Abstract
Ten months after an ineffective percutaneous coronary stent placement, a 53-year-old patient was rehospitalized with NYHA functional class IV congestive heart failure, ischemic heart disease and left ventricular aneurysm. Echocardiography revealed thrombus formation in the left ventricle with apical aneurysm. Even though left ventricular assist device (LVAD) implantation improves quality of patients’ lives with an increase of its overall use, it becomes more complicated in the presence of ventricular thrombus. We decided to perform ventricular reconstruction with thrombus extraction concomitant to HeartMate 3™ LVAD implantation. The patient was recovered uneventfully, and discharged on postoperative day 14. This report shares the patient’s case and the surgical procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Left ventricular assist device implantation is an accepted and efficient therapy of end-stage heart failure [1]. It is also common to have LV aneurysm following ischemic heart disease [2]. However, the presence of a ventricular thrombus during LVAD insertion increases operational complexity due to higher risk of embolism [3]. Hereby, we present a patient with antero-apical aneurysm undergoing LVAD implantation combined with LV reconstructive surgery.
Case
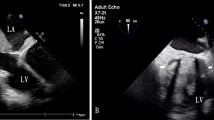
A 53-year-old man suffering from ischemic dilated cardiomyopathy with concomitant antero-apical aneurysm and thrombus formation was readmitted to our clinic in February 2017. His case had already been discussed at the transplant and assist device Medical Review Board of our institution and the final decision was to implement LVAD implantation or heart transplantation in September 2016. The patient’s history consisted of ineffective percutaneous coronary intervention stent placement of his left anterior descending artery in January 2016. Trans-thoracic echocardiography was inadequate because of poor echogenity; so the decision was to perform trans-esophageal echocardiography (TEE). TEE showed an antero-apical akinesis, pronounced diffuse thrombus with a max 2 cm thickness, with a decreased global LV function (LV ejection fraction 0.15) (Fig. 1). Elevated Pulmonary vascular resistance (PVR) with mild right ventricular (RV) dysfunction and grade 2 tricuspid regurgitation were noted. His preoperative assessment can be seen in Table 1.
Even though non-removable ventricular thrombus is hard to detect in preoperative period, the patient’s condition at the time of admission was poor enough to make him undergo LVAD placement as a bridge to transplantation. He was in level 3 according to Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) scale.
Surgical procedure
Under general anesthesia, the operation was performed with median sternotomy. Standard aortic arterial cannulation and unicaval venous cannulation was performed to institute cardiopulmonary bypass. The whole procedure was performed on cardiopulmonary bypass (CPB) without clamping the aorta. To avoid cerebral emboli, aortic valve closure was maintained by full CPB flow and vacuum-assisted venous drainage. We maintained the pump flow much more higher than the calculated ideal patient’s flow till we obtained a flat, nonpulsatile arterial tracing showing the lack of ejection from left ventricle. Intraoperative TEE confirmed the movement of aortic valve hardly opened in systolic phase. Ventriculotomy was performed with apical coring knife. Direct inspection as well as digital palpation revealed a diffuse thrombus and thin ventricular wall (Fig. 2a). Eventually, a longitudinal incision was extended, from LV apex through aneurysmal anterior wall away from LAD, because ventricular thrombus could not be easily resected via apical space. Subendocardial thrombus with a volume of 200 mL was removed aggressively with resection of 2 × 4 cm long scarred, dead tissue (Fig. 2b). This area was only limited to fibrinotic, thin aneurysmal sac without touching the residual dilated myocardium. The edges were then sewn longitudinally by leaving the one edge longer with individual 3–0 polypropylene mattress suture, buttressed with strips of Teflon felt. Extreme caution was given to form the elliptical geometry of ventricle. Giving the correct position of the inflow cannula, the physiologic axis of the ventricle (from the valvular midpoint to the apex) was confirmed by inspection and forcep. The longer edge was used to reinforce the suture line by overlapping it with continue locking suture to form a new neck for the sewing ring (Fig. 3a). The apical cannulation site was reevaluated under TEE prior to overlapping suture-line stitches. Twelve n-shaped stitches were put for sewing the ring fixation. One extra longitudinal mattress suture, securing the two free edges of ventricle to form a new neck, was then closed. After confirming that the new neck was strong enough to hold the device, subsequent steps of HeartMate 3™ LVAD implantation were performed in standard fashion (Fig. 3b).
a Left ventriculotomy was performed for the implantation of inflow cannula via a coring tool at apex. Thrombus formation was detected; however, extension of ventriculotomy was decided for further examination. b Ventriculotomy was extended posterolaterally to perform complete thrombus removal and aneurysmectomy properly. Aneurysm resection was performed together with endocardial scar tissue removal
a Linear reconstruction of the left ventricle was utilized by Teflon plegetted sutures in a horizontal mattress fashion, and the bleeding control was ensured with overlapping excessive tissue and reinforcing the suture line. b After the reconstruction of the left ventricle, LVAD was inserted in standard fashion at the apical part of incision
The patient woke up without any neurological deficit, and extubated on the 16th hour after operation. No postoperative complications were observed. Aggressive anticoagulation and antiplatelet treatment (ASA 150 mg/daily, clopidogrel 75 mg/daily and warfarin) were initiated with his concomitant thromboelastogram and platelet function study. Postoperative echocardiography revealed no residual thrombus, malposition of device or suction; the ventricular cavity was in well-elliptical shape with a LVEDD of 5.3 cm (volume of 160 mL) and the opening of aortic valve was obtained on each cycle (Fig. 4). The patient was transferred to inpatient clinic on postoperative day 4, and he was discharged on the 14th postoperative day with an INR value of 2.5. He was placed on the list for heart transplantation, and was doing well on third month outpatient clinic follow-up with no anticoagulation related complications or ventricular arrhythmias.
Discussion
Given the fact that surgical treatment of heart failure with ventricular assist devices has been significantly increasing, left ventricular aneurysms are still a surgical challenge during implantation due to surgeons’ limited experience. The annual thromboembolic incidence rate of advanced heart failure patients was reported to be between 1.5–3.5%; where the surgery becomes more complicated with the presence of thrombus [3]. Nevertheless, it is technically feasible to reconstruct aneurysm concomitant to LVAD implantation in selective patients [4,5,6].
Non-removable thrombus cannot be easily detected preoperatively. In our patient, preoperative trans-thoracic assessment did not show any evidence of diffuse thrombus until TEE evaluation was done. Total artificial heart implantation or heart transplantation could be another option for such patients. The reconstruction technique of left ventricular aneurysm should be individualized for each patient. Ensuring the adequate ventricular volume as well as its elliptical shape are the important points of the surgical approach. If the visibility of every portion is not sufficient, ventriculotomy should be extended for exploration. We had to extend the incision to excise the thinner wall, evacuate the diffused thrombus and trim the excessive trabecules. Preoperative remodeling of optimal ventricular cavity by echocardiography might be useful to estimate of the area that can be resected [7]. Palmen and colleagues described successful ventricular reconstruction around a Hegar 22 dilator from apex to the base in patients with history of prior surgical ventricular restoration [8]. In our patient, we use forceps to maintain the angle of inflow direction during the reconstruction of a neo-apex. And, we did not encounter any difficulty with the placement of the device; additionally, there was no bleeding around the new apical hole after reconstruction.
For the last two decades, reconstruction with patch turns into usage of the existing native apex if the residual wall is strong enough to hold the inflow cannula and the ventricle has enough volume to prevent suction. Chernyavskiy and Garbade et al. described the surgical reconstruction of ventricle with synthetic patch and defended that the patient would be at increased risk of repeat thromboembolism due to direct blood contact [6, 9]. More recently, there are successful implanted LVADs without patch plasty and the authors argued that the synthetic material could increase the risk of thrombosis, as well as it might alter the ventricular geometry unless it is measured appropriately. Fatullayev et al. were the first who described the reconstruction of the left ventricle without patch plasty [4]. They did not experience any difficulty at providing stability of sewing ring, and did not mention any changes in the inflow cannula position. Nevertheless, we have no direct evidence to conclude the surgical difference due to limited case availability. We preferred to close the ventricle without using a Dacron patch to minimize the thrombogenic predisposition, and the cavity was big enough for a primary closure while creating a good ventricular volume. There can still be a possibility of repeat thrombosis, but we believe that augmented anticoagulation would be safe enough for thrombosis.
One of the concerns during the procedure is the cerebrovascular event due to left ventricular thrombus. Lifting the apex of the heart may eventuate aortic insufficiency. Garbade et al. performed a reconstruction with a Dacron patch by cross-clamping the aorta for a better stabilization of the inflow cannula [9]. We believed that maintaining the aortic valve closure would be enough for avoiding cerebral emboli. It is crucial seeing the nonpulsatile arterial trace, which indicates the lack of ventricular ejection. Intraoperative TEE would be beneficial for following the movement of aortic valve. Chernyawskiy et al. implanted a temporary left ventricular support as a bridge to heart transplantation without clamping of the aorta [7]. In addition, Kervan and colleagues performed successful implantation of HeartWare LVAD following a ventricular reconstruction without cross-clamp and did not complain about perioperative cerebral complications [10].
As the ventricular wall was thin, overlapping the excessive tissue definitely helped to control the bleeding. Additionally, bioglue application on epicardial layers could also be helpful at bleeding control. No complication was seen due to previous thrombus or ventricular upward movement during CPB in the early postoperative period.
Conclusion
This combined surgery was a tailored approach that improves the patient’s physical condition, lowers operation time, and eases the procedure even without patch plasty. Furthermore, an aggressive anticoagulation therapy must be administered for these patients with close monitoring.
References
Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transpl. 2015;34:1495–504.
Wilensky RL, Jung SC. Thromboembolism in patients with decreased left ventricular function: incidence, risk, and treatment. J Cardiovasc Risk. 1995;2:91–6.
Diet F, Erdmann E. Thromboembolism in heart failure: who should be treated? Eur J Heart Fail. 2000;2:355–63.
Fatullayev J, Butters T, Sabashnikov A, Garcia Saez D, Mohite PN, Edwards G, Hoegerle B, et al. Left ventricular assist device implantation with concomitant left ventricular reconstruction without patchplasty. J Artif Organs. 2014;17:370–2.
Smith JW, Pal JD, Walters D, Mahr CM, Mokadam NA. Left ventricular assist device placement with concomitant ventricular reconstruction for aneurysmal disease. J Heart Lung Transpl. 2016;35:372.
Chernyavskiy AM, Marchenko AV, Lomivorotov V, Doronin D, Alsov SA, Nesmachnyy A. Left ventricular assist device implantation combined with surgical ventricular reconstruction. Tex Heart Inst J. 2012;39:627–9.
Cherniavsky AM, Karaskov AM, Marchenko AV, Mikova NV. Preoperative modeling of an optimal left ventricle volume for surgical treatment of ventricular aneurysms. Eur J Cardiothorac Surg. 2001;20:777–82.
Palmen M, Braun J, Beeres SL, Klautz RJ. Left ventricular assist device implantation in patients after left ventricular reconstruction. Interact Cardiovasc Thorac Surg. 2016;23:979–81.
Garbade J, Bittner HB, Barten MJ, Rastan A, Lehmann S, Mohr FW, et al. Combined surgical left ventricular reconstruction and left ventricular assist device implantation for destination therapy in end-stage heart failure. Circ Heart Fail. 2011;4:e14–5.
Kervan U, Unal EU, Sert DE, Pac M. Heartware left ventricular assist device implantation combined with surgical ventricular reconstruction. Exp Clin Transpl. 2016. https://doi.org/10.6002/ect.2016.0056.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Altas, O., Ozer, T., Ozgur, M.M. et al. Regenerating a ventricular cavity during left ventricular assist device implantation. J Artif Organs 22, 169–172 (2019). https://doi.org/10.1007/s10047-019-01089-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-019-01089-4