Abstract
The purpose of this study is to investigate the effect of urate-lowering therapies (ULTs) on renal uric acid excretion in gout patients. This prospective observational study involved 106 primary gout patients and 51 healthy controls. Gout patients received ULT with either xanthine oxidase inhibitors or the uricosuric agent benzbromarone. Parameters such as 24-h urinary uric acid, creatinine clearance, uric acid clearance, glomerular filtration load of uric acid, fractional excretion of uric acid, excretion of uric acid per volume of glomerular filtration, and urinary uric acid to urinary creatinine ratio were used to evaluate the pre- and post-treatment renal capacity for uric acid clearance in gout patients and were compared with the values in the healthy controls. Compared to healthy controls, gout patients had higher glomerular filtration load of uric acid and lower uric acid clearance, creatinine clearance, and fractional uric acid excretion. After ULT, both the xanthine oxidase inhibitor group and benzbromarone group patients showed reduction in glomerular filtration load of uric acid. Creatinine clearance was significantly improved in the xanthine oxidase inhibitor group. Excretion function was remarkably enhanced in patients who reached the treatment target (serum uric acid <6 mg/dl). Changes in glomerular uric acid filtration load were significantly correlated with changes in serum urate levels. Gout patients have impaired renal uric acid excretion. ULTs reduce renal urate load and enhance the renal capacity of uric acid clearance. Xanthine oxidase inhibitors showed superiority over benzbromarone in improving renal function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although uric acid overproduction, renal uric acid under-excretion, or both can contribute to the development of hyperuricemia and gout [1–3]; decreased renal excretion has been demonstrated to be the principal mechanism [4]. Uric acid is cleared through both renal and extra-renal pathways; however, the renal pathway is dominant, excreting more than two thirds of the uric acid produced in the body [5]. The classical four-compartment model [6], though not validated [7], helps to explain the renal excretion process. This model indicates that nearly all the uric acid is filtered by the glomeruli; then, 99 % of the filtered uric acid is absorbed in the proximal tubules (S1 segment). The proximal tubules secrete 45–50 % of the absorbed uric acid in the S1 and S2 segments, followed by post-secretory reabsorption (40–45 %) in the S3 segment. Thus, 5–10 % of the filtered uric acid is excreted from the body [8]. It was previously thought that renal handling of uric acid rested mainly on the secretion and reabsorption functions rather than on filtration [9]. However, recent research [10] has demonstrated that glomerular filtration is a key factor in serum urate regulation. It is estimated that only 2.9 % of gout patients have a normal glomerular filtration rate (GFR), while 24 % have a GFR <60 ml/min/1.73 m2. The majority of gout patients (73 %) have mild renal dysfunction. Overall, inadequate renal uric acid clearance caused by reduced glomerular filtration or tubular under-excretion is responsible for the development of gout.
Hyperuricemia is the independent risk factor for chronic kidney disease [11–13]. Uric acid, a pro-inflammatory and anti-oxidant substance, acts on renal epithelial cells [14] and causes tubular injury [5]. This raises the question of whether urate-lowering therapy (ULT) can improve renal function and uric acid handling, and if so, which type of ULT is optimal for this purpose. The most frequently used pharmacological ULT involves inhibition of uric acid synthesis by xanthine oxidase inhibitors (XOIs) and enhancement of urate excretion by uricosuric agents. In clinical practice, patients with low 24-h urinary uric acid (Uua) output are administered uricosuric therapy, while patients with normal or high 24-h urinary uric acid output are suggested XOIs. However, 24-h Uua output is easily affected by many factors such as diet, alcohol intake, urine volume, gender, height, weight, and serum uric acid (Sua) and creatinine (Scr) levels, and fluctuates with season and time [15]. Thus, it is not an effective indicator to guide gout treatment. Other indicators, including creatinine clearance (Ccr), uric acid clearance (Cua), fractional excretion of uric acid (FEua), glomerular filtration load of uric acid (FLua), excretion of uric acid per volume of glomerular filtration (EuaGF), and Uua to urinary creatinine ratio (Uua/Ucr), are less likely to be disturbed by physiological factors and the quality of urinary specimens and can therefore better assess renal excretion function. In this prospective observational study, we used these parameters to evaluate the effects of XOIs and a uricosuric agent on renal uric acid clearance and assessed the results to determine which type of ULT strategy is superior to improve renal uric acid excretion.
Methods
Study design and participant selection
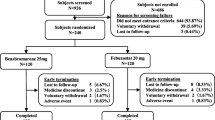
We enrolled patients who met the 1977 American Rheumatism Association (ARA) criteria [16] for the diagnosis of gout and age-matched healthy individuals between May 26, 2010 and November 15, 2013. Normal liver function, estimated glomerular filtration rate according to chronic kidney disease Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula >60 ml/min/1.73 m2 and routine blood test results were required in the patient group. The exclusion criteria were secondary gout, use of ULT in the past 2 weeks, contraindications to ULT, use of drugs interfering with renal uric acid handling (e.g., diuretics, aspirin, cyclosporine, niacin, and calcineurin inhibitors), nephrolithiasis, gestation or lactation, neoplasms, connective tissue diseases, and uncontrolled blood pressure (systolic, ≥160 mmHg and/or diastolic, ≥100 mmHg). The patients’ general information, medical history, and physical examination were recorded in detail. A self-restricted diet (no excessive purine intake and no alcohol) was recommended during treatment. Written informed consent was obtained from each participant. Comprehensive evaluations were made after the first (baseline) blood and urine analyses. All treatment decisions were made together by experienced rheumatologists and patients after excluding contraindications. An XOI (allopurinol, 300 mg/day or febuxostat, 40 or 80 mg/day) or a uricosuric agent (benzbromarone, 50 mg/day) was given to the gout patients. The ethics committee of Zhongshan Hospital approved this research.
Sample collection and laboratory tests
All the participants were given a three-liter plastic bottle containing acid preservatives and standardized written instructions on 24-h urine collection. Eight questions were asked as indicated by Wang et al. [17], and the 24-h Ucr excretion was calculated to check if the samples were eligible [18]. After the last urine voiding, concurrent fasting peripheral blood as well as 24-h urine samples were immediately tested using an autoanalyzer (Hitachi-7600, Tokyo, Japan). Scr and Ucr levels were measured by the enzymatic method. Uric acid in blood and urine was tested using the uricase method. After at least two months urate-lowering therapy, a second blood and urine measurement was performed in the same way as previously described.
Parameter calculations
Ccr was estimated by the CKD-EPI formula [19]. The equations for Cua, FEua, FLua, and EuaGF were as follows [20]:
Uv is the urine volume (ml/min). Ccr, Cua, 24-h Uua, FLua, and EuaGF were adjusted for a body surface area of 1.73 m2. After ULT, blood and urine tests were processed in the same way. The endpoint Sua concentration <6 mg/dl was considered the target of treatment [21].
Statistical analysis
All the data for the cases and controls were computerized, and statistical analysis was performed using SPSS, version 20.0 (SPSS Inc., Chicago, USA). Descriptive data were expressed as mean ± SD. A t test was used to estimate the difference between groups at the baseline. Comparisons of data at the baseline and after ULT were tested with a paired t test. Logistic regression analysis was conducted to find associations between changes in the tested variables, including Scr, Ccr, Cua, FLua, FEua, EuaGF, and Uua/Ucr, as well as the associations of these changes with changes in the Sua level. Pearson correlation was used to describe the correlations of pre-treatment Sua with excretion indicators as well as the changes of Sua with the changes of different variables. Statistical significance was set at P < 0.05.
Results
The comparison of uric acid excretion function between gout patients at baseline and healthy controls
This study involved 106 gout patients and 51 age-matched healthy controls. All the subjects were male. Eighteen of 106 patients had tophus by physical examinations and imaging tests. The mean disease duration was 23.4 ± 6.8 months. The mean age was 47.08 ± 15.14 years in the control group and 49.81 ± 13.11 years in the gout group. The median of 24 h Uua was 379.31 mg/day/1.73 m2 (range 132.65–924.10 mg/day/1.73 m2). The group characteristics at the baseline are listed in Table 1. The mean Sua level in gout patients was 9.56 ± 1.51 mg/dl. Compared to the controls, gout patients had lower Ccr (89.55 ± 14.86 ml/min/1.73 m2 vs. 103.78 ± 19.03 ml/min/1.73 m2, P < 0.05) and higher Scr (0.99 ± 0.13 mg/dl vs. 0.84 ± 0.18 mg/dl, P < 0.05), suggesting mild renal dysfunction. Neither 24-h Uua amount nor 24-h Uua concentration differed significantly between the groups. Gout patients had higher FLua (8.42 ± 2.30 mg/min/1.73 m2 vs. 4.78 ± 1.41 mg/min/1.73 m2, P < 0.05) and lower Cua (2.93 ± 1.12 ml/min/1.73 m2 vs. 6.72 ± 3.58 ml/min/1.73 m2, P < 0.05). The mean FEua was much lower in gout patients (5.01 % ± 2.35 %) than in control subjects (11.15 % ± 6.42 %, P < 0.05). Uua/Ucr was also significantly lower in gout patients (0.46 ± 0.20 vs. 0.58 ± 0.25, P < 0.05). EuaGF was similar in healthy controls (0.27 ± 0.11 mg/dl/1.73 m2) and patients (0.28 ± 0.14 mg/dl/1.73 m2; P > 0.05), indicating that the amount of uric acid excreted by the effective renal mass was similar in both groups. The main characteristics of gout patients were high glomerular filtration load of uric acid and uric acid under-excretion.
The characteristics of renal uric acid excretion between gout patients using anti-hypertensive drugs and without hypertension
Of the 106 gout patients, 34 had hypertension under control using anti-hypertensive drugs. The hypertensive drugs included angiotensin converting enzyme inhibitors (6 patients), angiotensin receptor blockers (ARB) (13 patients), calcium channel blockers (17 patients), and beta blocker (1 patient). Sixty-seven gout patients had no history of hypertension. The different characteristics of the renal clearance capacity of uric acid between hypertensive gout patients receiving medication and normotensive patients were displayed in Table 2. Patients using hypotensive drugs were older with the mean age of 58.09 ± 9.64 years in contrast to those without hypertension (43.91 ± 12.17 years, P < 0.001) and had lower Ccr (83.95 ± 13.00 ml/min/1.73 m2 vs. 93.32 ± 14.59 ml/min/1.73 m2, P = 0.002). The anti-hypertensive patients had lower FLua due to the reduced Ccr (7.60 ± 2.03 mg/min/1.73 m2 vs. 9.01 ± 2.32 mg/min/1.73 m2, P = 0.004) compared to the patients without hypertension. The rest of five patients with elevated blood pressure did not received anti-hypertensive medication. Their mean age was 62.20 ± 10.43 years, much older than the normotensive gout patients (P = 0.002). Besides, their Ccr (73.74 ± 8.56 ml/min/1.73 m2) was also reduced compared to the normotensive patients (P = 0.002). The Sua, Scr, FEua, EuaGF, Cua, and Uua/Ucr had no differences among these three groups.
Effect of different ULT strategies on renal handling of uric acid
Among the 106 gout patients, 88 received regular ULT with XOIs (62 patients) or benzbromarone (26 patients). In addition, sodium bicarbonate (600 mg/day) was given to all patients to alkalize the urine. Therapy in the XOI group lasted for 6 months with regular follow-up visits, while the mean treatment time of the benzbromarone group lasted for 4.87 months (95 % confidence interval [CI]: 2.51–7.22 months). Parameters at the baseline and after ULT are displayed in Table 3. At the observational endpoint, the improvement in Sua was greater in the XOI group (from 9.76 ± 1.57 mg/dl to 5.76 ± 1.93 mg/dl, P < 0.05) than in the benzbromarone group (from 8.03 ± 0.77 mg/dl to 5.74 ± 1.91 mg/dl, P < 0.05). With lowering of the Sua level, the FLua dropped in both groups: from 8.76 ± 2.39 mg/min/1.73 m2 to 5.37 ± 2.30 mg/min/1.73 m2 in the XOI group (P < 0.05) and from 7.20 ± 1.93 mg/min/1.73 m2 to 5.22 ± 2.46 mg/min/1.73 m2 (P < 0.05) in the benzbromarone group. Unlike the benzbromarone group in which 24-h Uua increased from 368.57 ± 134.76 mg/day/1.73 m2 to 437.22 ± 164.65 mg/day/1.73 m2 (P > 0.05), the XOI group showed a significant reduction in 24-h Uua (from 395.30 ± 145.08 mg/day/1.73 m2 to 247.91 ± 176.34 mg/day/1.73 m2, P < 0.05) paralleled by a decrease in FLua. The use of XOIs also improved renal function (Scr changed from 89.11 ± 14.16 ml/min/1.73 m2 to 92.33 ± 14.87 ml/min/1.73 m2, P < 0.05). Cua was significantly higher than the baseline level in both groups (from 2.87 ± 1.06 ml/min/1.73 m2 to 3.09 ± 2.34 ml/min/1.73 m2 in the XOI group, P < 0.05; from 3.31 ± 1.39 ml/min/1.73 m2 to 5.77 ± 2.46 ml/min/1.73 m2 in the benzbromarone group, P < 0.05). In the XOI group, FEua decreased to 4.54 % ± 2.77 % (P > 0.05) because Ccr improved more than Cua. In contrast, FEua was remarkably elevated in the benzbromarone group from 5.42 % ± 1.49 % to 8.93 ± 4.16 % (P < 0.05). EuaGF reduced markedly from 0.29 ± 0.16 mg/dl/1.73 m2 to 0.14 ± 0.08 mg/dl/1.73 m2 (P < 0.05) in the XOI group but remained at a similar level in the benzbromarone group. After achieving similar glomerular filtration load of uric acid (XOIs vs. benzbromarone: 5.37 ± 2.30 mg /min/1.73 m2 vs. 5.22 ± 1.77 mg/min/1.73 m2), XOIs produced a greater improvement in kidney function than did benzbromarone.
The influence of treat to target on uric acid clearance
We divided the XOI group patients into two subgroups according to their Sua levels at the observational endpoint. Individuals with Sua < 6 mg/dl were considered to have reached the treatment target (n = 38), and those with Sua ≥6 mg/dl were categorized into the target-failure group (n = 24; Table 4). The baseline Sua was 9.38 ± 1.10 mg/dl in the target-achieved group and 10.29 ± 2.02 mg/dl in the target-failure group. After 6 months of standardized therapy, Sua fell dramatically and was in the normal range (4.64 ± 0.80 mg/dl) in the target-achieved group. A reduction was also observed in the target-failure group (7.61 ± 1.61 mg/dl after treatment, P < 0.05). The 24-h Uua at the baseline (378.16 ± 139.52 mg/day/1.73 m2 vs. 404.01 ± 141.51 mg/day/1.73 m2; P > 0.05) and after ULT (234.68 ± 165.42 mg/day/1.73 m2 vs. 244.53 ± 171.35 mg/day/1.73 m2; P > 0.05) were similar in both groups. However, a significant increase in Ccr was noticed in the target-achieved group (88.54 ± 11.62 ml/min/1.73 m2 vs. 93.98 ± 13.77 ml/min/1.73 m2, P < 0.05). In addition, these patients showed higher Cua and FEur after XOI treatment than did the target-failure patients (Cua: 2.19 ± 1.28 ml/min/1.73 m2 vs. 3.63 ± 2.67 ml/min/1.73 m2, P < 0.05; FEur: 3.57 % ±1.72 % vs. 5.12 % ± 3.13 %, P < 0.05). FLua was consistent with Sua levels and fell drastically in the target-achieved group (8.33 ± 1.58 mg/min/1.73 m2 vs. 4.38 ± 1.10 mg/min/1.73 m2, P < 0.05). EuaGF and Uua/Ucr reduced in both groups (P < 0.05).
Univariate and multivariate logistic regression analysis for the relationship between treatment outcome (whether reached the goal of treat to target, Sua < 6 mg/dl) and the changes of different uric acid excretion parameters
Uua, Ccr, FLua, Cua, FEua, EurGF, and Uua/Ucr were selected as possible predictive factors. The univariate logistic regression analysis revealed that failure to reach the treatment target was related with increase in FLua (odds ratio [OR] = 0.55, 95 % confidence interval [CI]: 0.413–0.736, P < 0.05) and decrease in Cua (OR = 1.25, 95 % CI: 1.03–1.52, P < 0.05). In multivariate analysis, when adjusted with age, body mass index, hypertension, and drug species, reduce in FLua significantly associated with achieving Sua < 6 mg/dl (OR = 0.56, 95 % CI: 0.39–0.81, P = 0.002; Table 5).
The relationship of Sua and indicators for excretion function as well as the their changes
We used Pearson correlation analysis to determine whether baseline level of Sua was related to the excretion function indicators in patients who received ULT therapy (n = 88). FLua was positively correlated with Sua (r = 0.72, P = 0.001). Negative correlations were found between Sua and Cua (r = −0.280, P = 0.008), FEua (r = −0.458, P < 0.001), and EuaGF (r = −0.289, P = 0.007). The changes in Sua level in the XOI group were only correlated to the changes of FLua (r = 0.912, P < 0.001) and showed no correlation with renal uric acid excretion function (Cua: r = −0.086, P = 0.530; FEua: r = −0.222, P = 0.101; EuaGF: r = 0.091, P = 0.505). In the benzbromarone group, the change of FLua was positively correlated to changes in Sua levels (r = 0.943, P < 0.001), which showed a significant negative correlation with the changes of Cua (r = −0.565, P = 0.004) and FEua (r = −0.650, P = 0.002). The p values were listed in Table 6.
Discussion
When prescribing a self-restricted diet to gout patients, a 24-h Uua level of 800 mg/day is the most commonly used lower limit for uric acid overproducers, although this limit varies widely, ranging between 700 and 1000 mg/day. In Asians, 1000 mg/day has been suggested as the most appropriate cutoff, as less than 10 % of Asian gout patients are estimated to be overproducers [15]. In our study, only two patients had an adjusted 24-h Uua > 800 mg/day/1.73 m2, and the highest 24-h Uua was 859 mg/day/1.73 m2. Patients with EuaGF > 0.7 mg/dl/1.73 m2 [22] or Cua ≥ 6 ml/min/1.73 m2 [23] are classified as overexcretors. Only three of our patients were overexcretors (Cua, 6.64 ml/min/1.73 m2 in one patient; EuaGF, >0.7 mg/dl in two patients). Therefore, most of our patients were “underproducers” of uric acid. Compared to normal controls, gout patients had higher renal uric acid loads (FLua, 8.42 % ± 2.30 % vs. 4.78 % ± 1.41 %) and diminished renal Cua. This phenomenon has been previously demonstrated [24]. Perez-Ruiz et al. [20] reported a FLua of 5.58 ± 1.22 mg/min/1.73 m2 in healthy controls and 9.73 ± 2.02 mg/min/1.73 m2 in gout patients (P < 0.05). Moreover, their patients also showed impaired renal handling of uric acid compared to the normal individuals, with a lower Cua (4.96 ± 1.30 mg/min/1.73 m2 vs. 8.31 ± 1.85 mg/min/1.73 m2, P < 0.05) and FEua (4.59 ± 1.19 % vs. 7.57 ± 1.85 %, P < 0.05). However, the exclusion criteria differed between our study and their study. They chose subjects with normal renal function as their target population (Ccr: 111 ± 18 ml/min/1.73 m2 for healthy controls and 109 ± 18 ml/min/1.73 m2 for gout patients), whereas our patients had mild renal dysfunction with a mean Ccr of 89.55 ml/min/1.73 m2. Our selection criteria are in line with the real clinical situation, in that the vast majority of gout patients have renal dysfunction [10].
Lines of evidence support that hypertension is commonly associated with hyperuricemia. Almost 30 % of patients with hyperuricemia or gout have hypertension [25]. An activation of renin-angiotensin system (RAS) has been shown directly or indirectly associated with hyperuricemia either in animal models [26] or in patients [27]. However, one study reported that RAS-blocking drugs did not bring the protective effect of normalized Sua levels [28]. Therefore, whether and how hypertension could affect the Sua and uric acid excretion are still in debate. Another confounding factor is the anti-hypertensive drugs, especially diuretics and ARBs, which would act on uric acid excretion. In our study, ARBs were prescribed in 13 patients, including valsartan (11 patients) and candesartan (2 patients). Previous research has demonstrated that Losartan has the uricosuric effect by inhibiting urate transporter 1 (URAT-1) in the renal tubules [29]. Other ARBs like candesartan, olmesartan, and valsartan had no effect or slightly interfered with uric acid excretion [30]. Our data showed that indicators reflecting uric acid excretion function had no difference among the patients using hypotensive drugs, patients free of anti-hypertensive medication, and those without hypertension. The relationships between Sua and hypertension, as well as Sua and anti-hypertensive drugs need to be verified.
The renal handling of uric acid is regulated by glomerular filtration and proximal tubular transportation (mainly transporters for secretion and reabsorption). Uric acid causes the damage to the kidney epithelial cells leading to the deterioration of kidney function, which further aggregates hyperuricemia. Numerous animal models have proved that hyperuricemia induces glomerular hypoxia and eventually accelerates glomerulosclerosis [31]. In addition, impairment of proximal tubules occurs in gout patients. Uric acid is involved in the pathogenesis of acute kidney injury, chronic kidney disease, and hypertension [1]. Likewise, a diminished GFR or a proximal defect in clearing uric acid disrupts Sua homeostasis and eventually aggravates hyperuricemia. ULT is useful to improve the renal handling of uric acid. Obermayr et al. [32] found that Sua levels of 7–8.9 mg/dl nearly doubled the risk for a decline in renal function (OR = 1.74, 95 % CI: 1.45–2.09), while Sua levels >9.0 mg/dl tripled this risk (OR = 3.12, 95 % CI: 2.29–4.25). A few clinical trials have investigated the effect of ULTs on renal function. Feig et al. [33] reviewed seven studies of uric acid and chronic kidney disease (chronic kidney disease stage 2–4) published over the past 7 years; all these studies demonstrated that Sua reduction (by allopurinol 100–300 mg/day) increased estimated GFR and delayed the progression of chronic kidney disease. In our study, XOI group patients showed an obvious elevation of GFR after ULT, which supports the above results. FEua reflects tubular uric acid clearance adjusted for GFR, canceling out the impact of urinary volume. Our study showed that FEua was much lower in gout patients than in healthy individuals. Statistics have showed that this tubular defect has a genetic background, and its heritability has been estimated to be as high as 87 % [34]. After anti-hyperuricemia therapy with XOIs, patients who reached the treatment target had a higher FEua than the target-failure patients. This suggests that ULT may better preserve tubular uric acid excretion. However, this finding is far from conclusive and should be confirmed in basic research studies. Nevertheless, we can conclude that ULT promotes renal uric acid clearance, by improving glomerular filtration and/or increasing tubular excretion. Furthermore, the concept of treat to target is of great importance. Remarkable increase in kidney function was witnessed in patients with post-treatment Sua <6 mg/dl (Ccr increased from 88.54 ± 11.62 ml/min/1.73 m2 at the baseline to 93.98 ± 13.77 ml/min/1.73 m2 after treatment, P < 0.05). These patients had a stronger ability to clear uric acid, showing higher FEua and Cua than patients in the target-failure group. Interestingly, in the target-failure group, the Sua at the observational endpoint was ∼7 mg/dl (7.61 ± 1.61 mg/dl), which is the upper limit for normouricemia. This finding strongly supports that 6 mg/dl is more appropriate cutoff for the treat to target strategy.
The last issue that we tried to explore is which type of ULT is superior. Many studies have reported that hyperuricemia contributes to the development and progression of cardiovascular and metabolic diseases and causes vital organ damage [35–37]. Therefore, the necessity of standardized ULT is compelling. The two conventional cornerstones of ULT are XOIs and uricosuric agents. In our study, the representative XOIs, allopurinol and febuxostat, were compared with the classical uricosuric agent benzbromarone by observing the changes in renal urate excretion. XOIs were recommended as first-line drugs for gout in the 2012 ACR guidelines. A uricosuric agent like probenecid was recommended as an alternative first-line drug only in patients with contraindications or intolerance to XOIs. Although benzbromarone was withdrawn in 2003 in Europe and America due to serious hepatotoxicity, the classical uricosuric agent still has a large market in Asian countries, including China. There are two reasons for the continued use of benzbromarone in China: benzbromarone-related hepatotoxicity is rare in Asia, and other uricosuric agents, like probenecid, are unavailable in China. In addition, allopurinol-associated hypersensitivity syndrome is of great concern, as this syndrome occurs more frequently in Asian countries [38], including China, Japan, and Korea. Owing to the above-mentioned reasons, benzbromarone and XOIs are of equivalent importance in China. Pearson correlation analysis confirmed the different pharmacological mechanisms of XOIs and uricosuric agents on urate excretion. XOIs reduce renal uric acid load mainly by reducing uric acid production; they have no effect on tubular excretion of uric acid. Benzbromarone increases Cua by not only lightening uric acid load but also blocking uric acid reabsorption. It is crucial that after the achievement of similar uric acid loads, renal function was significantly improved in the XOI group (Ccr: from 94.06 ± 15.30 ml/min/1.73 m2 to 89.11 ± 14.16 ml/min/1.73 m2, P < 0.05) but not in the benzbromarone group. Since there are no consistent and systemic recommendations regarding specific ULTs in gout patients with renal impairment, we speculate that XOIs are optimal in gout patients with mild renal dysfunction. As observed in our study, Cua and FLua predict the effects of ULT. They are good parameters for monitoring Sua levels and evaluating the renal handling of uric acid.
A small sample size and short treatment duration are the limitations of our study. This difference was probably attributable to the shorter mean treatment duration in the benzbromarone group. We admit that further analyzing the impact of different dose of febuxostat on renal handling of uric acid will help us to compare different XOIs and the dose effect. Besides, hypertension and anti-hypertensive drugs like ARBs also acted on renal uric acid excretion. This impact was not eliminated in our study. In conclusion, gout patients show higher uric acid loads than do healthy controls and impaired renal handling of uric acid. ULTs can promote renal Cua by improving glomerular and tubular function. XOIs are superior to uricosuric agents in lowering Sua in patients with renal impairment.
Abbreviations
- GFR:
-
Glomerular filtration rate
- ULT:
-
Urate-lowering therapy
- XOI:
-
Xanthine oxidase inhibitors
- Ccr:
-
Clearance of creatinine
- Cua:
-
Clearance of uric acid
- FEua:
-
Fractional excretion of uric acid
- FLua:
-
Glomerular filtration load of uric acid
- Uua/Ucr:
-
Urinary uric acid to Urinary creatinine ratio
- ACR:
-
American College of Rheumatology
- AKI:
-
Acute kidney injury
- RAS:
-
Renin-angiotensin system
- ARB:
-
Angiotensin receptor blockers
- CKD:
-
Chronic kidney disease
References
Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, Yamanashi Y, Kasuga H, Nakashima H, Nakamura T, Takada Y, Kawamura Y, Inoue H, Okada C, Utsumi Y, Ikebuchi Y, Ito K, Nakamura M, Shinohara Y, Hosoyamada M, Sakurai Y, Shinomiya N, Hosoya T, Suzuki H (2012) Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun 3:764. doi:10.1038/ncomms1756
Khachadurian AK (1981) Hyperuricemia and gout: an update. Am Fam Physician 24(6):143–148
Nishida Y (1992) Relation between creatinine and uric acid excretion. Ann Rheum Dis 51(1):101–102
GUTMAN AB, Yu TF (1957) Renal function in gout; with a commentary on the renal regulation of urate excretion, and the role of the kidney in the pathogenesis of gout. Am J Med 23(4):600–622
Fathallah-Shaykh SA, Cramer MT (2013) Uric acid and the kidney. Pediatr Nephrol. doi:10.1007/s00467-013-2549-x
Diamond HS, Paolino JS (1973) Evidence for a post secretory reabsorptive site for uric acid in man. J Clin Invest 52(6):1491–1499. doi:10.1172/JCI107323
Mount DB (2005) Molecular physiology and the four-component model of renal urate transport. Curr Opin Nephrol Hypertens 14(5):460–463
Bellomo G (2013) Uric acid and chronic kidney disease: a time to act? World J Nephrol 2(2):17–25. doi:10.5527/wjn.v2.i2.17
Terkeltaub R, Bushinsky DA, Becker MA (2006) Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res Ther 8 Suppl 1:S4.doi:10.1186/ar1909
Krishnan E (2012) Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS One 7(11):e50046. doi:10.1371/journal.pone.0050046
Toda A, Ishizaka Y, Tani M, Yamakado M (2014) Hyperuricemia is a significant risk factor for the onset of chronic kidney disease. Nephron Clin Pract 126(1):33–38. doi:10.1159/000355639
Chung W, Kim AJ, Ro H, Chang JH, Lee HH, Jung JY (2013) Hyperuricemia is an independent risk factor for mortality only if chronic kidney disease is present. Am J Nephrol 37(5):452–461. doi:10.1159/000350534
Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Wang W, Li X, Wang H (2012) Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 379(9818):815–822. doi:10.1016/S0140-6736(12)60033-6
Koka RM, Huang E, Lieske JC (2000) Adhesion of uric acid crystals to the surface of renal epithelial cells. Am J Physiol Ren Physiol 278(6):F989–F998
Yu KH, Luo SF, Tsai WP, Huang YY (2004) Intermittent elevation of serum urate and 24-hour urinary uric acid excretion. Rheumatology (Oxford) 43(12):1541–1545. doi:10.1093/rheumatology/keh379
Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yü TF (1977) Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum 20(3):895–900. doi:10.1002/art.1780200320
Wang CY, Cogswell ME, Loria CM, Chen TC, Pfeiffer CM, Swanson CA, Caldwell KL, Perrine CG, Carriquiry AL, Liu K, Sempos CT, Gillespie CD, Burt VL (2013) Urinary excretion of sodium, potassium, and chloride, but not iodine, varies by timing of collection in a 24-hour calibration study. J Nutr 143(8):1276–1282. doi:10.3945/jn.113.175927
Kayatas S, Erdogdu E, Cakar E, Yılmazer V, Arınkan SA, Dayıcıoglu VE (2013) Comparison of 24-hour urinary protein and protein-to-creatinine ratio in women with preeclampsia. Eur J Obstet Gynecol Reprod Biol 170(2):368–371. doi:10.1016/j.ejogrb
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612
Perez-Ruiz F, Calabozo M, Erauskin GG, Ruibal A, Herrero-Beites AM (2002) Renal underexcretion of uric acid is present in patients with apparent high urinary uric acid output. Arthritis Rheum 47(6):610–613. doi:10.1002/art.10792
Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, Pillinger MH, Merill J, Lee S, Prakash S, Kaldas M, Gogia M, Perez-Ruiz F, Taylor W, Lioté F, Choi H, Singh JA, Dalbeth N, Kaplan S, Niyyar V, Jones D, Yarows SA, Roessler B, Kerr G, King C, Levy G, Furst DE, Edwards NL, Mandell B, Schumacher HR, Robbins M, Wenger N, Terkeltaub R, American College of Rheumatology (2012) 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 64(10):1447–1461. doi:10.1002/acr.21773
Simkin PA, Hoover PL, Paxson CS, Wilson WF (1979) Uric acid excretion: quantitative assessment from spot, midmorning serum and urine samples. Ann Intern Med 91(1):44–47. doi:10.7326/0003-4819-91-1-44
Perez-Ruiz F, Alonso-Ruiz A, Calabozo M, Herrero-Beites A, García-Erauskin G, Ruiz-Lucea E (1998) Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis 57(9):545–549. doi:10.1136/ard.57.9.545
Taniguchi A, Kamatani N (2008) Control of renal uric acid excretion and gout. Curr Opin Rheumatol 20(2):192–197. doi:10.1097/BOR.0b013e3282f33f87
Lin KC, Lin HY, Chou P (2000) The interaction between uric acid level and other risk factors on the development of gout among asymptomatic hyperuricemic men in a prospective study. J Rheumatol 27(6):1501–1505
Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ (2002) Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Ren Physiol 282(6):991–997
Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, Hollenberg NK, Fisher ND (2004) Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int 66(4):1465–1470
Berni A, Boddi M, Fattori EB, Cecioni I, Berardino S, Montuschi F, Chiostri M, Poggesi L (2010) Serum uric acid levels and renal damage in hyperuricemic hypertensive patients treated with renin-angiotensin system blockers. Am J Hypertens 23(6):675–680
Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H (2002) Molecular identification of a renal urate-anion exchanger that regulates blood urate levels. Nature 417(6887):447–452
Iwanaga T, Sato M, Maeda T, Ogihara T, Tamai I (2007) Concentration-dependent mode of interaction of angiotensin II receptor blockers with uric acid transporter. J Pharmacol Exp Ther 320(1):211–217
Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ (2002) A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13(12):2888–2897
Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R (2008) Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 19(12):2407–2413. doi:10.1681/ASN.2008010080
Feig DI (2014) Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol 26(2):176–185. doi:10.1097/BOR.0000000000000033
Emmerson BT, Nagel SL, Duffy DL, Martin NG (1992) Genetic control of the renal clearance of urate: a study of twins. Ann Rheum Dis 51(3):375–377
Storhaug HM, Norvik JV, Toft I, Eriksen BO, Løchen ML, Zykova S, Solbu M, White S, Chadban S, Jenssen T (2013) Uric acid is a risk factor for ischemic stroke and all-cause mortality in the general population: a gender specific analysis from The Tromsø Study. BMC Cardiovasc Disord 13:115. doi:10.1186/1471-2261-13-115
Nagahama K, Inoue T, Kohagura K, Ishihara A, Kinjo K, Ohya Y (2013) Hyperuricemia predicts future metabolic syndrome: a 4-year follow-up study of a large screened cohort in Okinawa, Japan. Hypertens Res 37(3):232–238
Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, Davos CH, Cicoira M, Shamim W, Kemp M, Segal R, Osterziel KJ, Leyva F, Hetzer R, Ponikowski P, Coats AJ (2003) Uric acid and survival in chronic heart failure: validation and application in metabolic, functional, and hemodynamic staging. Circulation 107(15):1991–1997
Ramasamy SN, Korb-Wells CS, Kannangara DR, Smith MW, Wang N, Roberts DM, Graham GG, Williams KM, Day RO (2013) Allopurinol hypersensitivity: a systematic review of all published cases, 1950–2012. Drug Saf 36(10):953–980
Acknowledgments
This research was supported by grant No. 11DJ1400101 (The Pathogenesis and Therapy of Hyperuricemia and Gout) from Shanghai Committee of Science and Technology Major Program.
Disclosure
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Lili Ma and Lei Wei contributed equally to this work
Rights and permissions
About this article
Cite this article
Ma, L., Wei, L., Chen, H. et al. Influence of urate-lowering therapies on renal handling of uric acid. Clin Rheumatol 35, 133–141 (2016). https://doi.org/10.1007/s10067-014-2806-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2806-9