Abstract
The prognostic nutritional index is an effective prognostic tool used in gastrointestinal surgeries. However, its value has not been verified in cardiovascular surgeries. This study aimed to investigate its utility in hemodialysis-dependent patients undergoing cardiac surgery. We retrospectively reviewed data of 110 hemodialysis-dependent patients who underwent cardiac surgery between January 2006 and July 2016. 20 variables were evaluated for short- and long-term mortality prediction. Patients were divided into high and low prognostic nutritional index groups with values > 34 (n = 90) and ≤ 34 (n = 20), respectively. Preoperative characteristics and surgical outcomes were compared between both groups. Overall, the in-hospital mortality rate was 9% (n = 10) and the 1-, 3-, and 5-year actual survival rates were 69%, 58%, and 40%, respectively. Univariate analysis for hospital death revealed age ≥ 70 years, body mass index ≤ 18 kg/m2, total cholesterol ≤ 120 mg/dl, concomitant procedures, albumin concentration ≤ 3.0 g/dl, and prognostic nutritional index ≤ 34 as risk factors. Multivariate logistic regression analysis confirmed age ≥ 70 years and concomitant procedures as independent risk factors. Whereas ejection fraction ≤ 30% and prognostic nutritional index ≤ 34 were strong independent predictors of long-term death. Patients in the low prognostic nutritional index group had significantly longer postoperative hospitalization, higher incidence of complications, significantly higher in-hospital mortality rate, and significantly lower actual survival rate. The low prognostic nutritional index affected surgical outcomes in hemodialysis-dependent patients undergoing cardiac surgery. Perioperative nutrition management based on the prognostic nutritional index may improve surgical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite recent advance in dialysis technology and surgical techniques, mortality and morbidity rates of hemodialysis (HD)-dependent patients undergoing cardiac surgery remain high [1,2,3,4]. HD-dependent patients frequently suffer from malnutrition and immune suppression, which can affect surgical outcomes [5]. To improve surgical outcomes, risk analysis based on immune-nutritional status is essential in this patient cohort. In gastroenterological surgeries, several indices were reportedly useful in assessing the immune-nutritional status of patients for preoperative risk analysis [6,7,8]. However, these indices are complex and their prognostic value has not been verified in HD-dependent and/or cardiac patients. Onodera et al. [9] proposed a simple prognostic tool, the prognostic nutritional index (PNI), which is calculated using the serum albumin concentration and total lymphocytic count. Several researchers reported its effectiveness in assessing the perioperative immune-nutritional status and surgical risk for patients undergoing gastrointestinal, hepatic, and lung surgeries [9,10,11,12]. However, the prognostic value of PNI has not been verified in cardiovascular surgeries. Thus, the aim of this study was to assess the value of PNI as a predictor of in-hospital and long-term mortalities in HD-dependent patients undergoing cardiac surgery.
Materials and methods
Informed consent was waived for this retrospective, observational study, which has been approved by my institutional committee on human research, and this protocol has been found acceptable by them (approval number A17-028).
Among 1694 consecutive patients who underwent valvular and coronary artery bypass grafting (CABG) at the Jichi Medical University Hospital between January 2006 and July 2016, 119 (7.0%) were chronic HD-dependent patients. Of those, data for PNI calculation were missing in 9 (7.6%) patients who were accordingly excluded. A total of 110 patients was included in this study [82 men and 28 women; mean age, 66 ± 9 years (range 33–83)].In 62 (56%) patients, renal failure was secondary to diabetes. Non-diabetic nephropathy was observed in 48 (44%) patients. Preoperatively, all patients were maintained on HD. The mean dialysis duration was 101 ± 35 months (range 1–432 months) prior to undergoing surgery.PNI was calculated according to the following formula: 10 × the serum albumin (g/dl) + 0.005 × the total lymphocytic count (1000/µL), as reported by Onodera et al. [9].
20 preoperative variables were evaluated to determine the risk factors for in-hospital and remote mortalities. Patients were divided into two groups of high (PNI > 34) (n = 90) and low PNI (PNI ≤ 34) (n = 20). Clinical characteristics of patients were compared between the two groups. In-hospital mortality rates, postoperative complication rates, length of hospital stay, and long-term survival rates were assessed. The cut-off value (PNI = 34) was determined using the first quintile point of PNI. The first quintile point is used to define the cut-off value as statistics method. When this set of patients’ data was divided into five equal parts with PNI value, one group included 22 patients. And these cut-off points are called quantiles. The first quintile point of PNI was 34.5. Based upon this statistical background, we defined cut-off value of PNI to be 34.
Surgical procedures
Surgical procedures are listed in Table 1. Isolated CABG was performed in 38 patients, valve surgery in 42, and combined surgery in 30. In valve surgeries, mechanical heart valves were used in 60 patients, bioprostheses in 8, and annular ring for annuloplasty in 4. The duration of surgery was 369 ± 108 (201–705) minutes. Emergent/urgent surgeries were defined as those performed within 24 h after consultation, and 6 patients underwent emergent/urgent surgeries.
Surgical techniques
Surgery was performed through a median sternotomy, except in four patients with porcine aortain in whom apico-aortic bypass was performed through a left anterolateral thoracotomy. Patients who underwent valve and concomitant cardiac procedures had moderate hypothermic cardiac arrest with standard cardiopulmonary bypass (apico-aortic bypass was performed under ventricular fibrillation). Cold blood cardioplegic solution was administered in an antegrade or a retrograde manner.
HD was performed on the day prior to the surgery using the standard method. We performed hemofiltration using a hemoconcentrator incorporated with a circuit to deal with excess hemodilution during cardiopulmonary bypass. Washed red blood cells treated with an autotransfusion system (Haemoneics Corporation, Braintree, MA) were used to avoid hyperkalemia associated with blood transfusion during surgery. Intraoperative HD was not routinely performed. Following surgery, most patients resumed their routine intermittent HD in the intensive care unit from the first postoperative day, except for those who were hemodynamically unstable or had advanced hyperkalemia, they alternatively underwent continuous veno-venous hemofiltration/hemodiafiltration.
Data collection and follow-up
The clinical characteristics of patients were acquired retrospectively from their medical records.
Preoperative and operative factors were evaluated. Mortality was defined as death occurring during the follow-up period due to any reason. Outcome data were acquired on follow-up. Patients were either examined at our outpatient clinic or contacted by telephone. The follow-up rate was 100%. The time between the surgery and examination or other contact ranged from 1 to 120 months (mean 28 ± 29 months).
Statistical analysis
Categorical variables were presented as numbers. Differences between groups were assessed using Fisher’s exact test. A p value of < 0.05 was used to identify variables for inclusion in the multivariate logistic regression analysis to predict in-hospital mortality. Overall survival was defined as the time from surgery to death from any cause. Time-related survival was estimated using the Kaplan–Meier method and compared by the log-rank test. Hazard ratios (HR) and 95% confidence intervals (CI) estimated using a multivariate Cox proportional hazards regression model were used. Long-term mortality prediction included in-hospital mortality. Statistical analysis was performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R foundation for Statistical Computing, Vienna, Austria). p values < 0.05 were considered statistically significant.
Results
In-hospital mortality occurred in 10 patients (9.0%). The causes of in-hospital mortality were pneumonia (n = 5 patients), heart failure (n = 2), myocardial infarction (n = 2), and cerebral infarction (n = 1).
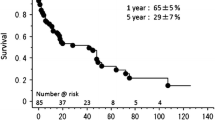
A total of 44 patients died following hospital discharge. The causes of death included heart failure (n = 6), withdrawal from dialysis (n = 6), pneumonia (n = 5), cerebral hemorrhage/infarction (n = 4), arrhythmia (n = 4), multiple organ failure (n = 2), myocardial infarction (n = 1), rupture of abdominal aortic aneurysm (n = 1), malignancy (n = 1), peritonitis (n = 1), ileus (n = 1), lower leg ischemia (n = 1), blood access trouble (n = 1), prosthetic valve endocarditis (n = 1), and unknown (n = 9). The 1-, 3- and 5-year actuarial survival rates including in-hospital deaths were 69 ± 5%, 58 ± 5%, and 40 ± 6%, respectively.
Risk factors for in-hospital death
Preoperative risk factors for in-hospital death are presented in Table 2. Univariate analysis revealed that age ≥ 70 years, concomitant procedures, body mass index (BMI) ≤ 18 kg/m2, total cholesterol level ≤ 120 mg/dl, albumin concentration ≤ 3.0 g/dl and PNI ≤ 34 were risk factors for in-hospital death. Multiple logistic regression analysis confirmed that age ≥ 70 years (HR 15.31, 95% CI 1.36–172.10, p = 0.027) and concomitant procedures (HR 15.20, 95% CI 1.69–137.00, p = 0.015) were risk factors for in-hospital death. While PNI ≤ 34 was not (HR 5.67, 95% CI 0.22–17.30, p = 0.29) (Table 3).
Risk factors for late death
Preoperative risk factors for long-term mortality are presented in Table 2. Univariate analysis (including in-hospital mortality) identified the following 4 risk factors as statistically significant predictors of long-term mortality: ejection fraction ≤ 30%, BMI ≤ 18 kg/m2, concomitant procedures, and PNI ≤ 34. Multivariate analysis using Cox proportional hazards modeling confirmed that ejection fraction ≤ 30% (HR 3.61, 95% CI 1.52–8.57, p = 0.0037) and PNI ≤ 34 (HR 2.09, 95% CI 1.08–4.05, p = 0.029) were statistically significant risk factors for long-term mortality (Table 4).
High PNI (> 34) versus low PNI (≤ 34)
Preoperative and operative characteristics of each group are shown in Table 5. Hemoglobin and BMI were significantly lower among patients in the low PNI group. A significantly higher incidence of infective endocarditis was observed among patients in the low PNI group (p = 0.005).
Patients in the low PNI group had a significantly higher in-hospital mortality rate compared to those in the high PNI group [low PNI group vs. high PNI group: 25.0% (5/20) vs. 5.6% (5/90), p = 0.017]. Additionally, they had a significantly higher postoperative complication rate [low PNI group vs. high PNI group: 55.0% (11/20) vs. 25.6% (23/90), p = 0.015] and a significantly longer postoperative hospitalization (low PNI group vs. high PNI group: 45.1 ± 28.2 vs. 22.7 ± 14.6 days, p = 0.000012) (Table 5).
Long-term results of high PNI (> 34) vs. low PNI (≤ 34) are shown in Fig. 1. Kaplan–Meier survival curves revealed that the respective 1-, 3-, and 5-year actual survival rates were 73 ± 5%, 62 ± 6%, and 42 ± 7% for patients in the high PNI group and 52 ± 11%, 40 ± 12%, and 32 ± 12% for those in the low PNI group. The log-rank test revealed that patients in the low PNI group had a significantly lower survival rate than those in the high PNI group (p = 0.046).
Discussion
Several studies reported the use of indexes for risk estimation of surgical patient based on nutritional assessment in HD-dependent patients [8, 13]. However, those indices and scoring systems were complex and not appropriate for use in the daily clinical practice prior to surgery.
Onodera’s PNI is a simple index that is calculated using the total lymphocytic count and serum albumin [9]. The principal finding of this study was that Onodera’s PNI was able to predict surgical outcomes in HD-dependent patients. In addition, low PNI (≤ 34) was a risk factor that impacted short- and long-term surgical outcomes.
It is known that malnutrition is common in HD-dependent patients and can impact surgical outcomes [5]. Malnutrition was correlated with systemic inflammation and impacted the prognosis of HD-dependent patients. This may be explained by the increased oxidative stress and cytokine production induced by malnutrition [14, 15]. Conversely, inflammation can cause hypoalbuminemia by suppressing albumin synthesis and promoting its transfer from the vascular to the extravascular space, which can occur in combination with reduced protein intake in chronic renal failure patients [15, 16]. Furthermore, low serum albumin levels were found to increase the risk of cardiovascular disease secondary to the development of an acute-phase response [17]. Thus, we believe that malnutrition is strongly correlated with inflammation, which can subsequently impact the prognosis of patients. Stenvinkel et al. reported that there was a strong correlation between malnutrition and inflammation in chronic renal failure patients. Additionally, the authors advocated the concept of “malnutrition, inflammation, and atherosclerosis (MIA syndrome)” [16]. They hypothesized that a vicious cycle of MIA syndrome may aggravate the deterioration of patients. Thus, the relationship between malnutrition and inflammation (and subsequent immunosuppression) should be considered in patients with end-stage renal failure undergoing cardiac surgery.
Our results indicated that PNI reflected the preoperative conditions of HD-dependent patients and effectively predicted their prognosis. While, hypoalbuminemia has been shown as a risk factor for poor prognosis [16]. In this study, we analyzed serum albumin concentration as a variable for hospital and remote death. Univariate analysis revealed albumin ≤ 3.0 g/dl as a risk factor for hospital death as well as PNI ≤ 34; however, multivariate analysis determined higher odds ratio in PNI than in serum albumin concentration. While, albumin ≤ 3.0 g/dl was not identified as a risk factor for remote death. Thus, PNI is more useful and realistic than serum albumin concentration to estimate surgical risk.
It was reported that the total lymphocytic count, which is another component of the PNI, was an indicator of cell-mediated immunity [18,19,20]. Previous studies revealed that cell-mediated immunosuppression was reflected by a decrease in the total lymphocytic count and that the magnitude of the decline in the number of lymphocytes predicted the prognosis of patients who underwent mechanical circulatory support and gastrointestinal surgeries [18,19,20]. This suggested that the coexistence of malnutrition and immunosuppression may aggravate the vicious cycle.
The current study indicated that low PNI (≤ 34) impacted short- and long-term survival, which may affect patient selection and surgical strategy. To improve outcomes of cardiac surgery in HD-dependent patients, patient selection and adequate surgical strategies based on PNI should be considered. In case of low PNI patients, surgical stress should be reduced as little as possible. For instance, prior procedures such as percutaneous coronary intervention and endovascular surgery might give one the idea to reduce surgical stress in combined surgeries. Additionally, intravenous hyperalimentation and serum albumin supply for aggressive perioperative nutritional management in addition to the conventional nutritional interventions such as dietary care and nutritional supplements should be considered to improve the immune-nutritional condition of patients. Owing to nutrition-inflammatory interactions, aggressive cytokine removal using online hemodiafiltration, continuous hemodiafiltration, or the use of polymethyl-methacrylate hemofilter can be potentially beneficial in controlling chronic microinflammation [5]. Thus, appropriate surgical strategies based on nutritional assessment and aggressive perioperative nutritional management may contribute to improving the clinical outcomes of HD-dependent patients who are undergoing cardiac surgery.
This study had several limitations. It was a non-randomized, retrospective study with a small sample size and a relatively short follow-up period. Furthermore, the data were collected over a 10-year period. This means that it is likely that the outcomes may have been influenced by the different surgical techniques and perioperative managements employed, including methods of hemodialysis.
Conclusion
Our results indicated that the low PNI affected short- and long-term surgical outcomes in HD-dependent patients undergoing cardiac surgery. Perioperative evaluations based on PNI may contribute to improving the surgical outcomes of HD-dependent patients who require cardiac surgery.
References
Zimmet AD, Almeida A, Goldstein J, Shardey GC, Pick AW, Lowe CE, Jolley DJ, Smith JA. The outcome of cardiac surgery in dialysis-dependent patients. Heart Lung Circ. 2005;14:187–90.
Filsoufi F, Rahmanian PB, Castillo JG, Silvay G, Carpentier A, Adams DH. Predictors and early and late outcomes of dialysis-dependent patients in contemporary cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:522–9.
Rahmanian PB, Adams DH, Castillo JG, Vassalotti J, Filsoufi F. Early and late outcome of cardiac surgery in dialysis-dependent patients: single-center experience with 245 consecutive patients. J Thorac Cardiovasc Surg. 2008;135:915–22.
Vohra HA, Armstrong LA, Modi A, Barlow CW. Outcomes following cardiac surgery in patients with preoperative renal dialysis. Interact Cardiovasc Thorac Surg. 2014;18:103–11.
Kawahito K, Aizawa K, Oki S, Saito T, Misawa Y. Heart valve surgery in hemodialysis-dependent patients: nutrition status impact on surgical outcome. J Artif Organs. 2016;19:134–40.
Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–7.
Iwasa M, Ogoshi S, Kitagawa S, Ohmori Y, Iwasa Y, Mizobuchi S, Tamiya T, Isono K. Effect of Preoperative Hyperalimentation for Patients with Esophageal Cancer and Usefulness of the Nutritional Assessment Index. In: Siewert JR, Hölscher AH, eds. Diseases of the Esophagus. Berlin: Springer; 1988. pp. 264–7.
Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–63.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–5.
Okada S, Shimada J, Teramukai S, Kato D, Tsunezuka H, Miyata N, Ishihara S, Furuya T, Nakazono C, Ishikawa N, Inoue M. Risk stratification according to the prognostic nutritional index for predicting postoperative complications after lung cancer surgery. Ann Surg Oncol. 2018; https://doi.org/10.1245/s10434-018-6368-y (Epub ahead of print).
Ke M, Xu T, Li N, Ren Y, Shi A, Lv Y, He H. Prognostic nutritional index predicts short-term outcomes after liver resection for hepatocellular carcinoma within the Milan criteria. Oncotarget. 2016;7:81611–20.
Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–92.
Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am J Clin Nutr. 2008;87:106–13.
Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82.
Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79:1635–41.
Stenvinkel P, Heimbu¨rger O, Lindholm B, Kaysen GA, Bergstro¨m J. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome). Nephrol Dial Transpl. 2000;15:953–60.
Kaysen GA, Stevenson FT, Depner TA. Determinants of albumin concentration in hemodialysis patients. Am J Kidney Dis. 1997;29:658–68.
Mitsusada M, Suzuki S, Kobayashi E, Enosawa S, Kakefuda T, Miyata M. Prevention of graft rejection and graft-versus-host reaction by a novel immunosuppressant, FTY720, in rat small bowel transplantation. Transpl Int. 1997;10:343–9.
Yamauchi H, Kobayashi E, Yoshida T, Kiyozaki H, Hozumi Y, Kohiyama R, Suminaga Y, Sakurabayashi I, Fujimura A, Miyata M. Changes in immune-endocrine response after surgery. Cytokine. 1998;10:549–54.
Kawahito K, Kobayashi E, Misawa Y, Adachi H, Fujimura A, Ino T, Fuse K. Recovery from lymphocytopenia and prognosis after adult extracorporeal membrane oxygenation. Arch Surg. 1998;133:216–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kurumisawa, S., Kawahito, K. Risk analysis using the prognostic nutritional index in hemodialysis-dependent patients undergoing cardiac surgery. J Artif Organs 21, 443–449 (2018). https://doi.org/10.1007/s10047-018-1056-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-018-1056-z