi-flexed above the head and the hands positioned in the center of the coil. Intravenous bolus injection of gadolinium-diethylene triamine pentaacetic acid (DTPA) (0.1 mmol/kg body weight) was performed after completion of the initial coronal scout view. In all patients, the imaging protocol consisted of fat-suppressed gadolinium-enhanced T1-weighted spin-echo in all orthogonal planes (axial, sagittal, and coronal planes), repetition time (TR) 855 ms, echo time (TE) 20 ms, field of view 15–20 cm, section thickness 4 mm with no intersection gap, one signal acquired, matrix 379 × 512, and time of acquisition 5 min 27 s. A total of 16 sections were obtained with this sequence. Other imaging variables were TR 36 ms, TE 9 ms, flip angle 50° field of view (FOV) 20–20 cm, one signal acquired, matrix 300 × 512, and time of acquisition 5 min 25 s. Axial slices consisted of two simultaneous series (2 × 8 and 2 × 30 sections with spin-echo, respectively). The first series covered the wrists from the distal radioulnar joints to the metacarpal bases and the second one the metacarpophalangeal (MCP) joints. Both feet were then scanned: patient supine position with a small pad under the knees for the duration of the examination. In all patients, post contrast fat suppressed spin-echo T1 (–two to eight slices) in all planes through the MTP joints was done [10]. For MRI knees, sagittal, coronal, and axial, T1-weighted, spin-echo MRI were obtained. Immediately after the acquisition of baseline images, bolus intravenous Gd-DTPA was administered 0.05 mmol Gd-DTPA. The following sequences were followed in all patients: coronal T2-weighted (TR/TE 2600-24/68, matrix size 256 × 160, section thickness 4 mm, spacing 0.5 mm, FOV 16 × 16), coronal STIR (TR/TE 5250-94/68, matrix size 256 × 160, section thickness 4 mm, spacing 0.5 mm, FOV 16 × 16), sagittal T2 (TR/TE 2600-24/68, matrix size 256 × 160, section thickness 4 mm, spacing 0.5 mm, FOV 16 × 16), sagittal STIR (TR/TE 5250-49, matrix size 256 × 160, section thickness 4 mm, spacing 0.5 mm, FOV 16 × 16), axial T1 (TR/TE 450-11.2, matrix size 320 × 192, section thickness 4 mm, spacing 0.5 mm, FOV 16 × 16), axial T2 (TR/TE 2600-23.5, matrix size 256 × 192, section thickness 4 mm, spacing 0.5 mm, FOV 16 × 16) [11].
MRI interpretation
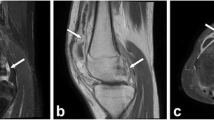
All MRI studies were evaluated for soft tissue edema (STE) (Fig. 1a), bone marrow edema (BME) (Figs. 1a, 2a, and 3a), joint effusion (Fig. 3c), tenosynovitis (Figs. 2c, 3d, e, 4c, d, and 5a, b) synovial enhancement in post contrast images (Fig. 1b, c, d), capsular edema (Figs. 2b and 3f), cartilaginous erosions (Fig. 4a, b), and bone erosions (Fig. 3b, 4a, b, and 5a, b). For tophaceous, lesions appear as intermediate signal intensity on TI-weighted images, low to intermediate signal on T2WI depending on mineral content of the lesion and intermediate with poor enhancement on T1 post contrast fat sat (Fig. 5c, d).
a Axial T2 fat sat, showing bone marrow edema of the first metatarsal head (arrow); b axial T2 fat sat showing capsular edema of the first metatarsophalngeal joint; c axial T1 fat sat showing tenosynovitis and soft tissue edema of the second toe; d axial T1 fat sat, showing non-enhancing tophaceous lesion close to the first metatarsophalangeal joint
a Sagittal PD fat sat, showing bone marrow edema of the head of first metatarsal bone (short arrow), together with overlying small tophecous lesion (long arrow); b sagittal PD fat sat showing eccentric bone erosion of the head of the first metatarsal bone; c sagittal PD fat sat showing ankle effusion; d, e coronal T1 fat sat showing extensor tendon tenosynovitis of the fourth toe with surrounding soft tissue edema in two different cases; f coronal PD fat sat, showing capsular edema and thickening of the first MTP joint
a, b Coronal T2 fat sat showing multiple gross tophi around the wrist and MCP joints indicated by white arrows; the related auricular cartilages are destroyed with subchondral erosions; c, d axial post contrast T1 fat sat showing variable degrees of flexor and extensor tenosynovitis as indicated by the white arrows
a, b Coronal PD fat sat showing signs of eccentric bone erosions around the ankle mortise with tenosynovitis of the peroneal and tibialis posterior tendons; c axial T1 post contrast fat sat showing tophaceous lesion (small arrow) and tenosynovitis (long arrow) related to the fourth and fifth toes, respectively; d sagittal T1 post contrast fat sat showing lobulated tophaceous lesion overlying the dorsal aspect of the first MTP joint
Statistical analysis
Quantitative variables were described using mean standard deviation (SD) and categorical variables using absolute values and percentages. Pearson’s correlation was used to measure the strength of the linear relationship between serum uric acid values and other variables like demographic, clinical characteristics, laboratory findings, and MRI findings.
Ethics
The design of the study has been approved by the local ethics committee, and all patients gave informed written consent to be enrolled into the study according to the Declaration of Helsinki.
Results
Twenty-seven male patients with established gouty arthritis could be included in our study. Their mean age (±SD) was 47.6 (±12.0) years, and their mean disease duration since first attack (±SD) in months was 43.2 (±31.8). A total of 50 MRI studies were performed at the following joints: 23 feet joints (n = 23), 18 ankles (n = 18), five knees (n = 5), and hand and wrist joints (n = 4). MRI studies identified capsular thickening in 19 patients, BME in 15, joint effusion in 21, STE in 20, bone erosions in 17, cartilaginous erosions in 4, tenosynovitis in 9, and synovial thickening in 7 cases as observed in post contrast MR images. Other clinical data, laboratory findings, and number of gouty attacks are summarized in Table 1.
Ten patients (37 %) showed no evidence of tophi on MRI, one joint was affected by tophaceous lesion in 14 (51.9 %), two joints in 2 (7.4 %) patients, and four joints in 1 (3.7 %) patient.
Serum uric acid levels showed no significant correlations with demographic features, disease characteristics, and markers of inflammation (Table 2), while positive correlations were observed between serum uric acid levels and MRI findings including capsular thickening (r = 0.552, p = 0.003), BME (r = 0.668, p ≤ 0.0001), STE (r = 0.559, p = 0.002), and tenosynovitis (r = 0.513, p = 0.006). Meanwhile, serum uric acid showed no significant correlations with other MRI findings: joint effusion (r = −0.195, p = 0.330), bone erosions (r = 0.203, p = 0.310), cartilaginous erosions (r = 0.152, p = 0.449), and number of tophi (r = 0.262, p = 0.187).
Discussion
There are few data in the literature regarding the bony and soft tissue changes of gout on MRI. The existing data focus primarily on the appearance of gouty tophi [4, 5, 8, 12–14].
Regarding tophi, we found that 10 patients (37 %) showed no evidence of tophi on MRI, one joint was affected by a tophaceous lesion in 14 (51.9 %), two joints in 2 (7.4 %) patients, and four joint in 1 (3.7 %) patient. Tophi have variable signal intensity on T2-weighted images. The most common pattern is heterogeneous intermediate to low signal intensity on T2-weighted images. Both the synovial membrane and tophi can show intense gadolinium enhancement, reflecting granulation tissue and increased vascularization [15].
In our study, other important MRI findings were identified: capsular thickening in 19 patients, BME in 15, joint effusion in 21, STE in 20, bone erosions in 17, cartilaginous erosions in 4, tenosynovitis in 9. and synovial thickening in 7 cases as observed in post contrast MR images. In previous work, Carter et al. [4] analyzed the prevalence of occult destructive arthropathy in subjects with gout and normal plain radiographs by utilizing both MRI and US. In their study, the authors defined the “index joint” as a joint that has had most acute attacks of gout and was free of erosive changes on plain radiographs. MRI and US studies were performed for the index joints as well as “asymptomatic joints” with no previous gouty attack. Their results showed that the first MTP was the most common index joint (n = 17) followed by the ankle (n = 5), mid-tarsal (n = 2), knee (n = 2), and wrist (n = 1). The knee was the most common asymptomatic joint (n = 21) followed by the wrist (n = 3), MTP (n = 2), and ankle (n = 1). Out of 27 subjects studied, 15 (56 %) had erosions on MRI of their index joint (p < 0.0001). Regarding the secondary endpoints on the index joint, the MRI detected synovial pannus (SP; n = 13), BME (n = 4), STE (n = 3), and none showed soft tissue tophi (STT). Regarding the asymptomatic joint, MRI findings included SP (n = 3), BME (n = 3), STE (n = 2), and bone erosions (n = 1), while US failed to show such findings in the asymptomatic joint. The authors concluded that MRI appears to be much more sensitive than US at detecting these findings. In another work, Chen et al. [12] examined 12 patients with intra-articular tophi in the knee joints by MRI and CT; the authors observed that tophi were located purely intra-articularly in 10 knees. The authors concluded that enhanced MR imaging could supplement, in some cases, accurate diagnosis with CT scanning of intra-articular tophaceous deposits [12]. In our study, we found that the number of gouty attacks as dependant variable showed only significant positive association with tophaceous lesions (p = 0.001). Puig et al. [16] evaluated with US 35 patients with asymptomatic hyperuricemia. The authors reported that urate deposits (tophi) in tendons, synovium, and other soft tissues were detected in 12 patients (34 %) and increased vascularity (inflammation) was evident in 8 of these patients (23 %). This study using US is the first to link the gap between asymptomatic hyperuricemia and symptomatic deposition of urate, namely gout. A characteristic sign by US is the double-contour sign which represents a hyperechoic band over anechoic cartilage [17]. In our study, we could not specify this sign which can be explained by the great difference between US and MRI techniques.
MRI is commonly used in autoimmune inflammatory arthritis to define disease activity and damage, but its role in gout remains unclear. MRI reveals that gout affects the joints, bones, and tendons [18]. In our study, we observed BME in 15 patients at different joints, and none of them showed evidence of concomitant osteomyleitis. This in contrast to the previous findings reported Poh et al. [18] who examined a total of 47 patients with established gout (51 scans); 33 (70 %) had uncomplicated gout, and 14 (30 %) had gout complicated by osteomyelitis. In their study, MRI showed tophi in 36 scans (71 %), erosions in 35 (69 %), BME in 27 (53 %), synovitis in 15 (29 %), tenosynovitis in 8 (16 %), and tendinosis in 2 (4 %). Uncomplicated gout and gout plus osteomyelitis did not differ for most MRI features. However, “severe BME” was much more common in gout plus osteomyelitis, occurring in 14/15 scans (93 %) compared with 3/36 scans (8 %) in uncomplicated gout (OR 154.0, 95 % CI 14.7–1612, p < 0.0001). Sensitivity and specificity of severe BME for concomitant osteomyelitis were 0.93 (95 % CI 0.68–0.99) and 0.92 (95 % CI 0.78–0.98), respectively [18]. The later findings need to be confirmed in further studies that incorporate larger number of patients.
In our study, MRI showed evidence of cartilaginous erosions in four patients. Cartilage damage is frequently observed in advanced destructive gout. MSU crystals have profound inhibitory effects on chondrocyte viability and function. Interactions between MSU crystals and chondrocytes may contribute to cartilage damage in gout through reduction of chondrocyte viability and promotion of a catabolic state. It was reported that MSU crystals rapidly reduced primary human chondrocyte and cartilage explant viability in a dose-dependent manner. Moreover, cartilage explants cultured with MSU crystals had a greater percentage of dead chondrocytes at the articular surface compared to untreated cartilage (p = 0.004). Relative mRNA expression of type II collagen and the cartilage matrix proteins aggrecan and versican was decreased in chondrocytes following culture with MSU crystals. In joints affected by gout, normal cartilage architecture was lost, with empty chondrocyte lacunae observed [19].
In the most recent work, Carter et al. [20] determined the percentage of patients with intercritical gout who have chronic synovial-based inflammation as evidenced by synovial pannus on a contrast-enhanced MRI of their most involved joint and determine if the presence and/or severity correlates with their serum urate levels. Although the authors found that the majority of patients with intercritical gout have evidence of chronic synovial-based inflammation, yet the presence and severity of this inflammation detected by enhanced MRI was not correlated with serum urate levels. In our study, all cases have already established gouty arthritis, and we observed positive correlations between serum uric acid levels and various MRI findings like capsular thickening (r = 0.552, p = 0.003), BME (r = 0.668, p ≤ 0.0001), STE (r = 0.559, p = 0.002), and tenosynovitis (r = 0.513, p = 0.006). In our study, we did not find ankyloses as found in a few joints in a study with combination of radiography and CT scanning in 800 joints of 20 patients with mostly tophaceous gout [21, 22]. We restricted ourselves to joints with active inflammation, and these will generally not be ankylotic joints.
Gerster and colleagues have suggested that CT provides more specific images than US or MRI for assessment of tophi [14]. In other study, in individual joints in patients with chronic gouty arthritis with CT, a strong relationship was demonstrated between bone erosion and intraosseous tophus. There was also a strong correlation between the CT erosion diameter and the intraosseous tophus diameter in individual joints (p < 0.0001) [23]. Recently, dual-energy CT (DECT) showed high sensitivity and specificity for the identification of urate crystals and diagnosis of acute gout. The risk factors for uric acid deposition include the disease duration, serum uric acid levels, the presence of tophi, and bone erosion [24]. In recent work, Shi et al. [25] evaluated the tophus urate volume, erosion volume, and the erosion score measurements in patients with gout by using DECT scans to compare the bone erosion volumes against bone erosion scores to determine a valid measure of joint destruction in chronic gout. The authors reported a strong positive correlation between individual erosion volumes and scores (p < 0.001) as well as total erosion volume and score per patient (p < 0.001). The authors suggested that the erosion volumes show to be a more direct and accurate method to evaluate bone erosion compared with erosion score, strongly supporting it as a superior and standard measure of structural joint damage in gout.
In a review, McQueen et al. summarize that high-resolution computed tomography is useful in investigating the relationship between bone erosions and tophi. Magnetic resonance imaging and ultrasonography can image the inflammatory nature of gouty arthropathy, revealing synovial and soft tissue inflammation, and can provide information about the composition and vascularity of tophi. Dual-energy computerized tomography is able to identify tophi by their chemical composition and reveal even small occult tophaceous deposits. All modalities could have important clinical applications in the patient for whom aspiration of monosodium urate crystals from the joint is not possible. Imaging can also provide outcome measures, such as change in tophus volume, for monitoring the response to urate-lowering therapy [26].
In our opinion, MRI study is superior compared to CT due to its ability to detect important signs that cannot be visualized with the use of CT like BME, minute cartilaginous erosions, and hypertrophic synovial inflammation in post contrast MR images with no exposure to ionizing radiation.
Likewise in our study, joints affected by tophi, synovial thickening, effusions, and bone erosion may be present, with BME adjacent to tophi [7, 12, 13]. Regarding hand joints, MRI studies have also shown that urate deposits spread along compartmental and fascial planes, rather than in a radial pattern in hand joint [7]. In a series of patients showing unexplained limitation of the knee joint, MRI was useful to evaluate tophaceous urate deposition as the cause of such limitation [27].
Epidemiological and experimental studies have shown that hyperuricaemia and gout are intricately linked with hypertension, metabolic syndrome, chronic kidney disease, and cardiovascular disease.
Hyperuricaemia and gout are independent risk factors for the development of these conditions and that these conditions account, in part, for the increased mortality rate of gouty patients [28]. For developing gout attacks, the serum urate level and specific crystal properties, i.e. specific crystal stereochemical/conformational properties, both are of importance [29]. Moreover, many crystals may be found in arthritic joints. Rheumatologists are able to diagnose, with a high degree of probability, which crystals induce arthritis in the individual case. A definite diagnosis supported by polarized microscopy may provide a firm basis for adequate long-term treatment and tight disease control [30].
Conclusion
MRI is a sensitive modality that can visualize the whole pathology related to gouty arthritis even in asymptomatic joints. In our opinion, MRI is generally superior compared to CT due to its ability to detect important signs that cannot be visualized with the use of CT like BME, minute cartilaginous erosions, and hypertrophic synovial inflammation in post contrast MR images with no exposure to ionizing radiation.
References
Gonzalez EB (2012) An update on the pathology and clinical management of gouty arthritis. Clin Rheumatol 31:13–21. doi:10.1007/s10067-011-1877-0
Bolzetta F, Veronese N, Manzato E, Sergi G (2012) Tophaceous gout in the elderly: a clinical case review. Clin Rheumatol 31:1127–1132. doi:10.1007/s10067-012-1956-x
Robier C, Stettin M, Quehenberger F, Neubauer M (2014) Cytospin preparations are superior to common smears in the detection of monosodium urate crystals in low-cellular synovial fluids. Clin Rheumatol 33:1797–1800. doi:10.1007/s10067-014-2619-x
Carter JD, Kedar RP, Anderson SR, Osorio AH, Albritton NL, Gnanashanmugam S, Valeriano J, Vasey FB, Ricca LR (2009) An analysis of MRI and ultrasound imaging in patients with gout who have normal plain radiographs. Rheumatology (Oxford) 48:1442–1446. doi:10.1093/rheumatology/kep278
Chen CK, Chung CB, Yeh L, Pan HB, Yang CF, Lai PH, Liang HL, Resnick D (2000) Carpal tunnel syndrome caused by tophaceous gout: CT and MR imaging features in 20 patients. Am J Roentgenol 175:655–659
Johnson TR, Weckbach S, Kellner H, Reiser MF, Becker CR (2007) Dual-energy computed tomographic molecular imaging of gout. Arthritis Rheum 56:2809
Popp JD, Bidgood WD Jr, Edwards LN (1996) Magnetic resonance imaging of tophaceous gout in the hands and wrists. Semin Arthritis Rheum 25:282–289
Narváez JA, Narváez J, Ortega R, De Lama E, Roca Y, Vidal N (2003) Hypointense synovial lesions on T2-weighted images: differential diagnosis with pathologic correlations. Am J Roentgenol 181:761–769
Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF (1997) Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 20:895–900
Boutry N, Lardé A, Lapègue F, Solau-Gervais E, Flipo RM, Cotten A (2003) Magnetic resonance imaging appearance of the hands and feet in patients with early rheumatoid arthritis. J Rheumatol 30:671–679
Emad Y, Ragab Y, Shaarawy A, Raafat H, El-Kiki HA, Rasker JJ (2008) Enhanced MRI in early undifferentiated oligoarthritis of the knee joints: improvements already visible after 2 months of DMARDs treatment. Clin Rheumatol 27:1177–1182. doi:10.1007/s10067-008-0935-8
Chen CK, Yeh LR, Pan HB, Yang CF, Lu YC, Wang JS, Resnick D (1999) Intra-articular gouty tophi of the knee: CT and MR imaging in 12 patients. Skelet Radiol 28:75–80
Yu JS, Chung C, Recht M, Dailiana T, Jurdi R (1997) MR imaging of tophaceous gout. Am J Roentgenol 168:523–527
Gerster JC, Landry M, Dufresne L, Meuwly JY (2002) Imaging of tophaceous gout: computed tomography provides specific images compared with magnetic resonance imaging and ultrasonography. Ann Rheum Dis 61:52–54
Perez-Ruiz F, Naredo E (2007) Imaging modalities and monitoring measures of gout. Curr Opin Rheumatol 19:128–133
Puig JG, de Miguel E, Castillo MC, Rocha AL, Martínez MA, Torres RJ (2008) Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids 27:592–595. doi:10.1080/15257770802136040
Thiele RG, Schlesinger N (2007) Diagnosis of gout by ultrasound. Rheumatology (Oxford) 46:1116–1121
Poh YJ, Dalbeth N, Doyle A, McQueen FM (2011) Magnetic resonance imaging bone edema is not a major feature of gout unless there is concomitant osteomyelitis: 10-year findings from a high-prevalence population. J Rheumatol 38:2475–2481. doi:10.3899/jrheum.110477
Chhana A, Callon KE, Pool B, Naot D, Gamble GD, Dray M, Pitto R, Bentley J, McQueen FM, Cornish J, Dalbeth N (2013) The effects of monosodium urate monohydrate crystals on chondrocyte viability and function: implications for development of cartilage damage in gout. J Rheumatol 40:2067–2074. doi:10.3899/jrheum.130708
Carter JD, Patelli M, Anderson SR, Prakash N, Rodriquez EJ, Bateman H, Sterrett A, Valeriano J, Ricca LR (2014) An MRI assessment of chronic synovial-based inflammation in gout and its correlation with serum urate levels. Clin Rheumatol. doi:10.1007/s10067-014-2644-9
Dalbeth N, Milligan A, Doyle AJ, Clark B, McQueen FM (2012) Characterization of new bone formation in gout: a quantitative site-by-site analysis using plain radiography and computed tomography. Arthritis Res Ther 14:R165. doi:10.1186/ar3913
Jansen TL (2012) Rheumatology meets radiology in the hot soup of Gutta. Arthritis Res Ther 14(6):126. doi:10.1186/ar4068
Dalbeth N, Collis J, Gregory K, Clark B, Robinson E, McQueen FM (2007) Tophaceous joint disease strongly predicts hand function in patients with gout. Rheumatology 46:1804–1807
Wu H, Xue J, Ye L, Zhou Q, Shi D, Xu R (2014) The application of dual-energy computed tomography in the diagnosis of acute gouty arthritis. Clin Rheumatol 33:975–979. doi:10.1007/s10067-014-2606-2
Shi D, Xu JX, Wu HX, Wang Y, Zhou QJ, Yu RS (2014) Methods of assessment of tophus and bone erosions in gout using dual-energy CT: reproducibility analysis. Clin Rheumatol. doi:10.1007/s10067-014-2725-9
McQueen FM, Doyle A, Dalbeth N (2011) Imaging in gout-what can we learn from MRI, CT, DECT and US? Arthritis Res Ther 13:246. doi:10.1186/ar3489
Schumacher HR Jr, Becker MA, Edwards NL, Palmer WE, MacDonald PA, Palo W, Joseph-Ridge N (2006) Magnetic resonance imaging in the quantitative assessment of gouty tophi. Int J Clin Pract 60:408–414
Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, Punzi L, So AK, Bardin T (2014) Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. doi:10.1038/nrrheum.2014.124
Jansen TL, Berendsen D, Crisan TO, Cleophas MC, Janssen MC, Joosten LA (2014) New gout test: enhanced ex vivo cytokine production from PBMCS in common gout patients and a gout patient with Kearns-Sayre syndrome. Clin Rheumatol 33:1341–1346. doi:10.1007/s10067-014-2620-4
Jansen TL, Rasker JJ (2011) Therapeutic consequences of crystals in the synovial fluid: a review for clinicians. Clin Exp Rheumatol 29:1032–1039
Conflict of interest
All the authors responsible for this work declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emad, Y., Ragab, Y., El-Naggar, A. et al. Gadolinium-enhanced MRI features of acute gouty arthritis on top of chronic gouty involvement in different joints. Clin Rheumatol 34, 1939–1947 (2015). https://doi.org/10.1007/s10067-015-2895-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-2895-0