Abstract
Background
To assure adequate and efficient treatment in the emergency department (ED) despite increasing patient numbers, early risk stratification might be helpful for directing resource allocation.
Objective
To determine whether routine clinical data can predict in-hospital mortality in nonsurgical ED patients and to specifically identify the best predictive parameters.
Materials and methods
This retrospective cohort study investigated 34,333 nonsurgical adult patients who attended one of the two participating EDs in Berlin, Germany, within 1 year. Routine clinical data were analysed for their potential to predict in-hospital mortality using logistic regression as well as classification and regression tree (CART) analysis. A validation dataset contained 35,646 patients of the following year.
Results
In-hospital mortality was 1.8 % (634/34,333). C-reactive protein (CRP) and red cell distribution width (RDW) were the best predictors of mortality. A model with nine predictors (CRP, RDW, age, potassium, sodium, WBC, platelets, RBC and creatinine) achieved an area under the receiver operating characteristic curve (AUROC) of 0.870 (95 % confidence interval, CI: 0.857–0.883). A three-marker model (CRP, RDW, age) resulted in an AUROC of 0.866 (95 % CI: 0.853–0.878). In the independent validation dataset the AUROC for this three-marker model was 0.837 (95 % CI: 0.825–0.850). CART analysis corroborated the importance of CRP and RDW, and a clinical algorithm for risk stratification was developed (Emergency Processes in Clinical Structures, EPICS score).
Conclusion
Two different statistical procedures and independent validation revealed similar results, suggesting a combination of CRP and RDW as a score (EPICS score) for early identification of high-risk patients. This might be particularly helpful in overcrowded situations and where resources are limited. The suggested score should be validated and potentially adapted to diverse ED settings and patient populations in international multicentre trials.
Zusammenfassung
Hintergrund
Um die adäquate und effiziente Behandlung von Notfallpatienten bei steigenden Patientenzahlen gewährleisten zu können, könnte eine frühe Risikostratifizierung der Ressourcenallokation in der Notaufnahme dienen.
Ziel der Arbeit
Welche routinemäßig erhobenen Daten sind geeignet, die intrahospitale Mortalität von internistischen Notfallpatienten vorherzusagen?
Material und Methoden
In dieser retrospektiven Kohortenstudie wurden 34.333 erwachsene internistische Notfallpatienten ausgewertet, welche die Notaufnahmen Charité Campus Virchow Klinikum oder Benjamin Franklin binnen eines Jahres aufsuchten. Klinische Routinedaten wurden mit logistischer Regression und „Classification and Regression trees“ (CART)-Analyse auf ihr Potential der Vorhersage der intrahospitalen Mortalität untersucht. Ein Validierungsdatensatz des Folgejahres enthielt 35.646 Patienten.
Ergebnisse
Die intrahospitale Mortalität war 1,8 % (634/34.333). C-reaktives Protein (CRP) und die Erythrozytenverteilungsbreite (RDW) waren die besten Prädiktoren der Mortalität. Ein Modell mit 9 Prädiktoren (CRP, RDW, Alter, Kalium, Natrium, Leukozyten, Thrombozyten, Erythrozyten, Kreatinin) erzielte eine AUROC von 0,870 (95 %-KI 0,857–0,883). Ein 3-Marker-Modell (CRP, RDW, Alter) resultierte in einer AUROC von 0,866 (95 %-KI 0,853–0,878), welche in der Validierung bei 0,837 (95 %-KI 0,825–0,850) lag. Die CART-Analyse bestätigte CRP und RDW als beste Risikoprädiktoren. Ein möglicher klinischer Anwendungsscore wurde entwickelt („Emergency Processes in Clinical Structures“, EPICS-Score).
Diskussion
Zwei verschiedene statistische Verfahren und die unabhängige Validierung führten zu ähnlichen Ergebnissen, aus denen sich eine Kombination aus CRP und RDW als ein Score (EPICS-Score) ableiten lässt, um Hochrisikopatienten frühzeitig zu erkennen. Der entwickelte Risikoscore könnte nach der erforderlichen externen Validierung in internationalen Multizenterstudien und Anpassung an verschiedene Notfallsettings und Patientenpopulationen hilfreich sein, die Priorisierung der ärztlichen Behandlung insbesondere bei Überfüllung zu steuern.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In order to assure adequate and efficient treatment in the emergency department (ED) in spite of increasing patient numbers, early risk stratification is becoming increasingly important for guiding treatment priority. Clinical information obtained early after presentation to the ED might be useful for improving risk stratification and characterizing the patient populations of different EDs. Data on risk stratification of unselected ED patients are sparse [5]. Therefore, the objective of this analysis was to assess the value of routine data obtained early after admission for risk categorization in terms of in-hospital mortality.
In light of the trend towards increasing patient numbers and limited resources, effective triage systems, early risk stratification and good quality management with detailed standard operating procedures (SOPs) are becoming increasingly important, in order to be able to continue to assure adequate and efficient treatment in the ED [3, 6, 8, 9, 10, 28, 33, 36, 38, 42].
Triage systems such as the Manchester Triage System (MTS) and the Emergency Severity Index (ESI) are predominantly symptom-based approaches. Essential clinical information usually obtained during the early minutes after presentation to the ED and laboratory parameters available after a short time period might be useful for improving risk stratification; however, these currently remain unconsidered in the majority of ED patients. Prior research on risk stratification in nonsurgical ED patients with respect to this routine clinical information has often been conducted in small subpopulations with specific diagnoses, such as myocardial infarction or heart failure, or with specific symptoms [1, 5, 35]. As the initial evaluation of a patient is symptom based, the value of diagnosis-based scores for risk stratification early after admission is limited. For patients with cardiac chief complaints like chest pain or dyspnoea, several studies have assessed risk factors for different endpoints [7, 16, 29, 32] and some of these are commonly used for risk stratification in clinical routine. Data on characterization and risk stratification in the majority of patients with other or no chief complaints, or unselected ED patients, are sparse [2, 5, 14, 15, 19, 20–22, 34], even though these groups represent the majority of ED patients.
The objective of this analysis was to assess the value of the routine vital and laboratory parameters obtained early after admission and usually available within the first hour for their potential role in risk categorization in terms of the endpoint in-hospital mortality.
The results of this study might aid improvement of current risk stratification strategies and thus promote efficient resource allocation in unselected nonsurgical ED patients.
Materials and methods
Patients
This retrospective cohort study included all nonsurgical patients who attended one of the two participating EDs of the University Hospital Charité Berlin (n = 34,333) between February 15, 2009 and February 14, 2010. In a validation analysis, the data of all patients attending the same EDs from February 15, 2011 until February 14, 2012 were included (n = 35,646).
The participating EDs are both central EDs at different sites of the Charité in Berlin, Germany. At both study sites, the decision whether patients are primarily surgical or nonsurgical is taken by the triage. All patients are initially assessed and treated in the ED; and then admitted to a special ward, transferred to another hospital or discharged home after a tentative diagnosis has been made and appropriate treatment initiated. Only adult (above 18 years of age), internal and neurological ED patients were included in this study.
The routine data of all patients were retrieved from the hospital information system (HIS) in an IT-supported automated manner. Data preparation and statistical analyses were accomplished according to the guidelines on good clinical practice in secondary data analysis [17].The Charité ethics committee confirmed that the analysis was acceptable with respect to standard ethical rules in science and research. This study is an investigator-initiated trial and received no funding. The study was registered on November 16, 2009 in the German Clinical Trials Register: universal trial number: U1111-1112-5093.
Biomarker measurement
C-reactive protein (CRP), creatinine, sodium and potassium were measured on the cobas device (Roche Diagnostics, Basel, Switzerland): CRP was determined using a human immunoassay with a coefficient of variation (CV) of 1.2 % at 3.4 mg/l. The CV of creatinine was 2.8 % at 98.2 µmol/l and was determined by the Jaffé Method. The CV for sodium was between 0.3 and 0.4 % at different concentrations and the CV for potassium between 0.3 and 0.5 %. White blood cell count (WBC), red blood cell count (RBC) and platelets were measured by flow cytometry on a Sysmex device (XN series; Sysmex, Hyogo, Japan).
Statistical analysis
The primary endpoint was in-hospital mortality as assessed for every patient during their entire hospital stay (inpatients). For patients who were discharged or transferred from the ED (outpatients), only the stay in the ED was assessed. These outpatients were regarded as survivors. Demographic characteristics (age, gender), as well as chief complaints, vital signs and laboratory parameters were assessed for their potential to predict in-hospital mortality. Only the first value after admission to the ED was analysed. In order to assess the bivariate association between predictors and in-hospital mortality, receiver operating characteristic (ROC) curves and the area under these curves (AUROC) were used. For all variables associated with the investigated endpoint in bivariate analysis, a correlation matrix was constructed to check for multicollinearity (supplementary table 1 “Correlation between all predictors in the final model as well as patient gender”). All analyses stated above were performed using the statistical software SPSS (Statistical Package for Social Science version 19; SPSS, IBM, Armonk, NY, USA). All analyses reported below were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA). Relative risks (RRs) as well as the 95 % exact binomial confidence intervals (CI) were calculated for each single predictor in bivariate analyses. Eleven factors with the highest resulting RRs were further investigated in a multivariate model by logistic regression analysis, as the model did not converge when log-binomial regression was used [37]. However, since the investigated endpoint was rare in the overall cohort as well as in all investigated subgroups, the odds ratios (ORs) obtained from logistic regression analyses are a good estimate of the RR. ROC curves for the final regression model were computed and all variables associated with the endpoint in bivariate analysis were additionally subjected to classification and regression tree (CART) analysis to validate the findings from the logistic regression and to achieve improved interpretation of their effects.
Results
Patient characteristics
Patient characteristics and their association with in-hospital mortality are shown in Table 1.
In-hospital mortality
Overall, 34,333 patients were analysed in this study. Of these, 1.8 % died during their hospital stay (n = 634). Mortality was higher in men (2.1 %) than in women (1.6 %; p = 0.003) and higher in those above 65 years of age (3.5 %) compared to younger patients (0.8 %; p < 0.001, Table 2). An additional dataset containing 35,646 ED patients was used to validate the findings of the derivation dataset. In this validation dataset, the overall in-hospital mortality was 2.1 % (765/35,646).
Diagnoses
The most frequent diagnosis—in both survivors and non-survivors—was stroke (International Classification of Diseases, ICD code: I63). Other frequent diagnoses in non-survivors were sepsis, acute myocardial infarction and heart failure (Table 3).
Multivariate analysis
In multivariate analysis, 26,235 observations were analysed. The final model included nine predictors (Table 4). A CRP value above 71.9mg/l and a red cell distribution width (RDW) value above 16.2 % had the highest ORs.
The ROC curve of this model including all nine factors covered an AUROC of 0.863 (95 % CI: 0.848–0.877). The AUROC for this model including the numeric variables instead of dichotomised values was 0.870 (95 % CI: 0.857–0.883).
Three-marker model
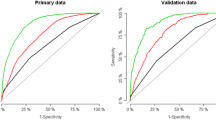
A model containing only the three best predictors for mortality (CRP, RDW, age) revealed an AUROC of 0.866 (95 % CI: 0.853–0.878; Fig. 1a) when numeric variables were modelled and an AUROC of 0.825 (95 % CI: 0.809–0.841) for the binary predictors (Table 5). The best predictor within the model was CRP, with an OR of 5.56.
Validation of the three-marker model
An independent validation of the three-marker model was performed in a validation dataset with 35,646 patients. Of these, 32,493 observations with complete information were available. The AUROC for the three-marker model with numeric variables in the validation was 0.837 (95 % CI: 0.825–0.850; Fig. 1b). Logistic regression for the three binary parameters revealed almost identical ORs for CRP and RDW (Table 5).
Classification and regression tree analysis
All factors associated with the endpoint in bivariate analysis were further analysed in a classification and regression tree (CART) analysis. The identified risk groups ranged in their respective probabilities for in-hospital mortality from 0.6 to 16.2 %. CRP with a cutoff value of 7.2 mg/l was the best predictor for in-hospital mortality. At the second level, RDW with a cutoff value of 14.9 % had the best discriminatory power (Fig. 2). In further splits, age was also identified as separating marker in two of the four risk groups (data not shown). The identified split groups were also applied to the validation dataset and resulted in absolutely comparable risk groups between 0.9 and 15.1 % (Fig. 3). The results of both decision trees could be translated into a clinical scoring system (supplementary figure 1 “The EPICS score” ; EPICS: Emergency Processes In Clinical Structures).
Sensitivity analyses
The proposed EPICS score was analysed for short-term mortality and for inpatients only (supplementary table 2 “Sensitivity analyses in the derivation dataset (n = 34,333)”). Of all 634 patients with a fatal outcome during their initial hospitalisation, 21.6 % (n = 137) died within the first day and 51.4 % (326) within 1 week after admission. Risk categorization remained valid, but risks were lower for short-term mortality and higher when only inpatients were analysed.
Discussion
This is the first study to identify CRP and RDW as the best predictors of in-hospital mortality in unselected medical ED patients and also includes a validation of the identified predictors. With respect to discriminatory power, the inclusion of six further clinical parameters did not improve the prediction significantly, as assessed by the AUROC. The main discriminatory results could be reproduced in a second equally large dataset and in sensitivity analyses, thus validating the initially identified markers as a stable instrument for risk stratification. The CART analysis illustrating the risk actually observed in the resultant subgroups also identified CRP and RDW as the best predictors—albeit with slightly different cutoff points. The resultant risk groups were also reproducible in the independent validation dataset, thus confirming the stability of the identified groups. A risk score was suggested (EPICS score), but needs to be validated and potentially adapted in a prospective multicentre trial.
In-hospital mortality
Overall, 1.8 % of patients died during their initial hospital stay. The mortality in this study is low when compared to other studies investigating ED patients and reporting a 4.7–6.1 % mortality [2, 11, 21, 34]. However, all these studies applied criteria which already contained a risk selection. The mortality of all in-hospital patients in our study was 4.7 % and thus comparable to other studies [18].
Predictors of in-hospital mortality
External validity
Both the original (n = 34,333) and the validation datasets (n = 35,646) of this study represent particularly large datasets of unselected ED patients. Data from two Berlin EDs were analysed. One ED is located in the north of Berlin, in an area with a younger population, lower social status and a higher proportion of immigrants; the other is in the south of Berlin, in a district with a higher social status and an older population.
Internal validity
Only a few studies have investigated risk stratification of truly unselected, nonsurgical ED patients based on routine parameters available early after admission ([5]; Table 6). The AUROCs of the developed scores revealed values between 0.757 and 0.930, and were thus comparable to the AUROC of our three-marker model (0.866). One limitation with respect to the clinical implication of scores developed in other studies is that considerably more predictors were necessary in all bar one of the studies, thus substantially restricting their practical use in an ED setting [2, 11, 15, 21–23, 28, 34, 39]. Most of the scores performed well in the derivation dataset, but some were not validated in independent validation datasets [11, 13, 15]. Some studies showed that laboratory parameters were better predictors of in-hospital mortality as compared to the vital signs used in most of the aforementioned risk scores [4, 15, 26, 27]. The current study achieved an AUROC of 0.870 for a model with nine predictors and an AUROC of 0.866 for a model with three predictors (CRP, age, RDW = EPICS score); thus both values indicate good discriminatory ability [31]. In the validation data set, the AUROC was comparably good (0.837). Therefore, in comparison to other studies, the CAR-I score is practical; has been developed and validated in truly unselected ED patients; and its discriminatory ability is comparable to other experimental scores.
Content validity of identified predictors
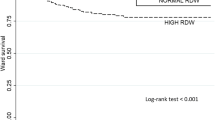
Some of our predictors were also identified by other investigators. Age is included in most scores. CRP, an inflammatory marker, was not part of any of the prediction models in other studies, even though it has been identified as a prognostic marker in several studies, reviews and meta-analyses in different patient populations, as well as in healthy individuals [30, 41]. RDW was only integrated into a model by one study group. RDW reflects the variance in the volume of circulating red blood cells. A high value for RDW equates to a high variance in red blood cell volume (anisocytosis; [24]). Possible causes for changes in the RDW value are chronic anaemia (i.e.: leukaemia, immunhaemolytic anaemia), iron deficiency or disturbances of erythropoiesis (i.e.: erythropoietin resistance, renal failure; [24, 40]). In some studies the prognostic impact of RDW has already been shown. In a review by Patel et al., RDW was a strong predictor for mortality in population-based studies of an older population [25]. O'Sullivan et al. identified RDW as one of six predictors in a model for in-hospital mortality [21]. In a study by Fukuta et al., CRP and RDW did not show any association [12] and the correlation was also low in our population, indicating the independency of their abilities in risk prediction.
Sources of potential bias
The analysis of secondary data is associated with limitations regarding missing values and data quality. Missing values in this study should be regarded as differentially missing, since measurement of laboratory values depends on initial symptoms and the status of the individual patient. Of the patients with missing laboratory values, 48.8 % were outpatients without any blood sampling. These patients are likely to represent a low-risk subpopulation with a low clinical impact on risk scores, particularly as missing laboratory data had a protective impact in additional regression analyses.
Additionally, not only patients admitted to the hospital, but also those transferred to other hospitals and those sent home from the ED were investigated. However, these patients could have died shortly after having been discharged and could thus also have introduced bias into the reported effect measures. Follow-up data should be further considered in future research. Sensitivity analyses were performed to address potential bias. The advantage of using routine data is that no artificial population is specifically selected for study purposes; as such, a real-life ED population was investigated.
Clinical utility
The benefit of risk stratification depends highly on the ED situation in general as well as on the patient population. Using a risk stratification score in addition to established triage systems could help to identify patients in need of early therapeutic measures. The variables in our score are related to common and dangerous diseases (heart failure, infection and sepsis). The most frequent diagnoses of 38.2 % of all non-survivors were attributable to acute conditions (stroke, sepsis, acute myocardial infarction, intracerebral haemorrhage, acute renal failure and pneumonia). The suggested score has the potential to identify these high-risk patients and therefore to improve their allocation to early diagnostic workup and subsequent treatment. Another important potential use of the identified parameters is in benchmarking to better characterize the disease severity of the population of a specific ED, for adequate resource allocation and comparison of EDs.
Practical conclusion
In this study two different statistical procedures and an independent validation revealed similar results, suggesting a combination of CRP and RDW as a score (the EPICS score) for early identification of high-risk patients. This score might prove particularly helpful in situations of overcrowding and where resources are limited. Based on future international multicentre trials, the suggested score should be validated and potentially adapted to the diversity of ED settings and patient populations. Whether the prioritized attention and fast initiation of treatment in patients with a higher risk is an appropriate measure to improve patients' outcome needs to be investigated in an interventional process trial comparing a new risk stratification process guided by the EPICS score with current standards.
Abbreviations
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- CART:
-
classification and regression tree
- CRP:
-
C-reactive protein
- EPICS:
-
Emergency Processes in Clinical Structures
- ESI:
-
Emergency Severity Index
- GCS:
-
Glasgow Coma Scale
- HIS:
-
hospital information system
- MTS:
-
Manchester Triage System
- RAPS:
-
Rapid Acute Physiology Score
- RDW:
-
red cell distribution width
- REMS:
-
Rapid Emergency Medicine Score
- SOP:
-
standard operating procedure
References
Arenja N, Breidthardt T, Socrates T et al (2011) Risk stratification for 1-year mortality in acute heart failure: classification and regression tree analysis. Swiss Med Weekly 141:w13259
Asadollahi K, Hastings IM, Gill GV et al (2011) Prediction of hospital mortality from admission laboratory data and patient age: a simple model. Emerg Med Australasia 23:354–363
Bernstein SL, Aronsky D, Duseja R et al (2009) The effect of emergency department crowding on clinically oriented outcomes. Acad Emerg Medicine 16:1–10
Bland RD, Shoemaker WC, Abraham E et al (1985) Hemodynamic and oxygen transport patterns in surviving and nonsurviving postoperative patients. Critical Care Med 13:85–90
Challen K, Goodacre SW (2011) Predictive scoring in non-trauma emergency patients: a scoping review. Emergency Med J 28:827–837
Christ M, Grossmann F, Winter D et al (2010) Modern triage in the emergency department. Dtsch Arztebl Int 107:892–898
Cinar O, Cevik E, Acar A et al (2012) Evaluation of mid-regional pro-atrial natriuretic peptide, procalcitonin, and mid-regional pro-adrenomedullin for the diagnosis and risk stratification of ED patients with dyspnea. Am J Emerg Med 30(9):1915–1920
Diercks DB, Roe MT, Chen AY et al (2007) Prolonged emergency department stays of non-ST-segment-elevation myocardial infarction patients are associated with worse adherence to the American College of Cardiology/American Heart Association guidelines for management and increased adverse events. Ann Emerg Med 50:489–496
Fee C, Weber EJ, Maak CA et al (2007) Effect of emergency department crowding on time to antibiotics in patients admitted with community-acquired pneumonia. Ann Emerg Med 50:501–509, 509 e501
Fitzgerald G, Jelinek GA, Scott D et al (2010) Republished paper: emergency department triage revisited. Postgrad Med J 86:502–508
Froom P, Shimoni Z (2006) Prediction of hospital mortality rates by admission laboratory tests. Clin Chem 52:325–328
Fukuta H, Ohte N, Mukai S et al (2009) Elevated plasma levels of B-type natriuretic Peptide but not C-reactive protein are associated with higher red cell distribution width in patients with coronary artery disease. Int Heart J 50:301–312
Goodacre S, Turner J, Nicholl J (2006) Prediction of mortality among emergency medical admissions. Emerg Med J 23:372–375
Grimaldi D, Nonino F, Cevoli S et al (2009) Risk stratification of non-traumatic headache in the emergency department. J Neurol 256:51–57
Hucker TR, Mitchell GP, Blake LD et al (2005) Identifying the sick: can biochemical measurements be used to aid decision making on presentation to the accident and emergency department. Br J Anaesth 94:735–741
Kelly AM (2012) How useful are the Heart Foundation risk criteria for assessment of emergency department patients with chest pain? Emerg Med Australasia 24:260–265
Maisel A, Neath SX, Landsberg J et al (2012) Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnoea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Failure 14:278–286
Mockel M, Searle J, Muller R et al (2012) Chief complaints in medical emergencies: do they relate to underlying disease and outcome? The Charite Emergency Medicine Study (CHARITEM). Eur J Emerg Med 20(2):103–108
Nemec M, Koller MT, Nickel CH et al (2010) Patients presenting to the emergency department with non-specific complaints: the Basel Non-specific Complaints (BANC) study. Acad Emerg Med 17:284–292
Nickel CH, Ruedinger J, Misch F et al (2011) Copeptin and peroxiredoxin – 4 independently predict mortality in patients with nonspecific complaints presenting to the emergency department. Acad Emerg Med 18:851–859
O'sullivan E, Callely E, O’riordan D et al (2012) Predicting outcomes in emergency medical admissions—role of laboratory data and co-morbidity. Acute Med 11:59–65
Olsson T, Lind L (2003) Comparison of the rapid emergency medicine score and APACHE II in nonsurgical emergency department patients. Acad Emerg Med 10:1040–1048
Olsson T, Terent A, Lind L (2004) Rapid Emergency Medicine score: a new prognostic tool for in-hospital mortality in nonsurgical emergency department patients. J Int Med 255:579–587
Park KI, Kim KY (1987) Clinical evaluation of red cell volume distribution width (RDW). Yonsei Med J 28:282–290
Patel KV, Semba RD, Ferrucci L et al (2010) Red cell distribution width and mortality in older adults: a meta-analysis. J Gerontol A Biol Sci Med Sci 65:258–265
Pine M, Jones B, Lou YB (1998) Laboratory values improve predictions of hospital mortality. Int J Qual Health Care 10:491–501
Pine M, Norusis M, Jones B et al (1997) Predictions of hospital mortality rates: a comparison of data sources. Ann Int Med 126:347–354
Prytherch DR, Sirl JS, Schmidt P et al (2005) The use of routine laboratory data to predict in-hospital death in medical admissions. Resuscitation 66:203–207
Razzouk L, Fusaro M, Esquitin R (2012) Novel biomarkers for risk stratification and identification of lifethreatening cardiovascular disease: troponin and beyond. Curr Cardiol Rev 8:109–115
Ridker PM, Rifai N, Clearfield M et al (2001) Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med 344:1959–1965
Ridley S (1998) Severity of illness scoring systems and performance appraisal. Anaesthesia 53:1185–1194
Schaub N, Reichlin T, Twerenbold R et al (2012) Growth differentiation factor – 15 in the early diagnosis and risk stratification of patients with acute chest pain. Clin Chem 58:441–449
Schull MJ, Vermeulen M, Slaughter G et al (2004) Emergency department crowding and thrombolysis delays in acute myocardial infarction. Ann Emerg Med 44:577–585
Silke B, Kellett J, Rooney T et al (2010) An improved medical admissions risk system using multivariable fractional polynomial logistic regression modelling. QJM 103:23–32
Simms AD, Reynolds S, Pieper K et al (2012) Evaluation of the NICE mini-GRACE risk scores for acute myocardial infarction using the Myocardial Ischaemia National Audit Project (MINAP) 2003–2009: National Institute for Cardiovascular Outcomes Research (NICOR). Heart 99(1):35–40
Slagman A (2013) Prädiktoren für die intrahospitale Mortalität internistischer Notfallpatienten. http://www.diss.fuberlin.de/docs/servlets/MCRFileNodeServlet/FUDOCS_derivate_000000002899/MSE_Masterarbeit_final_ohne_MNr.pdf
Spiegelman D, Hertzmark E (2005) Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162:199–200
Stolte E, Iwanow R, Hall C (2006) Capacity-related interfacility patient transports: patients affected, wait times involved and associated morbidity. CJEM 8:262–268
Van Der Wulp I, Schrijvers AJ, Van Stel HF (2009) Predicting admission and mortality with the Emergency Severity Index and the Manchester Triage System: a retrospective observational study. Emerg Med J 26:506–509
Van Kimmenade RR, Mohammed AA, Uthamalingam S et al (2010) Red blood cell distribution width and 1-year mortality in acute heart failure. Eur J Heart Failure 12:129–136
Wilhelm J, Hettwer S, Hammer D et al (2012) Outcome prediction using clinical scores and biomarkers in patients with presumed severe infection in the emergency department. Med Klin Intensivmed Notfallmed 107(7):558–563
Wilper AP, Woolhandler S, Lasser KE et al (2008) Waits to see an emergency department physician: U.S. trends and predictors, 1997–2004. Health Aff (Millwood) 27:w84–w95
Acknowledgements
Angela Ale-Abaei and Matthias Koch are leading physicians in the ED of the Charité Benjamin Franklin and were involved in the development of the conceptual design of the study. Marlene Walsh, Rebecca Resendiz, Daniela Vlazny, Martin Stierholz, Anne Jöns, Sara Matern, Franziska Fritzsch, Marie Hesse and Gültekin Bozkurt are medical students who were involved in the data assessment of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
A. Slagman, J. Searle, J.O. Vollert, C. Müller, R. Muller, R. Somasundaram and M. Möckel state that there are no conflicts of interest.
All procedures followed were in accordance with the ethical standards of the responsi ble committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975 (in its most recently amended version).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Slagman, A., Searle, J., Vollert, J. et al. Predicting in-hospital mortality using routine parameters in unselected nonsurgical emergency department patients. Notfall Rettungsmed 18, 501–509 (2015). https://doi.org/10.1007/s10049-015-0055-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10049-015-0055-3