Abstract
Treating a patient with heparin-induced thrombocytopenia can be challenging particularly when the patient requires urgent cardiac surgery that uses heparin for anticoagulation. We herein report a case of a 61-year-old man with idiopathic dilated cardiomyopathy associated with heparin-induced thrombocytopenia and who underwent plasma exchange to remove heparin-induced thrombocytopenia antibodies before undergoing left ventricular assist device implantation. The surgery was performed using cardiopulmonary bypass and unfractionated heparin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune-mediated heparin-induced thrombocytopenia (HIT) is a prothrombotic and life-threatening side effect of heparin. Patients with HIT are at high risk for a fatal thrombotic event due to anti-platelet factor 4 (PF4)/heparin IgG antibodies, which activate platelets in a heparin-dependent fashion and consequently increase thrombin generation [1].
The first-line treatment of HIT is heparin discontinuation and replacing heparin with another anticoagulant. However, in the setting of cardiac surgery, replacing heparin with another drug is generally hesitated because uncertainties exist in the management of the alternative drug due to the scarcity of relevant clinical data.
We herein report a case of a HIT patient who underwent left ventricular assist device (LVAD) implantation using unfractionated heparin (UFH) for anticoagulation after plasma exchange (PE), which was performed to remove the HIT antibodies.
Case report
A 61-year-old man with idiopathic dilated cardiomyopathy diagnosed 7 years ago was transferred to our hospital for the treatment of uncontrolled congestive heart failure. Serial echocardiography and Swan–Ganz catheter pressure tracings revealed a severely impaired heart (central venous pressure 7 mmHg; mean pulmonary arterial pressure 34 mmHg; pulmonary wedge pressure 20 mmHg; cardiac index 1.7 L/min/m2; left ventricular end-diastolic/systolic diameter 70/66 mm; left ventricular ejection fraction 13%). After admission, the patient was given increased doses of inotropes and diuretics, and intravenous UFH was initiated. Despite maximum medical therapy, the patient continued to suffer from the symptoms of heart failure, and an intra-aortic balloon pump (IABP) was inserted via the femoral approach at day 7 of admission. Heart transplantation was deemed necessary for this patient; thus, LVAD implantation was indicated.
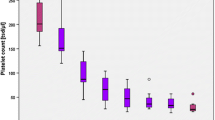
Serial blood examinations showed a platelet count that began to drop at day 7 of UFH infusion (Fig. 1). This decline in the platelet count was initially thought to be due to the mechanical effects of the IABP; however, subsequent hematological data suggested the involvement of HIT (4Ts score [2], 5 points). HIT was confirmed by the positive results from the enzyme-linked immunosorbent assay (ELISA, PF4-IgG, Genetic Testing Institute, Waukesha, WI, USA) for anti-PF4/heparin IgG antibodies (optical density value, 1.00; cutoff value, 0.4) and the washed platelet activation assay. At day 21 of admission, all heparin infusions were ceased and replaced with argatroban (0.07–0.08 µg/kg/min, titrated to obtain an activated partial thromboplastin time of 45 –55 s); a prompt recovery of the platelet count was then observed. No signs of thrombosis were identified, and the stable hemodynamics with the IABP in place suggested that LVAD implantation could be delayed until the tests showed negative for the HIT antibodies. However, at 1 month of isolation from heparin, both the ELISA and the washed platelet activation assay still showed a positive result. As the patient’s mental condition began to grow unstable due to the IABP placement, which prohibited ambulation, an earlier LVAD implantation was considered necessary and PE was undertaken to facilitate the removal of the HIT antibodies. Continuous hemodiafiltration using fresh frozen plasma as fluid replacement was performed; a total of 5.6 L of plasma was exchanged. After the exchange, negative results were obtained from the assays (Fig. 1). Another PE was performed on the day before surgery (2 days after the first PE) due to concerns of a potentially suboptimal HIT antibody level at the time of surgery. The patient underwent implantation of the Jarvik 2000 LVAD (Jarvik Heart Inc., New York, NY, USA) concomitantly with tricuspid annuloplasty using a 28-mm Edwards MC3 tricuspid ring (Edwards Lifesciences, Irvine, CA, USA). CPB was used during the procedures. UFH was administered as a bolus (300 U/kg) before commencing CPB and additionally dosed based on the activated clotting time during CPB. After weaning off CPB, UFH was reversed with intravenously administered protamine sulfate. No clots were detected in either the reservoir or the oxygenator throughout the surgery. The total operation time and the CPB time were 408 and 101 min, respectively. The amount of intraoperative bleeding was 2115 mL. Anticoagulation with argatroban and warfarin was resumed 2 days after the surgery. Despite the reuse of UFH during surgery, HIT-related thrombosis was not detected, and a steady rise in the platelet count was observed postoperatively. Thrombin-antithrombin complex, D-dimer, and fibrin/fibrinogen degradation product levels remained low for a month, and all results of the ELISA and the washed platelet activation assay remained negative for 3 weeks after the surgery (Fig. 2). Postoperative course was uneventful and he discharged on postoperative day 87.
Comment
Heparin-induced thrombocytopenia is an immune-mediated adverse drug reaction to unfractionated or low-molecular-weight heparin, and its frequency has been reported to be 11% in patients receiving a mechanical circulatory support [3]. HIT is caused by the formation of antibodies against the heparin–platelet factor (PF4) complex; the binding of the antibodies to the heparin–PF4 complex activates platelet aggregation and thrombin generation. The treatment of HIT is generally discontinuation of heparin or replacement of heparin with another anticoagulant; currently, the use of other anticoagulants remains unestablished in the setting of cardiac surgery. Therefore, treating a HIT patient who needs cardiac surgery can be challenging, particularly if the need is urgent.
UHF is the most commonly used anticoagulant in cardiac surgery because of its rapidly titratable and easily reversible properties. Argatroban, which is a direct thrombin inhibitor, is an alternative drug that can replace UFH. However, the usefulness of argatroban in the surgical setting has remained controversial; several studies have contradicted its efficacy because bleeding complications are common [4, 5]. There is no specific reversal agent for argatroban, and reports have shown that the anticoagulation effect of argatroban may continue up to 29 h after cessation of the drug due to its increased elimination half-life and decreased clearance from the blood circulation [4]. The consequence of these effects is prolonged bleeding time, with which, in our case, despite the cessation of argatroban 3 h before surgery, we had trouble dealing during and after surgery.
The current American College of Chest Physicians guideline suggests that short-term use of heparin during CPB is acceptable in HIT patients who are sufficiently cleared of the platelet-activating HIT antibodies [6]. The reported median time for the HIT antibodies to become undetectable after isolation from heparin is 50–80 days, depending on the type of assay performed [7]. Thus, in patients who need an urgent CPB-involving procedure, such waiting time may be intolerable.
Vender et al. first reported PE as a rescue therapy for HIT patients who require cardiac surgery [8]. They reported uneventful CPB using heparin after performing multiple PEs. A recent study has also demonstrated that PE can rapidly remove the HIT antibodies from the circulation, thus further implicating the potential of PE for HIT treatment [9]. Other studies have shown similar efficacy of PE as a prophylaxis before CPB, although, notably, there were patients who were not tested for adequate clearance of the circulating HIT antibodies before commencing CPB [10, 11]. Welsby et al. published a case series of 11 patients who were diagnosed as HIT from the ELISA results and received a single PE [10]. All patients underwent an uneventful cardiac surgery using heparin. A follow-up ELISA was performed postoperatively in 9 of the 11 patients: 6 had negative antibody titers (optical density < 0.4) and the rest had equivocal results but showed reduction in the titers (optical density 0.5–1.3); none had an assay performed before reexposure to heparin. Whereas the guideline suggests that HIT patients should avoid heparin re-exposure until the antibody levels are undetectable [6], it must be noted that the cutoff for a clinically “safe” HIT antibody level has not been determined. In our case, we set the cutoff, which was based on the suggestions from previous studies [10, 12], as either < 0.4 for optical density on the ELISA or a negative result on the washed platelet activation assay. Although a “safe” level was reached after the initial PE, there was a 2-day interval between PE and surgery; thus, a second PE was conducted the day before surgery to ensure safety during CPB. Our approach may have been too aggressive; however, provided with no universally agreed cutoff, we considered it safer to reduce the antibody level further using PE than to risk a complication related to HIT during CPB. A limitation exists in emergent situations where there is no time for a negative result before surgery. In such situations, it would be helpful to know the minimum amount of PE required for a given HIT antibody level. Further studies are thus necessary.
In conclusion, this case report supports the efficacy of PE in HIT patients undergoing cardiac surgery that uses UFH for anticoagulation. Although the use of non-heparin anticoagulants has been described in the literature, safety issues associated with the monitoring of these drugs during CPB remain unsolved. Delaying the surgery until the HIT antibodies are undetectable may be an option if the patient can tolerate the delay. If urgent surgery is required, preoperative PE may facilitate the removal of the antibodies; however, further studies are necessary to optimize the treatment strategy of HIT using PE.
References
Greinacher A. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373:252–61.
Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–65.
Schenk S, El-Banayosy A, Prohaska W. el al. Heparin-induced thrombocytopenia in patients receiving mechanical circulatory support. J Thorac Cardiovasc Surg. 2006;131:1373–81.
Follis F, Filippone G, Montalbano G, et al. Argatroban as a substitute of heparin during cardiopulmonary bypass: a safe alternative? Interact CardioVasc Thorac Surg. 2010;10:592–6.
Hillebrand J, Sindermann J, Schmidt C, Mesters R, Martens S, Scherer M. Implantation of left ventricular assist devices under extracorporeal life support in patients with heparin-induced thrombocytopenia. J Artif Organs. 2015;18:291–9.
Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012; 141:e495S–530S.
Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med. 2001;344:1286–92.
Vender JS, Matthew EB, Silverman IM, Konowitz H, Dau PC. Heparin-associated thrombocytopenia: alternative managements. Anesth Analg. 1986;65:520–2.
Warkentin TE, Sheppard JI, Chu FV, Kapoor A, Crowther MA, Gangji A. Plasma exchange to remove HIT antibodies: dissociation between enzyme-immunoassay and platelet activation test reactivities. Blood. 2015;125:195–8.
Welsby IJ, Um J, Milano CA, et al. Plasmapheresis and heparin reexposure as a management strategy for cardiac surgical patients with heparin-induced thrombocytopenia. Anesth Analg. 2010;110:30–5.
Jaben EA, Torloni AS, Pruthi RK, Winters JL. Use of plasma exchange in patients with heparin-induced thrombocytopenia. A report of two cases and a review of the literature. J Clin Apher. 2011;26:219–24.
Maeda T, Nakagawa K, Murata K, et al. Identifying patients at high risk of heparin-induced thrombocytopenia-associated thrombosis with a platelet activation assay using flow cytometry. Thromb Haemost. 2017;117:127–38.
Acknowledgements
The permission to publish this case report has been granted by the patient.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Koizumi, S., Kohno, H., Watanabe, M. et al. Left ventricular assist device implantation after plasma exchange for heparin-induced thrombocytopenia. J Artif Organs 21, 462–465 (2018). https://doi.org/10.1007/s10047-018-1055-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-018-1055-0